Abstract
Purpose
Hyperglycemia has been associated with poor outcomes in many disease states. This retrospective study assessed the association between hyperglycemia and survival in patients with newly diagnosed glioblastoma multiforme (GBM).
Patients and Methods
Between 1999 and 2004, before the standard use of temozolomide, 191 patients were accrued onto New Approaches to Brain Tumor Therapy CNS Consortium trials with similar eligibility criteria. Time-weighted mean glucose and mean glucocorticoid dose were calculated for each patient using all values collected regularly in follow-up. The primary outcome was survival.
Results
Mean glucose levels ranged between 65 and 459 mg/dL. These were divided into quartiles: quartile one (< 94 mg/dL), quartile two (94 to 109 mg/dL), quartile three (110 to 137 mg/dL), and quartile four (> 137 mg/dL). Median survival times for patients in quartiles one, two, three, and four were 14.5, 11.6, 11.6, and 9.1 months, respectively. The association between higher mean glucose and shorter survival persisted after adjustment for mean daily glucocorticoid dose, age, and baseline Karnofsky performance score (KPS). Compared with patients in the lowest mean glucose quartile, those in quartile two (adjusted hazard ratio [HR], 1.29; 95% CI, 0.85 to 1.96), quartile three (adjusted HR, 1.35; 95% CI, 0.89 to 2.06), and quartile four (adjusted HR, 1.57; 95% CI, 1.02 to 2.40) were at progressively higher risk of dying (P = .041 for trend).
Conclusion
In these patients with newly diagnosed GBM and good baseline KPS, hyperglycemia was associated with shorter survival, after controlling for glucocorticoid dose and other confounders. The effect of intensive management of glucocorticoid-related hyperglycemia on survival deserves additional study in patients with GBM.
INTRODUCTION
A growing body of evidence in the diabetes literature suggests that short-term, and even relatively mild, hyperglycemia leads to excess morbidity and mortality in acute illness.1,2 In the setting of cancer, in particular, large epidemiologic studies have suggested that survival is shorter in patients with comorbid diabetes.3–7
Patients with glioblastoma multiforme (GBM) are at particular risk for hyperglycemia, because their peritumoral edema is routinely treated with high-dose glucocorticoids, which are known to increase plasma glucose, due to impairing glucose transport.8–10 Furthermore, because patients with GBM have a poor prognosis and are thus unlikely to be at risk for long-term diabetes complications, the goal of hyperglycemic management in these patients is, in general, the avoidance of acute hyperglycemic complications, rather than intensive management. In this study, we tested the hypothesis that hyperglycemia is associated with shorter survival in patients with newly diagnosed GBM.
PATIENTS AND METHODS
This retrospective study, approved by the institutional review board, analyzed the association between hyperglycemia and survival among patients with newly diagnosed GBM accrued onto five New Approaches to Brain Tumor Therapy CNS Consortium phase II trials. These trials were conducted between 1999 and 2004, before the standard use of temozolamide. They assessed the effect on survival of noncytotoxic therapies, including penicillamine, carboxyamido-triazole, suramin, gadolinium-texaphyrin, and celecoxib, which were administered in combination with postoperative external-beam radiation therapy (60 Gy in 30 fractions).11–15 None of the experimental agents are known to affect glucose homeostasis, and none were associated with improved survival.
Patients
Patients eligible for this review had histologically confirmed supratentorial grade 4 astrocytoma (GBM), which was untreated except for biopsy or surgery, and glucocorticoid administration. Other eligibility criteria for accrual onto the five trials included: age ≥ 18 years, Karnofsky performance score (KPS) ≥ 60, estimated life expectancy ≥ 2 months, absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 100,000 μL, hemoglobin ≥ 9.0 g/dL, creatinine ≤ 1.7 mg/dL, bilirubin ≤ 1.2 to 1.5 mg/dL, aminotransferases ≤ 2 to 4 times above the upper limit of normal, and ability to provide informed consent. Exclusion criteria included the presence of an intercurrent illness that could interfere with protocol treatment, and concurrent malignancy unless disease free for ≥ 5 years. Prior diabetes was not an exclusion criterion.
Glycemia and Glucocorticoid Assessment
Each patient had basic chemistries, which included glucose, performed approximately weekly during radiation therapy, and every 1 to 2 months thereafter. The fasting status of the patient at the time of testing was not known. Mean glucose was calculated for each patient using all available glucose results. The primary analysis used a time-weighted approach for calculating mean glucose, in which each glucose value was weighted according to the number of days between it and the next record or the censor date.
Glucocorticoids were prescribed by the patients' oncologists based on clinical need. The doses were recorded during follow-up approximately every 1 to 2 months. Three patients had no recorded glucocorticoid doses, and were excluded from the analysis. A time-weighted mean dexamethasone dose was calculated using the same method used for glucose.
Statistical Analysis
Mean glucose was divided into four groups according to the quartiles of distribution. Patient characteristics—including mean daily dexamethasone dose, age, KPS, sex, race, extent of surgical resection (craniotomy for resection v biopsy), and trial onto which accrued—were compared across quartiles using linear regression for continuous variables, and logistic regression for dichotomous variables.16–19
The primary end point of this study was survival. Overall survival time was calculated as time from histologic diagnosis to death as a result of any cause. Event times were censored if the patient was alive at the time of last follow-up.
Univariate Cox proportional hazards regression analysis was used to assess the association between individual patient characteristics and overall survival status.20 We used P ≤ .05 as the criterion for selecting covariates to be included in the multivariate Cox regression model. The unadjusted and adjusted hazard ratios (HRs) with respect to the mean glucose quartiles were reported. Moreover, multivariate Cox proportional hazards regression was also employed to estimate adjusted HRs associated with each increase of 10 mg/dL in mean glucose.
A secondary end point was infection, which was recorded prospectively by medical record review. Multivariate logistic regression was used to evaluate the effect of mean glucose on the development of infection, and Cox proportional hazards regression was used to assess the effect of infection on survival. All analyses were performed using STATA software (STATA Corp, College Station, TX; Computing Resource Center, Santa Monica, CA), and all reported P values are two sided.
RESULTS
Of the 191 patients studied, the mean age was 55.7 years (standard deviation, 11.2 years), the median KPS was 90 (range, 60 to 100), 64% were male, and 93% were white. Mean daily dexamethasone dose ranged between 0 and 48 mg (median, 5 mg). The mean time-weighted glucose varied between 65 and 459 mg/dL, and was divided into quartiles: quartile one (< 94 mg/dL), quartile two (94 to 109 mg/dL), quartile three (110 to 137 mg/dL), and quartile four (> 137 mg/dL).
Comparisons of demographic and clinical characteristics according to quartile of time-weighted mean glucose are listed in Table 1. Consistent with the glucose-elevating effect of glucocorticoids, mean daily dexamethasone dose increased with increasing quartile of mean glucose (P = .006 for trend). Additionally, mean age increased and KPS decreased with increasing quartile of mean glucose (P = .001 and .040 for trend, respectively). There was no difference in the distribution of sex, race, or surgical debulking versus biopsy with regard to quartile of mean time-weighted glucose.
Table 1.
Patient Characteristics According to Quartile of Time-Weighted Mean Glucose
| Characteristic | Quartile of Mean Glucose |
P | |||
|---|---|---|---|---|---|
| One (n = 46) | Two (n = 50) | Three (n = 48) | Four (n = 47) | ||
| Dexamethasone dose, mg | .006 | ||||
| Median | 3 | 4 | 6 | 8 | |
| 25%-75% percentiles | 0-6 | 1-10 | 2-15 | 4-14 | |
| Age, years | .001 | ||||
| Mean | 53 | 54 | 55 | 60 | |
| SD | 14 | 10 | 9 | 10 | |
| Sex | .926 | ||||
| Male, % | 65 | 66 | 54 | 70 | |
| Race | .459 | ||||
| White, % | 96 | 94 | 88 | 94 | |
| KPS | .053 | ||||
| ≥ 90, % | 74 | 76 | 67 | 54 | |
| Surgery | .257 | ||||
| Debulked, % | 96 | 74 | 83 | 83 | |
Abbreviations: SD, standard deviation; KPS, Karnofsky performance score.
Of the 191 patients accrued onto the studies, 187 (98%) died in the follow-up period, which encompassed 214.3 person-years. The mortality rate was 0.87 deaths per person-year, and the median survival was 11.0 months. The four surviving patients were observed between 35 and 82 months.
Median survival was 14.5 months in quartile one, 11.6 months in quartile two, 11.6 months in quartile three, and 9.1 months in quartile four. In an unadjusted Cox proportional hazards model, relative to quartile one, the estimated HRs were 1.30 for quartile two (95% CI, 0.86 to 1.95), 1.56 for quartile three (95% CI, 1.02 to 2.37), and 2.06 for quartile four (95% CI, 1.35 to 3.14, with a P value of .001 for trend; Table 2). For every 10 mg/dL increase in time-weighted mean glucose, the HR was 1.05 (95% CI, 1.02 to 1.07; P < .0001). In addition, mean daily dexamethasone dose, age, and KPS were each statistically significantly associated with survival in the univariate analysis (Table 2).
Table 2.
Univariate Association Between Patient Characteristics and Survival
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Mean glucose | .001 | ||
| Quartile one, n = 46 | Reference | ||
| Quartile two, n = 50 | 1.30 | 0.86 to 1.95 | |
| Quartile three, n = 48 | 1.56 | 1.02 to 2.37 | |
| Quartile four, n = 47 | 2.06 | 1.35 to 3.14 | |
| Mean daily dexamethasone dose, per 10 mg/d | 1.29 | 1.14 to 1.45 | < .0001 |
| Age, per 10 years | 1.50 | 1.30 to 1.73 | < .0001 |
| KPS, per 10 points | 0.82 | 0.71 to 0.94 | .006 |
| Race, white v other | 1.47 | 0.84 to 2.59 | .181 |
| Sex, male v female | 1.04 | 0.77 to 1.41 | .794 |
| Biopsy v surgery | 1.34 | 0.91 to 1.98 | .133 |
| Trial | .908 | ||
| Penicillamine | Reference | ||
| Carboxyamido-triazole | 0.95 | 0.63 to 1.44 | |
| Suramin and XRT | 1.20 | 0.79 to 1.81 | |
| Gadolinium-texaphyrin | 0.83 | 0.35 to 1.97 | |
| Cox-2 inhibitor | 0.99 | 0.62 to 1.57 | |
NOTE. The time-weighted mean glucose values for quartiles one to four are < 94, 94 to 109, 110 to 137, and > 137 mg/dL, respectively.
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance score; XRT, radiation therapy.
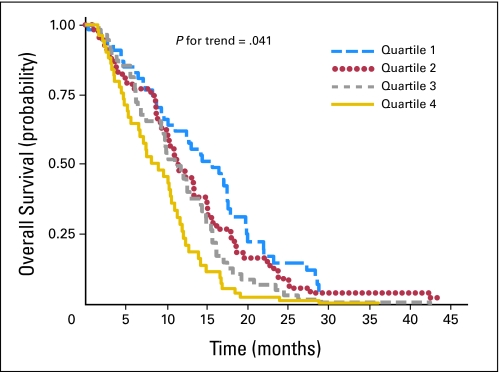
After we controlled for mean daily dexamethasone dose, age, and KPS in a multivariate Cox proportional hazards regression model, the association between hyperglycemia and survival persisted. Figure 1 displays the adjusted survival curves for the four quartiles of time-weighted mean glucose. Compared with patients in the lowest quartile of mean glucose, those in quartile two (adjusted HR, 1.29; 95% CI, 0.85 to 1.96), quartile three (adjusted HR, 1.35; 95% CI, 0.89 to 2.06), and quartile four (adjusted HR, 1.57; 95% CI, 1.02 to 2.40) were at progressively higher risk of dying (P = .041 for trend; Table 3). An incremental increase of 10 mg/dL in time-weighted mean glucose was associated with an adjusted HR of 1.03 (95% CI, 1.00 to 1.06; P = .035).
Fig 1.
Adjusted survival curves comparing quartiles of mean time-weighted glucose, constructed using multivariate Cox proportional hazards regression, and adjusting for mean daily dexamethasone dose, age, and Karnofsky performance score. The ranges of time-weighted mean glucose for quartiles one, two, three, and four were < 94, 94 to 109, 110 to 137, and > 137 mg/dL, respectively.
Table 3.
Multivariate Association Between Patient Characteristics and Survival
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Mean glucose | 0.041 | ||
| Quartile one, n = 46 | Reference | ||
| Quartile two, n = 50 | 1.29 | 0.85 to 1.96 | |
| Quartile three, n = 48 | 1.35 | 0.89 to 2.06 | |
| Quartile four, n = 47 | 1.57 | 1.02 to 2.40 | |
| Mean daily dexamethasone dose, per 10 mg/d | 1.34 | 1.17 to 1.53 | < .001 |
| Age, per 10 years | 1.47 | 1.27 to 1.72 | < .001 |
| KPS, per 10 points | 0.84 | 0.72 to 0.97 | .021 |
NOTE. The time-weighted mean glucose values for quartiles one to four are < 94 mg/dL, 94 to 109 mg/dL, 110 to 137 mg/dL, and > 137 mg/dL, respectively.
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance score.
The strength of our findings was demonstrated by several sensitivity analyses. Our results were unchanged when we excluded 68 patients with gaps longer than 2 months between glucose or glucocorticoid recordings (adjusted HR associated with a 10 mg/dL increase in time-weighted mean glucose, 1.05; 95% CI, 1.00 to 1.11; P = .036), and when we excluded 18 patients with outlying survival times (HR, 1.03; 95% CI, 1.00 to 1.06; P = .051). The results were also largely unaffected when average glucose not weighted for time was used as the exposure variable (HR, 1.04; 95% CI, 1.00 to 1.07; P = .032), and when the standard deviation of each patient's mean glucose was adjusted for in the model (HR, 1.03; 95% CI, 1.00 to 1.06; P = .081).
The secondary outcome (infection) was experienced in follow-up by 27 patients (14%), 18 of whom had pneumonia. Patients who developed infections had worse survival compared with patients without infections, with an HR of 1.64 (95% CI, 1.08 to 2.48; P = .02). In a multivariate logistic regression analysis, there was a trend toward an association between infection and mean glucose, with an odds ratio for infection of 1.06 (95% CI, 0.99 to 1.13; P = .09) for every 10 mg/dL increase in time-weighted mean glucose. However, adjusting for infection in the multivariate model did not alter the significant association between mean glucose and survival (adjusted HR associated with a 10 mg/dL increase in time-weighted mean glucose remained 1.03; 95% CI, 1.00 to 1.06; P = .035).
DISCUSSION
In this study, we report a shorter survival time associated with hyperglycemia in a group of patients with GBM and good baseline KPS. Even after adjustment for mean daily dexamethasone dose and other prognostic variables, the HR increased with increasing quartile of time-weighted mean glucose.
Recent large population-based studies have suggested that diabetes and impaired glucose tolerance are risk factors for developing certain types of cancer.21,22 Furthermore, pre-existing diabetes at the time of cancer diagnosis has also been shown to be associated with increased all-cause mortality and cancer recurrence, most definitively in colon and breast cancers.4–7,23 In the specific case of glioma, small studies have not shown that premorbid diabetes increases cancer incidence,24–28 but the effect of hyperglycemia on survival after GBM diagnosis has never before been assessed.
One mechanism that has been proposed to explain the association between diabetes and worse survival in colon and breast cancers is that hyperinsulinemia resulting from hyperglycemia stimulates tumor growth. Insulin is a member of a family of growth factors, and similar to its cousins (insulin-like growth factors 1 and 2), may promote tumor proliferation.29 Patients with colorectal cancer have demonstrated higher fasting insulin levels than have control patients,30 and a prospective study has shown that patients with breast cancer with higher fasting insulin concentrations have decreased survival and higher rates of recurrence.31 In vivo studies have shown that high insulin levels enhance colorectal and breast cancer cell proliferation via receptors on the tumors.32,33 Furthermore, treatment with metformin, which lowers insulin levels, decreases the incidence and size of mammary adenocarcinomas in tumor-prone transgenic mice.34 Human glial tumors possess insulin receptors with binding activities and characteristics identical to peripheral insulin receptors, and insulin has been shown to stimulate glucose uptake in cultures of human GBM cells.35
Another mechanism may be that hyperglycemia itself promotes tumor growth. Glucose is the major substrate for cerebral metabolism, and high-grade brain tumors have high glucose consumption.36–38 The glucose content in the brains of healthy mice increases minimally after an intraperitoneal bolus of glucose, whereas mice with gliomas experience an increase in intratumoral glucose content of 2.5 times after induction of hyperglycemia.39 High glucose levels within GBMs could provide extra substrate for glycolytic metabolism, and support unchecked tumor growth. Unfortunately, we were not able to directly evaluate this proposed biologic mechanism in this study, because radiation therapy for GBM commonly results in pseudoprogression, which would have complicated this assessment.40,41
It is also possible that the association between hyperglycemia and worse survival is mediated not by tumor growth, but rather by other adverse effects of hyperglycemia, such as increased infection rates. Hyperglycemia is known to increase the risk of infection in outpatients and inpatients,42–44 and this risk is likely magnified when glucocorticoids are received, because glucocorticoids suppress the immune system and predispose patients to infection.45,46 Although we did observe a trend toward a relationship between hyperglycemia and infection in our study sample, infection had no impact on the association between hyperglycemia and survival. This finding suggests that the mechanism by which hyperglycemia may shorten survival does not include increasing infection rates.
To our knowledge, this is the first study to evaluate the association between glucose levels during cancer treatment and survival. An important strength of our study was our large study population, which was homogeneous with regard to eligibility criteria and treatment. Data collection was reliable and relatively uniform, because all patients were receiving research protocols. Moreover, patients with GBM, in particular, are at high risk for hyperglycemia, given the routine use of glucocorticoids in their management. Because glucocorticoid dose would also be expected to reflect the extent of residual disease and peritumoral edema, the validity of our results was strengthened by carefully adjusting for glucocorticoid dose in the analysis.
This study had several limitations inherent to its retrospective design. As with any observational study, there remains a possibility that unmeasured confounders influenced our findings. For example, residual tumor burden, which would have shortened survival and might have increased glucose because of physiologic stress, may have been incompletely controlled for in this analysis, despite our adjustment for glucocorticoid dose. Also, there was heterogeneity in the frequency of glucose and glucocorticoid recordings, although a sensitivity analysis did not suggest that this biased our results. Furthermore, the glucose measurements available were performed at random. Hemoglobin A1c levels would have provided a better index for evaluating long-term glycemia. Other potentially important variables that were not assessed in this retrospective study of patients diagnosed and treated from 1999 to 2004 include O6-methylguanine–DNA methyltransferase methylation status, exogenous insulin dose, and serum insulin level. These will be measured in a future prospective study aimed at confirming our results in the post-temozolomide era.
Our findings raise the question of whether intensive management of hyperglycemia would lead to improved survival in patients with newly diagnosed GBM. To answer this question, a randomized trial is being planned to compare survival between hyperglycemic patients with GBM whose hyperglycemia is managed intensively by an endocrinologist, and hyperglycemic patients with GBM whose hyperglycemia is managed by an oncologist according to the standard of care.
In conclusion, we observed significantly shorter survival in patients with GBM who experienced hyperglycemia, compared with patients with GBM who did not experience hyperglycemia, after controlling for several important confounders, including glucocorticoid therapy. Future studies evaluating whether lowering glucose improves cancer survival are warranted, especially considering the relatively benign nature of diabetes interventions.
Footnotes
Supported by Grant No. CA062475 from the National Institutes of Health. R.L.D. was supported by an institutional research training grant (T32 DK062707-03) for clinical diabetes research.
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology, June 2, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rachel L. Derr, Xiaobu Ye, Melissa U. Islas, Serena Desideri, Christopher D. Saudek, Stuart A. Grossman
Financial support: Christopher D. Saudek, Stuart A. Grossman
Administrative support: Serena Desideri, Stuart A. Grossman
Provision of study materials or patients: Stuart A. Grossman
Collection and assembly of data: Rachel L. Derr, Xiaobu Ye, Melissa U. Islas, Serena Desideri, Stuart A. Grossman
Data analysis and interpretation: Rachel L. Derr, Xiaobu Ye, Melissa U. Islas, Christopher D. Saudek, Stuart A. Grossman
Manuscript writing: Rachel L. Derr, Xiaobu Ye, Melissa U. Islas, Christopher D. Saudek, Stuart A. Grossman
Final approval of manuscript: Rachel L. Derr, Xiaobu Ye, Melissa U. Islas, Serena Desideri, Christopher D. Saudek, Stuart A. Grossman
REFERENCES
- 1.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction—Long-term results from the diabetes and insulin-glucose infusion in acute myocardial infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 3.Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–1185. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 4.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 5.Polednak AP. Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: A population-based study. Cancer Detect Prev. 2006;30:466–472. doi: 10.1016/j.cdp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21:433–440. doi: 10.1200/JCO.2003.07.125. [DOI] [PubMed] [Google Scholar]
- 7.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 8.Donihi AC, Raval D, Saul M, et al. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12:358–362. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- 9.Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975;56:1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest. 1983;72:1814–1820. doi: 10.1172/JCI111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen T, Lush R, Grossman SA, et al. Phase II clinical and pharmacologic study of radiation therapy and carboxyamido-triazole (CAI) in adults with newly diagnosed glioblastoma multiforme. Invest New Drugs. 2007;25:259–263. doi: 10.1007/s10637-006-9023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laterra JJ, Grossman SA, Carson KA, et al. Suramin and radiotherapy in newly diagnosed glioblastoma: Phase 2 NABTT CNS Consortium study. Neuro Oncol. 2004;6:15–20. doi: 10.1215/S1152851703000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw EG, Grossman SA, Carson KA, et al. Phase I study of gadolinium texaphyrin in newly diagnosed glioblastoma. J Clin Oncol. 2007;25(suppl):91s. abstr 2064. [Google Scholar]
- 15.Grossman SA, Olson J, Batchelor T, et al. Effect of phenytoin on celecoxib pharmacokinetics in patients with glioblastoma. Neuro Oncol. 2008;10:190–198. doi: 10.1215/15228517-2007-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato V, Papaleo A, Castrichino A, et al. Prognostic implication of clinical and pathologic features in patients with glioblastoma multiforme treated with concomitant radiation plus temozolomide. Tumori. 2007;93:248–256. doi: 10.1177/030089160709300304. [DOI] [PubMed] [Google Scholar]
- 17.Shinojima N, Kochi M, Hamada J, et al. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg. 2004;101:219–226. doi: 10.3171/jns.2004.101.2.0219. [DOI] [PubMed] [Google Scholar]
- 18.Reavey-Cantwell JF, Haroun RI, Zahurak M, et al. The prognostic value of tumor markers in patients with glioblastoma multiforme: Analysis of 32 patients and review of the literature. J Neurooncol. 2001;55:195–204. doi: 10.1023/a:1013845004294. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 21.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 22.Saydah SH, Loria CM, Eberhardt MS, et al. Abnormal glucose tolerance and the risk of cancer death in the united states. Am J Epidemiol. 2003;157:1092–1100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 23.Barone BB, Peairs KS, Yeh HC, et al. Pre-existing diabetes and effects on mortality in patients diagnosed with cancer. JAMA. in press. [Google Scholar]
- 24.Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development: Results from the international adult brain tumour study. Int J Cancer. 1999;82:155–160. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Cicuttini FM, Hurley SF, Forbes A, et al. Association of adult glioma with medical conditions, family and reproductive history. Int J Cancer. 1997;71:203–207. doi: 10.1002/(sici)1097-0215(19970410)71:2<203::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99:252–259. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 27.Schwartzbaum J, Jonsson F, Ahlbom A, et al. Prior hospitalization for epilepsy, diabetes, and stroke and subsequent glioma and meningioma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:643–650. doi: 10.1158/1055-9965.EPI-04-0119. [DOI] [PubMed] [Google Scholar]
- 28.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 29.Gupta K, Krishnaswamy G, Karnad A, et al. Insulin: A novel factor in carcinogenesis. Am J Med Sci. 2002;323:140–145. doi: 10.1097/00000441-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Suehiro T, Matsumata T, Shikada Y, et al. Hyperinsulinemia in patients with colorectal cancer. Hepatogastroenterology. 2005;52:76–78. [PubMed] [Google Scholar]
- 31.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 32.Tran TT, Naigamwalla D, Oprescu AI, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147:1830–1837. doi: 10.1210/en.2005-1012. [DOI] [PubMed] [Google Scholar]
- 33.Frasca F, Pandini G, Vigneri R, et al. Insulin and hybrid insulin/IGF receptors are major regulators of breast cancer cells. Breast Dis. 2003;17:73–89. doi: 10.3233/bd-2003-17108. [DOI] [PubMed] [Google Scholar]
- 34.Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Grunberger G, Lowe WL, Jr, McElduff A, et al. Insulin receptor of human cerebral gliomas: Structure and function. J Clin Invest. 1986;77:997–1005. doi: 10.1172/JCI112402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes CG, Wise RJ, Gibbs JM, et al. In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Ann Neurol. 1983;14:614–626. doi: 10.1002/ana.410140604. [DOI] [PubMed] [Google Scholar]
- 37.Di Chiro G, DeLaPaz RL, Brooks RA, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology. 1982;32:1323–1329. doi: 10.1212/wnl.32.12.1323. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler A, von Kienlin M, Decorps M, et al. High glycolytic activity in rat glioma demonstrated in vivo by correlation peak 1H magnetic resonance imaging. Cancer Res. 2001;61:5595–5600. [PubMed] [Google Scholar]
- 39.Simões RV, García-Martín ML, Cerdán S, et al. Perturbation of mouse glioma MRS pattern by induced acute hyperglycemia. NMR Biomed. 2008;21:251–264. doi: 10.1002/nbm.1188. [DOI] [PubMed] [Google Scholar]
- 40.Jefferies S, Burton K, Jones P, et al. Interpretation of early imaging after concurrent radiotherapy and temozolomide for glioblastoma. Clin Oncol (R Coll Radiol) 2007;19(suppl 3):S33. [Google Scholar]
- 41.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 42.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–554. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 43.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 44.Golden SH, Peart-Vigilance C, Kao WH, et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408–1414. doi: 10.2337/diacare.22.9.1408. [DOI] [PubMed] [Google Scholar]
- 45.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: Clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 46.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]