Summary
Hosts in nature will often acquire infections by different helminth species over their lifetime. This presents the potential for new infections to be affected (particularly via the host immune response) by a host’s history of previous con- or hetero-specific infection. Here we have used an experimental rat model to investigate the consequences of a history of primary infection with either Nippostrongylus brasiliensis, Strongyloides venezuelensis or S. ratti on the fitness of, and immunological response to, secondary infections of S. ratti. We found that a history of con-specific, but not hetero-specific, infection reduced the survivorship of S. ratti; the fecundity of S. ratti was not affected by a history of either con- or hetero-specific infections. We also found that a history of con-specific infection promoted Th2-type responses, as shown by increased concentrations of total IgE, S. ratti-specific IgG1, rat mast cell protease II (RMCPII), IL4 (but decreased concentrations of IFNγ) produced by mesenteric lymph nodes (MLNs) in response to S. ratti antigen. Additionally, S. ratti-specific IgG1 was positively related to the intensity of both primary and secondary infections of S. ratti. Hetero-specific primary infections were only observed to affect the concentration of total IgE and RMCPII. The overall conclusion of these experiments is that the major immunological effect acting against an infection is induced by the infection itself and that there is little effect of prior infections of the host.
Keywords: density-dependence, parasite interactions, immuno-epidemiology, immuno-ecology
Introduction
In nature, hosts are usually exposed to infection by a range of infectious organisms, including different species of gastrointestinal helminths (Behnke, 2008). Yet, laboratory studies typically analyse only single species infections. While such analyses have given a good understanding of the biology and host immunology of specific infections, studies of single infections are not able to investigate how this is affected by infection with other species.
Interactions between co-infecting species may be direct, where two parasite species compete for a limited resource such as food or space within the host. Interactions between parasite species may also be indirect, particularly where they are mediated via the host immune response (Keymer, 1982). Thus, parasites do not need to infect at the same site within a host to interact if immune cross-reactivity (via either immune initiators or effectors) occurs between them. Similarly, an infection may be affected by the host’s previous infection history where immunological memory acts to mediate indirect interactions between parasites.
Helminth parasites cause a characteristic T-helper 2 (Th2)-style immune response, in contrast to most micro parasites that result in a Th1-style immune response (Finkelman et al., 1997). In view of this, there may be greater potential for immunologically-mediated indirect interactions between different helminth species compared with potential interactions between helminths and non-helminths.
Previously, we have found that the gastrointestinal nematode Strongyloides ratti is subject to immune-dependent density-dependent effects (Paterson and Viney 2002). Thus, the probability of survival and the fecundity of individual parasitic female worms is negatively affected by the density of co-infecting con-specifics, with this effect dependent on the host immune response. We have also found that the host anti-S. ratti immune response is qualitatively and quantitatively affected by the density of infection (Bleay et al., 2007), moving from a Th1 to a Th2 type profile with dose. This suggests that this dose-dependent qualitative and quantitative change of the host immune response causes the density-dependent effects on components of fitness of S. ratti. We also found that these immune-dependent effects could occur via immunological memory (Paterson and Viney, 2002). Previous studies have shown the possibility of hetero-specific effects on S. ratti infections. Thus, immunisation with a comparatively high dose (e.g. 3,000 larvae) of Nippostrongylus brasiliensis significantly reduced the number of worms recovered from a secondary S. ratti infection (Nawa et al., 1982). Similarly, a primary Trichinella spiralis infection resulted in reduced survival of a subsequent S. ratti infection (Moqbel and Wakelin, 1979). The development of the S. ratti free-living generation of S. ratti is affected by the anti-S. ratti immune response (Harvey et al., 2000). However, hetero-specific host immunisation also affects this development, though to a lesser degree than con-specific immunisation (West et al., 2001).
We wished to determine the extent to which a history of either con- or hetero-specific nematode infection affects the fitness of, and immunological response to, subsequent S. ratti infection. We hypothesise that prior infection of the host will (i) negatively affect subsequent S. ratti infection, (ii) enhance the host anti-S. ratti immune response, (iii) that both these effects will be comparatively greater for prior infection with species that are most closely related to S. ratti, and (iv) that both these effects will be comparatively greater in more high intensity prior infections.
Methods
Parasites and experimental design
The S. ratti isofemale line ED321 Heterogonic was used throughout (Viney, 1996). A strain of S. venezuelensis was obtained from H. Maruyama (Nagoya City University Medical School) and a strain of Nippostrongylus brasiliensis was obtained from R.M. Maizels (University of Edinburgh). These three parasite species were maintained by serial passage in Wistar rats.
The experimental design was to infect rats with different doses of one of three gastrointestinal nematodes (S. ratti, S. venezuelensis or N. brasiliensis), to clear these infections and to subsequently infect the rats with different doses of an S. ratti infection. In this way, the effect of prior infection (both species and dose) on components of fitness of the secondary S. ratti infection and on the hosts’ immune response could be investigated. The overall experimental design is shown in Table 1. Eighty four female Wistar rats of c.100g weight were randomly assigned among these 21 treatments (i.e., 4 rats per treatment), with the experiment conducted in two equal experimental blocks (i.e., 2 rats per treatment per experimental block), with the blocks separated by 28 days. The primary infection was given on day 0 post infection (p.i.) by the subcutaneous administration of infective third stage larvae (iL3s), as previously described (Wilkes et al., 2007); control animals were given a sham inoculation of PBS only. Faeces were collected on days 5, 8 and 12 p.i., cultured appropriately to the infecting parasite species (Table 1). For Strongyloides spp. this is as described previously (Viney, 1996); for N. brasiliensis, host faeces were mixed with an equal volume of wetted charcoal, and this maintained in a large Petri dish at 25°C for six days. These faecal cultures were performed to confirm that these primary infections occurred. On days 15 and 16 p.i. all rats were administered thiabendazole, as described by Paterson and Viney (2002), to remove the worm infections. We confirmed that this treatment was effective by faecal culture of treated animals.
Table 1.
Experimental design showing the 21 treatments. Immunology: 1 indicates that immunoglobulin and RMCP concentrations were measured, 2 indicates that cytokine concentrations were additionally measured, all as detailed above
| Primary infection | Secondary infection | |||
|---|---|---|---|---|
| Species | Dose | Species | Dose | Immunology |
| S. ratti | 30 | S. ratti | 0 | 2 |
| S. ratti | 30 | S. ratti | 30 | 2 |
| S. ratti | 30 | S. ratti | 750 | 2 |
| S. ratti | 750 | S. ratti | 0 | 2 |
| S. ratti | 750 | S. ratti | 30 | 2 |
| S. ratti | 750 | S. ratti | 750 | 2 |
| S. venezuelensis | 30 | S. ratti | 0 | 1 |
| S. venezuelensis | 30 | S. ratti | 30 | 1 |
| S. venezuelensis | 30 | S. ratti | 750 | 1 |
| S. venezuelensis | 750 | S. ratti | 0 | 1 |
| S. venezuelensis | 750 | S. ratti | 30 | 1 |
| S. venezuelensis | 750 | S. ratti | 750 | 1 |
| N. brasiliensis | 30 | S. ratti | 0 | 1 |
| N. brasiliensis | 30 | S. ratti | 30 | 1 |
| N. brasiliensis | 30 | S. ratti | 750 | 1 |
| N. brasiliensis | 750 | S. ratti | 0 | 1 |
| N. brasiliensis | 750 | S. ratti | 30 | 1 |
| N. brasiliensis | 750 | S. ratti | 750 | 1 |
| Control (PBS) | 0 | S. ratti | 0 | 2 |
| Control (PBS) | 0 | S. ratti | 30 | 2 |
| Control (PBS) | 0 | S. ratti | 750 | 2 |
Thirty two days later (i.e., day 48 p.i.) the secondary infection was given (Table 1), which is day 0 post secondary infection (p.s.i.). Faecal samples were collected on days 6, 9, 13, 16 and 20 p.s.i., the faeces cultured as previously described (Viney, 1996) at 19°C for three days, and the number of larvae that developed in these cultures was used as a measure of the total viable egg output of the infection (Gemmill et al., 1997). Two animals from each treatment group were killed on days 7 and 21 p.s.i. each, the small intestine removed and stored at −20°C for subsequent determination of the number of S. ratti parasitic females, as previously described (Wilkes et al., 2004). Survivorship was calculated as the number of parasitic females in the rat divided by the secondary dose. The per capita fecundity was calculated as the number of larvae that developed in faecal cultures divided by the number of parasitic females in the rat (Paterson and Viney, 2002).
Previous work has shown that in the presence of a host immune response, S. ratti parasitic females become positioned more posteriorly in the host small intestine (Kimura, et al., 1999; Wilkes et al., 2004). In this work, we have used part of the small intestine for immunological analyses (below). Therefore we will have underestimated the number of parasitic females (i.e. survivorship) and, consequently, overestimated per capita fecundity (Bleay et al., 2007). It is not known how the different primary infection treatments (Table 1) may affect the intestinal position of S. ratti. Therefore the possibility exists that these different treatments may affect differently the underestimate of survivorship and the overestimate of per capita fecundity.
For all treatments, the concentration of S. ratti-specific immunoglobulin G1 (IgG1), IgG2a and IgG2b and total IgE all in serum and S. ratti-specific IgA and rat mast cell protease II both in intestinal tissue, was determined for animals sacrificed at days 7 and 21 p.s.i., all as previously described (Wilkes et al., 2007). The intestinal tissue used for immunological analyses was 5cm of gut distal to the first 10% by length of the small intestine. Thus, the measures of the number of parasitic females in the gut, excludes any that were in this region (above). For two animals additional tissue was taken: for one rat with the PBS control primary - 30 dose secondary sacrificed on day 21 p.s.i., 12cm of gut was used and, for one rat with the treatment S. ratti 750 dose primary - 750 dose secondary sacrificed on day 21 p.s.i., 6 cm was used.
In addition, for the same animals in the S. ratti primary - S. ratti secondary and the PBS control primary - S. ratti secondary treatments (Table 1) the concentration of the following cytokines were measured: interleukin 4 (IL4), interleukin 13 (IL13), and interferon γ (IFNγ) from both spleen and mesenteric lymph node (MLN) cells stimulated with S. ratti parasitic female antigen all as previously described (Wilkes et al., 2007). Spleens and MLNs were collected from animals sacrificed at days 7 and 21 p.s.i.
Statistical analysis
All analyses were conducted in R v2.7.0 (www.r-project.org). Analyses of S. ratti survivorship and per capita fecundity were performed using a generalised linear model (GLM) with a negative binomial error distribution (using the parameterization described in Wilson and Grenfell (1997)) and followed that described previously (Paterson and Viney, 2002). For survivorship, the dependent variable was the number of parasitic females. In order to express survivorship as the proportion of parasitic females in a host relative to the dose of iL3s administered, the dose of iL3s administered was used as an offset variable (i.e. a parameter value specified a priori rather than estimated from the data) (Crawley, 2002; Paterson and Viney, 2002). For per capita fecundity, the number of larvae developing in faecal cultures was the dependent variable and the number of parasitic females was used as an offset variable. Analyses of survivorship and per capita fecundity excluded those animals receiving PBS controls in the secondary infection (Table 1).
Deletion testing was used to derive minimal models for survivorship and per capita fecundity; i.e. models that contained only significant terms and for which no further significant terms could be added (Crawley, 2002). Likelihood ratio (LR) tests were used to assess significance of terms. All terms were fitted as factors (i.e. discrete variables). For both survivorship and per capita fecundity, these minimal models were derived by successive deletion of terms from a maximal model that consisted of Block as a main effect (two level factor: replicates 1 and 2) and of the main effects of, and second order interactions between, Secondary Dose (two level factor: 30 vs. 750 S. ratti iL3s), Primary Dose (7 level factor: PBS vs. 30 N. brasiliensis iL3s vs. 750 N. brasiliensis iL3s vs. 30 S. venezuelensis iL3s vs. 750 S. venezuelensis iL3s vs. 30 S. ratti iL3s vs. 750 S. ratti iL3s) and Time (two level factor: days 7 vs. 21 p.s.i.). Thus the formula for the terms to be tested in the maximal model was: Block + (Primary Dose + Secondary Dose + Time)2.
Deletion testing was also used to determine the significance of factor levels. Thus, to determine whether the primary dose affected either survivorship or per capita fecundity, animals that received either 30 or 750 iL3s as a secondary dose were grouped together within each species to give a four level factor of primary infection that specified only the species, not the number, of iL3s (Primary Spp.: PBS vs. N. brasiliensis vs. S. venezuelensis vs. S. ratti). LR tests were then performed between models containing either the 7 level Primary Dose factor or the four level Primary Spp. factor to determine the significance of deleting factor levels associated with number of iL3s administered in the primary dose. Similarly, to determine whether a hetero-specific primary infection affected either S. ratti survivorship or per capita fecundity (i.e. whether primary infection with N. brasiliensis or S. venezuelensis was equivalent to a sham primary infection with PBS), animals receiving either PBS, N. brasiliensis or S. venezuelensis in a primary infection were combined into a single group and LR tests performed to determine the significance of factor levels for N. brasiliensis and S. venezuelensis.
Analyses of immune parameters included all animals (i.e. including those of secondary infection dose 0 (Table 1)) and followed the methods described in Paterson et al. (2008). Briefly, immune parameters were, where possible, normalised by a Box-Cox transformation identified by maximum likelihood such that the residuals from a linear model of (Time x Secondary dose + Primary dose) conformed as closely as possible to a normal distribution. Values of λ found were −0.4 for IgE, −0.18 for IgG1, −1.01 for RMCPII, 0.02 for IL4 MLN, 0.02 for IFNγ MLN, −0.1 for IFNγ Spleen, 0.1 for IL13 MLN and 0.26 for IL13 Spleen and these were then analysed using linear models. No satisfactory transformations for IgA, IgG2a, IgG2b or IL4 Spleen were found and so these were analysed using GLMs with presence/absence of a detectable level of immunoglobulin isotype or cytokine (relative to corresponding negative controls). Significance of terms was determined using deletion testing from maximal models as for survivorship and per capita fecundity (as above) with the exception that secondary dose was fitted as a three level factor (0 vs. 30 vs. 750 iL3s) and that the significance of factor levels within the secondary infection were tested. Thus, animals that received either 30 or 750 S. ratti iL3s in a secondary infection were grouped together to give a two level factor (0 vs. 30 or 750 iL3s) and compared against the three level factor (0 vs. 30 vs. 750 iL3s). F tests were used in the case of linear models and LR tests were used in the case of GLMs.
Results and Discussion
Effects of primary infections on the survivorship of S. ratti secondary infections
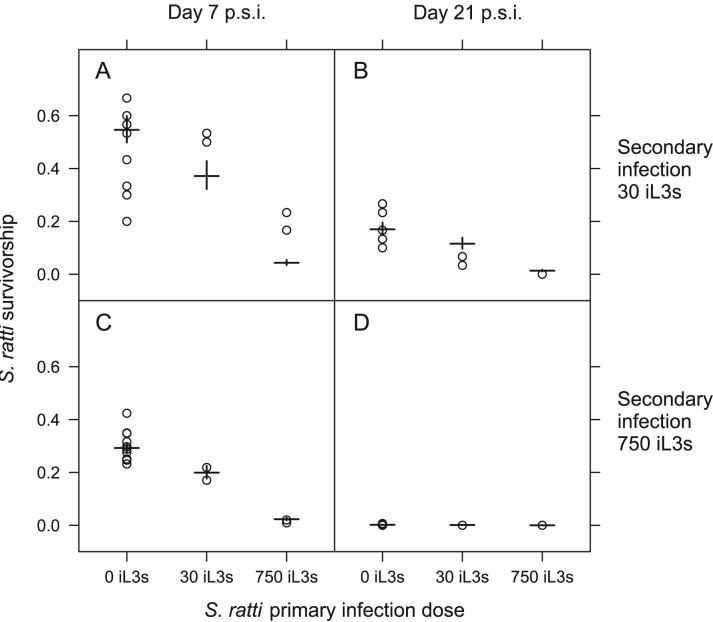
Fewer S. ratti parasitic females were present in hosts previously exposed to S. ratti and this effect was density-dependent; the greater the dose given in a primary infection the fewer parasitic females were observed in the secondary infection (Primary dose, LR test = 168.1, d.f. = 1, p < 0.001) (Table 2 and Figure 1). Analogously, there was a density-dependent effect of the secondary infection to reduce the survivorship of the secondary infection (Secondary dose x Time, LR test = 198.5, d.f. = 1, p < 0.001) (Table 2). These results are fully consistent with previous observations of these phenomena (Paterson and Viney, 2002; Bleay et al., 2007). A hetero-specific primary infection of N. brasiliensis or of S. venezuelensis did not affect the S. ratti survivorship in the secondary infection. That is, there was no significant difference between control (PBS), S. venezuelensis or N. brasiliensis primary infections in the survivorship of a secondary S. ratti infection. Therefore, S. ratti survivorship is not affected by these hetero-specific prior infections.
Table 2.
The effects of primary infections on S. ratti survivorship and per capita fecundity
| Term | Coefficient | LR testa | P valuea | |
|---|---|---|---|---|
| Survivorship b | Intercept | −0.605 ± 0.094 | - | |
| Secondary dose (750 iL3s) | −1.17 ± 0.17 | 34.38 | <0.001 | |
| Time (Day 21 p.s.i.) | −0.624 ± 0.106 | 55.13 | <0.001 | |
| Primary dose (30 S. ratti iL3s)c | −0.385 ± 0.126 | |||
| Primary dose (750 S. ratti iL3s)c | −2.54 ± 0.21 | 168.13 | <0.001 | |
| Secondary dose × Time | −3.96 ± 0.32 | 198.50 | <0.001 | |
| Fecundity d | Intercept | 2.27 ± 0.31 | ||
| Secondary dose (750 iL3s) | 0.87 ± 0.18 | 21.01 | <0.001 | |
| Time (Day 21 p.s.i.) | −3.45 ± 0.63 | 39.40 | <0.001 | |
| Primary spp. (N. brasiliensis) | 0.375 ± 0.366 | |||
| Primary spp. (S. venezuelensis) | 0.755 ± 0.366 | |||
| Primary spp. (S. ratti) | −0.157 ± 0.367 | 13.18 | <0.01 | |
| Time × Primary spp. (N. brasiliensis) | 2.13 ± 0.70 | |||
| Time × Primary spp. (S. venezuelensis) | 2.04 ± 0.70 | |||
| Time × Primary spp. (S. ratti) | 3.15 ± 0.82 | 15.31 | <0.01 |
Likelihood ratio test presented for deletion of individual terms. For factors, tests refer to simultaneous deletion of all factor levels.
2 × log-likelihood = −318.31 with 50 residual degrees of freedom, overdispersion parameter k = 29.6 ± 14.5.
Factor levels for primary dose were grouped such that animals receiving either PBS, N. brasiliensis or S. venezuelensis in a primary infection were compared with those receiving 30 and 750 S. ratti iL3s as a primary infection.
2 × log-likelihood = −560.87 with 40 residual degrees of freedom, overdispersion parameter k = 2.83 ± 0.63.
Figure 1.
The S. ratti survivorship for three primary S. ratti doses and secondary S. ratti infections at doses of 30 iL3s (A, B) and 750 iL3s (C, D) and at days 7 (A, C) and 21 p.s.i. (B, D). Results for individual animals are circles, with model estimates from Table 2 shown as horizontal bars with standard errors on these estimates indicated by vertical bars. Animals receiving N. brasiliensis or S. venezuelensis primary infections are included in the ‘0 iL3s’ group since these hetero-specific infections had no detectable effect on subsequent S. ratti survivorship.
Effects of primary infections on the per capita fecundity of S. ratti secondary infections
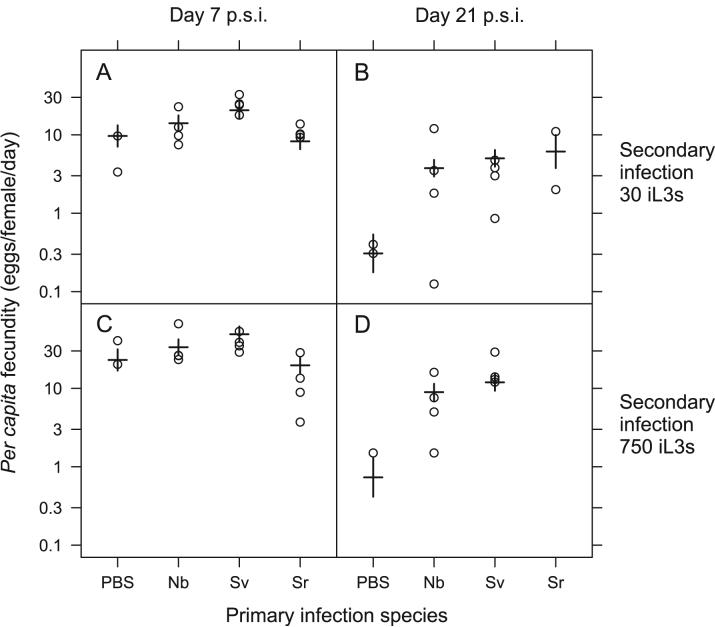
Per capita fecundity declined between days 7 and 21 p.s.i. in all groups, consistent with our previous findings (Paterson and Viney, 2002). There was no consistent effect of primary infection on the fecundity of secondary infections on both days 7 and 21 p.s.i. However, the per capita fecundity of S. ratti secondary infections on day 21 p.s.i. was different between the control, PBS treated group and the infected groups (Time x Primary spp., LR test = 15.3, d.f. = 1, p < 0.001) (Table 2 and Figure 2). But, the direction of this effect is counterintuitive, because the S. ratti fecundity of the control, PBS treated group was lower than that of the primary infected groups (Figure 2). Among the primary infected groups, the reduction in fecundity between days 7 and 21 p.s.i. was approximately equivalent (and not statistically distinguishable) between the N. brasiliensis, S. venezuelensis and S. ratti infection groups (Figure 2); no effect of primary dose was observed. We note that accurate estimates of per capita fecundity are difficult for day 21 p.s.i., since approximately half of the animals no longer had worms and hence are excluded from the analysis; the remainder had very low numbers of parasitic females. Moreover, the sample size for control, PBS treated group on day 21 p.s.i. is four, which is half that of the other primary infection groups (because different primary dose groups (Table 1) are grouped together). Overall, our results clearly show that neither hetero- nor con-specific primary infection reduced the fecundity of subsequent S. ratti infection; the observed effect of the PBS treatment should be interpreted with caution.
Figure 2.
The S. ratti per capita fecundity for con- or hetero-specific primary infections and secondary S. ratti infections at doses of 30 iL3s (A, B) and 750 iL3s (C, D) and at days 7 (A, C) and 21 p.s.i. (B, D). Results for individual animals are circles, with model estimates from Table 2 shown as horizontal bars with standard errors on these estimates indicated by vertical bars. Data are plotted on a log scale. The primary infections were: PBS controls (PBS), N. brasiliensis (Nb), S. venezuelensis (Sv) and S. ratti (Sr). The different doses delivered in the primary infection (Table 1) for each species are grouped together since the dose of the primary infection had no detectable effect on the per capita fecundity of S. ratti secondary infections.
Therefore, our hypothesis of the effect of prior infection on S. ratti components of fitness is only supported for con-specific infection. However, it has been previously observed that immunisation with 3,000 larvae of N. brasiliensis significantly affected the survivorship and fecundity of a secondary S. ratti infection (Nawa et al., 1982). This therefore suggests that comparatively very high doses, can induce detectable hetero-specific effects.
Associations between immune parameters and the survivorship and per capita fecundity of secondary infections
We extended this analysis (above) to determine whether there were any additional statistically detectable effects of measures of the host immune response on the survivorship and fecundity of secondary S. ratti infections. To do this, each of the immune parameters were added to these statistical models (Table 2) either as main effects or as interactions with time. Data for the concentration of immunglobulins and RMCPII were available for all infection groups (Table 1); data for the concentration of cytokines were only available for S. ratti or PBS control primary infections (Table 1).
S. ratti survivorship was negatively associated with the concentration of IL13 produced by MLN cells (IL13 MLN, LR test = 56.0, d.f. = 1, p < 0.001) (Table 3). This occurred as a main effect, such that there was no difference in the effect between days 7 and 21 p.s.i. IL13 has previously been identified to be important in protective immune responses in helminth infections (Finkelman et al., 1999).
Table 3.
The association between S. ratti survivorship and IL13 produced by mesenteric lymph node cells
| Term | Coefficient | LR testa | P valuea |
|---|---|---|---|
| Intercept | 0.418 ± 0.229 | ||
| Time (Day 21 p.s.i.) | −2.23 ± 0.32 | 49.37 | <0.001 |
| Secondary dose (750 iL3s) | −0.828 ± 0.209 | 28.98 | <0.001 |
| Primary spp. S. ratti (30 iL3s) | −0.797 ± 0.260 | - | - |
| Primary spp. S. ratti (750 iL3s) | −2.31 ± 0.20 | 45.49 | <0.001 |
| Time × Secondary dose (750 iL3s) | −4.16 ± 0.78 | 49.85 | <0.001 |
| IL13 MLN | −0.646 ± 0.088 | 56.01 | <0.001 |
Likelihood ratio test presented for deletion of individual terms. For factors, tests refer to simultaneous deletion of all factor levels.
2 × log-likelihood = −75.20 with 13 residual degrees of freedom, overdispersion parameter k > 100
S. ratti per capita fecundity was associated with the concentration of S. ratti-specific IgG1 and the concentration of IL4 and IL13 produced by MLN cells, as interactions with time p.s.i. (Time x IgG1, LR test = 4.9, d.f. = 1, p < 0.05; Time x IL4 MLN, LR test = 19.1, d.f. = 1, p < 0.001; Time x IL13 MLN, LR test = 16.2, d.f. = 1, p < 0.001) (Table 4). These effects occurred such that IgG1, IL4 and IL13 concentrations were negatively associated with fecundity on day 7 p.s.i.
Table 4.
The association between S. ratti fecundity and immune parameters
| Term | Coefficient | LR testa | P valuea | |
|---|---|---|---|---|
| IgG1 b | Intercept | 2.06 ± 0.35 | ||
| Time (Day 21 p.s.i.) | −3.48 ± 0.67 | 11.67 | <0.001 | |
| Secondary dose (750 iL3s) | 0.723 ± 0.195 | 12.87 | <0.001 | |
| Primary spp. (N. brasiliensis) | 0.390 ± 0.346 | - | ||
| Primary spp. (S. venezuelensis) | 0.759 ± 0.346 | - | ||
| Primary spp. (S. ratti) | 0.241 ± 0.444 | 12.12 | <0.01 | |
| Time × Primary spp. (N. brasiliensis) | 2.09 ± 0.68 | - | ||
| Time × Primary spp. (S. venezuelensis) | 2.19 ± 0.69 | - | ||
| Time × Primary spp. (S. ratti) | 2.64 ± 0.85 | 12.64 | <0.01 | |
| IgG1 | −0.320 ± 0.184 | 0.23 | 0.63 | |
| Time × IgG1 | 0.686 ± 0.297 | 4.99 | <0.05 | |
| IL4 c | Intercept | 1.28 ± 0.42 | ||
| Time (Day 21 p.s.i.) | −3.26 ± 0.73 | 7.40 | <0.01 | |
| Secondary dose (750 iL3s) | 0.988 ± 0.261 | 14.66 | <0.001 | |
| Primary spp. (S. ratti) | 1.57 ± 0.63 | 1.08 | 0.30 | |
| Time × Primary spp. (S. ratti) | 2.13 ± 0.94 | 5.50 | <0.05 | |
| IL4 MLN | −1.30 ± 0.43 | 1.015 | 0.31 | |
| Time × IL4 MLN | 2.23 ± 0.54 | 19.17 | <0.001 | |
| IL13 d | Intercept | 3.16 ± 0.23 | ||
| Time (Day 21 p.s.i.) | −5.02 ± 0.62 | 7.40 | <0.01 | |
| Secondary dose (750 iL3s) | 0.724 ± 0.163 | 19.10 | <0.001 | |
| Primary spp. (S. ratti) | −0.510 ± 0.170 | 1.08 | 0.30 | |
| Time × Primary spp. (S. ratti) | 3.46 ± 0.58 | 42.55 | <0.001 | |
| IL13 MLN | −0.479 ± 0.152 | 0.41 | 0.52 | |
| Time × IL13 MLN | 2.07 ± 0.53 | 16.25 | <0.001 |
Likelihood ratio test presented for deletion of individual terms. For factors, tests refer to simultaneous deletion of all factor levels.
2 × log-likelihood = −555.89 with 38 residual degrees of freedom, overdispersion parameter k = 3.18 ± 0.73
2 × log-likelihood = −168.595 with 10 residual degrees of freedom, overdispersion parameter k = 3.18 ± 0.73
2 × log-likelihood = −106.54 with 6 residual degrees of freedom, overdispersion parameter k = 33.1 ± 18.9
Previously, we found that S. ratti survivorship was negatively related to the concentration of parasite-specific IgG1, IgA and IL4 MLN, whereas fecundity was negatively related to the concentration of IgA only (Bleay et al., 2007). Therefore there is some qualitative overlap between the immunological results of these two studies, in that the concentration of IL4 MLN and IgG1 is associated with components of S. ratti fitness in both studies, though the details of these effects differ between the studies. Note, the prior study did not analyse IL13. It is notable that here we have not detected any effect of the concentration of IgA, in contrast to the previous study (Bleay et al., 2007). These apparently different observations may be due to temporal effects. Previously it was observed that the concentration of IgA changed with time (Bleay et al., 2007). Here, IgA concentration was only measured at two points, and this may not have detected such temporal changes in its concentration.
Effects of primary and secondary infections on immune parameters
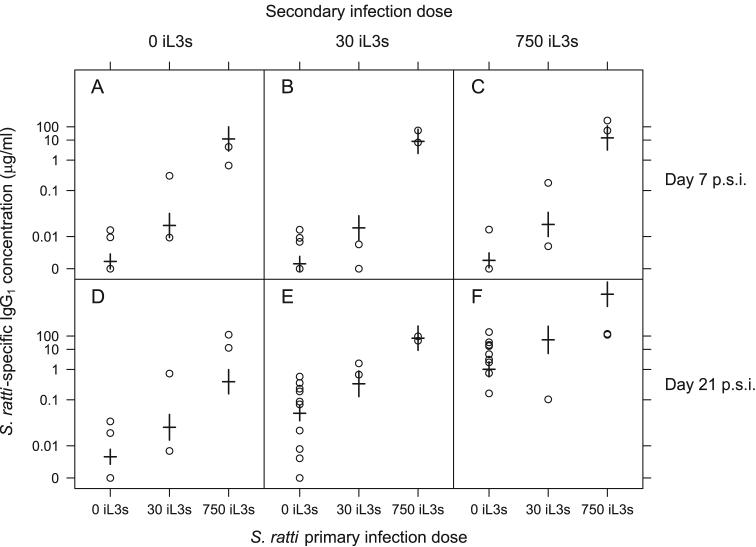
A secondary infection with S. ratti resulted in a significantly greater concentration of the following measured immune parameters, compared with a control PBS secondary infection treatment: IL4, IL13 and IFNγ all produced by MLN cells, IL13 produced by spleen cells, S. ratti-specific IgG1, total IgE and RMCPII (Table 5). The concentration of IL4 produced by MLN cells was positively affected by the dose of the secondary infection (Secondary dose, F2,32 = 14.29, p < 0.001) (Table 5). This has been previously observed in S. ratti primary infections (Bleay et al., 2007). Further, as shown in Figure 3, the concentration of IgG1 was also affected by the dose of the secondary infection at 21 days p.s.i., but not at 7 days p.s.i. (Time x Secondary dose, F2,73 = 18.81, p < 0.001) (Table 5). The effect of IL13 concentration produced by both MLN and spleen cells are consistent with the observed association between the concentration of this cytokine and survivorship.
Table 5.
The effects of primary and secondary infections on immune parameters
| Term | Coefficient | ta | P valuea | |
|---|---|---|---|---|
| IgE b | Intercept | −1.31 ± 0.31 | −4.28 | <0.001 |
| Time (Day 21 p.s.i.) | 0.513 ± 0.194 | 2.65 | <0.01 | |
| Secondary dose (30 or 750 iL3s)c | 0.639 ± 0.206 | 3.11 | <0.01 | |
| Primary spp. (N. brasiliensis) | 0.600 ± 0.314 | 1.91 | 0.06 | |
| Primary spp. (S. venezuelensis) | 0.574 ± 0.314 | 1.83 | 0.07 | |
| Primary spp. (S. ratti, 30 iL3s) | 0.866 ± 0.363 | 2.39 | <0.05 | |
| Primary spp. (S. ratti, 750 iL3s) | 1.18 ± 0.36 | 3.24 | <0.01 | |
| IgG1 d | Intercept | −0.830 ± 0.133 | −6.24 | - |
| Time (Day 21 p.s.i.) | 0.250 ± 0.189 | 1.32 | 0.19 | |
| Secondary dose (30 iL3s) | −0.043 ± 0.177 | −0.24 | 0.81 | |
| Secondary dose (750 iL3s) | 0.019 ± 0.177 | 0.11 | 0.92 | |
| Primary spp. S. ratti (30 iL3s) | 0.644 ± 0.209 | 3.08 | <0.01 | |
| Primary spp. S. ratti (750 iL3s) | 2.19 ± 0.21 | 10.5 | <0.001 | |
| Time × Secondary dose (30 iL3s) | 0.822 ± 0.250 | 3.29 | <0.01 | |
| Time × Secondary dose (750 iL3s) | 1.55 ± 0.25 | 6.13 | <0.001 | |
| Time × Primary spp. S. ratti (30 iL3s) | −0.116 ± 0.309 | −0.38 | 0.71 | |
| Time × Primary spp. S. ratti (750 iL3s) | −0.849 ± 0.296 | −2.87 | <0.01 | |
| RMCPII e | Intercept | −1.55 ± 0.34 | −4.58 | - |
| Secondary dose (30 or 750 iL3s)c | 0.902 ± 0.182 | 4.97 | <0.001 | |
| Time (Day 21 p.s.i.) | 0.564 ± 0.469 | 1.2 | 0.23 | |
| Primary spp. (N. brasiliensis) | 1.45 ± 0.39 | 3.75 | <0.001 | |
| Primary spp. (S. venezuelensis) | 1.14 ± 0.40 | 2.95 | <0.01 | |
| Primary spp. (S. ratti) | 1.79 ± 0.39 | 4.63 | <0.001 | |
| Time × Primary spp. (N. brasiliensis) | −1.56 ± 0.56 | −2.76 | <0.01 | |
| Time × Primary spp. (S. venezuelensis) | −1.19 ± 0.56 | −2.1 | <0.05 | |
| Time × Primary spp. (S. ratti) | −1.44 ± 0.56 | −2.55 | <0.05 | |
| IL4 MLN f | Intercept | −1.27 ± 0.27 | −4.73 | - |
| Secondary dose (30 iL3s) | 0.969 ± 0.295 | 3.28 | <0.01 | |
| Secondary dose (750 iL3s) | 1.56 ± 0.30 | 5.30 | <0.001 | |
| Primary spp. S. ratti (30 or 750 iL3s)g | 0.646 ± 0.256 | 2.53 | <0.05 | |
| IL13 MLN h | Intercept | −0.315 ± 0.242 | −1.30 | - |
| Time (Day 21 p.s.i.) | −0.856 ± 0.255 | −3.36 | <0.01 | |
| Secondary dose (30 or 750 iL3s)c | 1.27 ± 0.26 | 4.88 | <0.001 | |
| IL13 spleen i | Intercept | −0.97 ± 0.21 | −4.63 | - |
| Secondary dose (30 or 750 iL3s)c | 1.46 ± 0.26 | 5.67 | <0.001 | |
| IFNγ MLNj | Intercept | −0.529 ± 0.318 | −1.67 | - |
| Time (Day 21 p.s.i.) | 0.983 ± 0.259 | 3.79 | <0.001 | |
| Primary spp. S. ratti (30 or 750 iL3s)g | −0.628 ± 0.275 | −2.28 | <0.05 | |
| Secondary dose (30 or 750 iL3s)c | 0.685 ± 0.275 | 2.49 | <0.05 |
Significance of individual terms are presented as estimates against a t distribution. F tests from deletion tests are presented in the text.
2 × log-likelihood = −211.92 with 78 residual degrees of freedom
Factor levels for secondary dose were grouped such that animals receiving a dose of 0 were compared with those receiving either 30 or 750 S. ratti iL3s.
2 × log-likelihood = −98.70 with 73 residual degrees of freedom
2 × log-likelihood = −183.40 with 74 residual degrees of freedom
2 × log-likelihood = −74.56 with 32 residual degrees of freedom
Factor levels for primary dose were grouped such that animals receiving either PBS, N. brasiliensis or S. venezuelensis were compared with those receiving 30 or 750 S. ratti iL3s as a primary infection.
2 × log-likelihood = −65.88 with 29 residual degrees of freedom
2 × log-likelihood = −77.16 with 34 residual degrees of freedom
2 × log-likelihood = −79.89 with 32 residual degrees of freedom
Figure 3.
Anti-S. ratti IgG1 concentrations for three primary S. ratti doses and secondary S. ratti infections at doses of 0 (A, D), 30 (B, E) and 750 iL3s (C, F) at days 7 (A, B, C) and 21 p.s.i. (D, E, F). Results for individual animals are circles, with model estimates from Table 5 shown as horizontal bars with standard errors on these estimates indicated by vertical bars. Data are plotted on a Box-Cox transformed scale. Animals receiving N. brasiliensis or S. venezuelensis primary infections are included in the ‘0 iL3s’ group since these hetero-specific infections had no detectable effect on subsequent anti-S. ratti IgG1 concentration.
A primary infection significantly affected the concentration of the following measured immune parameters in a secondary S. ratti infection: IL4 and IFNγ produced by MLN cells, S. ratti-specific IgG1, total IgE and RMCPII. A primary S. ratti infection, regardless of the dose, resulted in a higher concentration of IL4 and lower concentration of IFNγ by MLN cells (IL4 MLN, Primary spp. S. ratti, F1,32 = 6.38, p < 0.05; IFNγ MLN, Primary spp. S. ratti, F1,32 = 5.21, p < 0.05) (Table 5). Thus, prior history of exposure to S. ratti suppresses inflammatory Th1-type responses (i.e. IFNγ) and further promotes Th2-type responses (i.e. IL4) in an S. ratti secondary infection, consistent with the Th2-bias associated with protective, acquired immune responses to nematode infections (Bancroft, 1994; Turner, 2003) Further, there was a positive S. ratti primary dose-dependent effect on the S. ratti-specific IgG1 concentration in the secondary infection (Time x Primary spp. S. ratti, F2,73 = 4.13, p < 0.05). That is, a S. ratti primary infection of 750 iL3s resulted in a greater concentration of IgG1 in a secondary infection, compared with primary dose of 30 iL3s. This effect occurred on both days 7 and 21 p.s.i., but was comparatively somewhat stronger on day 7 p.s.i. (Figure 3). These results suggest that IgG1 has a central role in the host immune response to S. ratti, since it is affected in a density-dependent manner by both prior and current infections. These results are therefore consistent with our hypothesis, namely that host prior infection can enhance anti-S. ratti immune responses.
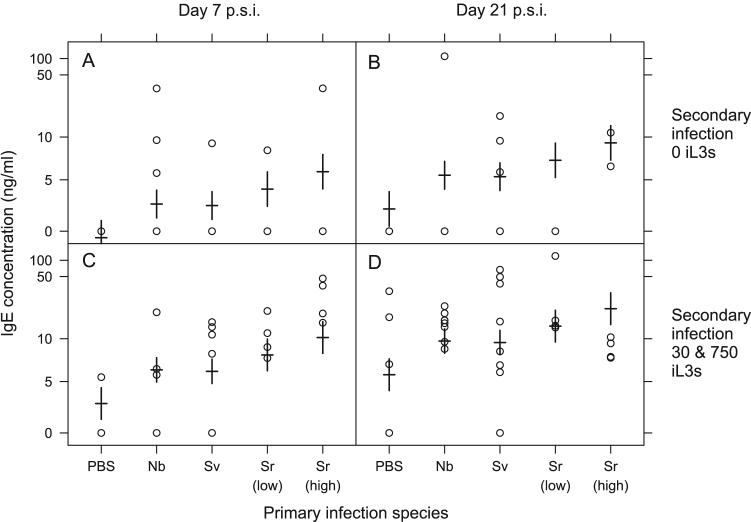
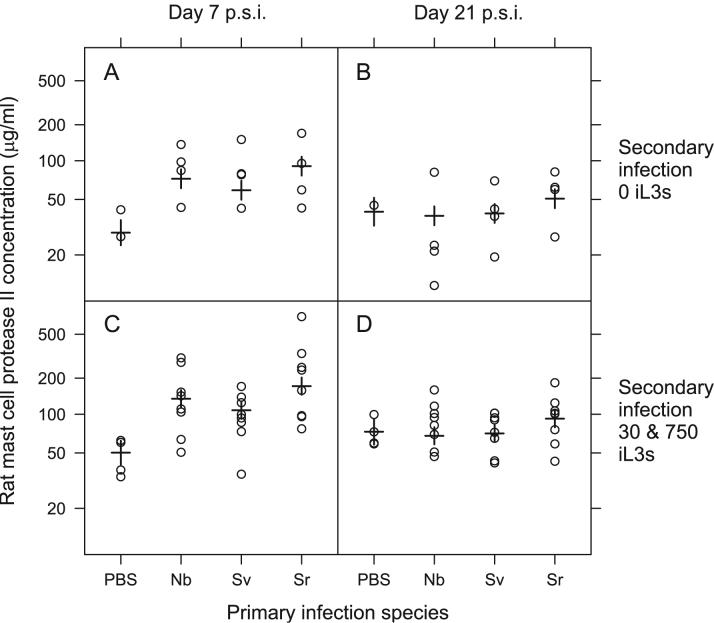
Hetero-specific primary infections only affected the concentration of total IgE and RMCPII. A primary infection of either N. brasiliensis or S. venezuelensis increased the concentration of IgE, compared with control (PBS) treated animals, during the secondary infection (Primary spp., F4,78= 2.89, p <0.05) (Table 5). Both N. brasiliensis and S. venezuelensis had effects of the same magnitude on the concentration of IgE; these effects were, in turn, less than those caused by a primary S. ratti infection (Table 5). There was no effect of the dose of these hetero-specific primary infections on the concentration of IgE during the secondary infection. However, there was a positive effect of the dose of the primary S. ratti infection on the concentration of IgE during the secondary infection (Table 5). Primary infections with N. brasiliensis, S. venezuelensis or S. ratti increased the concentration of RMCPII compared with control, PBS treated animals, on day 7 p.s.i., but not on day 21 p.s.i. (Time x Primary spp.,F3,74 = 2.81, p < 0.05, Table 5). There was no difference between the effect of the primary infecting species (N. brasiliensis, S. venezuelensis or S. ratti) on the concentration of RMCPII during the secondary infection (Figure 4).
Figure 4.
Total IgE concentrations for con- or hetero-specific primary infections and secondary S. ratti infections having received either a dose 0 iL3s (A, B) or a dose of either 30 or 750 iL3s (C, D) at days 7 (A, C) and 21 p.s.i. (B, D). Results for individual animals are circles, with model estimates from Table 5 shown as horizontal bars with standard errors on these estimates indicated by vertical bars. Data are plotted on a Box-Cox transformed scale. The primary infections were: PBS controls (PBS), N. brasiliensis (Nb), S. venezuelensis (Sv), S. ratti 30 iL3s (Sr low) and S. ratti 750 iL3s (Sr high). The different doses delivered in the primary infection for N. brasiliensis and, S. venezuelensis are grouped together since the doses of these primary infections were not found to significantly affect IgE concentration during S. ratti secondary infections. Animals receiving 30 or 750 iL3s in a secondary infection were grouped together since there was no detectable difference between these doses with respect to their effect on IgE concentration.
Both the concentration of total IgE and RMCPII are measures of non-specific effectors of the host immune response elicited by gastrointestinal nematode infection. Therefore these hetero-specific prior infections resulted in some enhancement of the anti-S. ratti immune response, though there was no difference between S. venezuelensis and N. brasiliensis prior infection, nor was there an effect of their dose. These results therefore support, in-part, our hypothesis of the effect of host prior infection on anti-S. ratti immune responses.
In conclusion, this experiment has shown that the strongest effect on S. ratti is the effect of a con-specific prior infection on S. ratti survivorship. We have also found that a host con- or hetero-specific prior infection can enhance the host immune response against a secondary S. ratti infection, but in different ways. Thus, there is a primary con-specific, dose-dependent effect on the concentration of IgG1, an isotype previously identified to be important in S. ratti infections (Wilkes et al., 20007; Bleay et al., 2007). In contrast there are dose-independent non-specific immune effects (IgE and RMCPII) of hetero-specific host prior infection. These results therefore suggest that the principal immunological effect against a nematode infection is elicited by that infection itself and by its dose, that prior infections have smaller, mainly dose-independent, effects and hetero-specific infections have the least effect.
Figure 5.
RMCPII concentrations for con- or hetero-specific primary infections and secondary S. ratti infections having received either a dose 0 iL3s (A, B) or either 30 or 750 iL3s (C, D) at days 7 (A, C) and 21 p.s.i. (B, D). Results for individual animals are circles, with model estimates from Table 5 shown as horizontal bars with standard errors on these estimates indicated by vertical bars. Data are plotted on a Box-Cox transformed scale. The primary infections were: PBS controls (PBS), N. brasiliensis (Nb), S. venezuelensis (Sv) and S. ratti (Sr). The different doses delivered in the primary infection for each species are grouped together since the dose of the primary infection was not found to significantly affect RMCPII concentration during S. ratti secondary infections. Animals receiving 30 or 750 iL3s in a secondary infection were grouped together since there was no detectable difference between these doses with respect to their effect on RMCPII concentration.
Acknowledgments
We would like to thank Maayan Navellou for technical help. This work was funded by a grant from the Wellcome Trust.
References
- Bancroft AJ, Else KJ, Grencis RK. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. European Journal of Immunology. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- Behnke JM. Structure in parasite component communities in wild rodents: predictability, stability, associations and interactions .... or pure randomness? Parasitology. 2008;135:751–766. doi: 10.1017/S0031182008000334. [DOI] [PubMed] [Google Scholar]
- Bleay C, Wilkes CP, Paterson S, Viney ME. Density-dependent immune responses against the gastrointestinal nematode Strongyloides ratti. International Journal for Parasitology. 2007;37:1501–1509. doi: 10.1016/j.ijpara.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ. Statistical computing: an introduction to data analysis using S-plus. Wiley; Chichester: 2002. [Google Scholar]
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JFJ. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annual Review of Immunology. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Wynn TA, Donaldson DD, Urban JFJ. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Current Opinion in Immunology. 1999;11:420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- Gemmill AW, Viney ME, Read AF. Host immune status determines sexuality in a parasitic nematode. Evolution. 1997;51:393–401. doi: 10.1111/j.1558-5646.1997.tb02426.x. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Gemmill AW, Read AF, Viney ME. The control of morph development in the parasitic nematode Strongyloides ratti. Proceedings of the Royal Society of London Series B. 2000;267:2057–2063. doi: 10.1098/rspb.2000.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymer AE. Density-dependent mechanisms in the regulation of intestinal helminth populations. Parasitology. 1982;84:573–587. doi: 10.1017/s0031182000052847. [DOI] [PubMed] [Google Scholar]
- Kimura E, Shintoku Y, Kadosaka T, Fujiwaram M, Kondom S, Itoh M. A second peak of egg excretion in Strongyloides ratti-infected rats: Its origin and biological meaning. Parasitology. 1999;119:221–226. doi: 10.1017/s0031182099004631. [DOI] [PubMed] [Google Scholar]
- Moqbel R, Wakelin D. Trichinella spiralis and Strongyloides ratti: Immune interaction in adult rats. Experimental Parasitology. 1979;47:65–72. doi: 10.1016/0014-4894(79)90008-0. [DOI] [PubMed] [Google Scholar]
- Nawa Y, Mimori T, Korenaga M, Tada I. Stage-specific cross-resistance between Nippostrongylus brasiliensis and Strongyloides ratti (Nematoda) in rats. Journal of Parasitology. 1982;68:804–808. [PubMed] [Google Scholar]
- Paterson S, Viney ME. Host immune responses are necessary for density-dependence in nematode infections. Parasitology. 2002;125:283–292. doi: 10.1017/s0031182002002056. [DOI] [PubMed] [Google Scholar]
- Paterson S, Wilkes CP, Bleay C, Viney ME. Immunological responses elicited by different infection regimes with Strongyloides ratti. PLoS One. 2008;3:e2509. doi: 10.1371/journal.pone.0002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, Faulkner H, Kamgno J, Cormont F, van Snick J, Else KJ, Grencis RK, Behnke JM, Boussinesq M, Bradley JE. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. Journal of Infectious Diseases. 2003;188:1768–1775. doi: 10.1086/379370. [DOI] [PubMed] [Google Scholar]
- West SA, Gemmill AW, Graham A, Viney ME, Read AF. Immune stress and facultative sex in a parasitic nematode. Journal of Evolutionary Biology. 2001;14:333–337. [Google Scholar]
- Wilkes CP, Bleay C, Paterson S, Viney ME. The immune response during a Strongyloides ratti infection of rats. Parasite Immunology. 2007;29:339–346. doi: 10.1111/j.1365-3024.2007.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes CP, Thompson FJ, Gardner MP, Paterson S, Viney ME. The effect of the host immune response on the parasitic nematode Strongyloides ratti. Parasitology. 2004;128:661–669. doi: 10.1017/s0031182004005062. [DOI] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT. Generalized linear modelling for parasitologists. Parasitology Today. 1997;13:33–38. doi: 10.1016/s0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]
- Viney ME. Developmental switching in the parasitic nematode Strongyloides ratti. Proceedings of the Royal Society of London Series B. 1996;263:201–208. doi: 10.1098/rspb.1996.0032. [DOI] [PubMed] [Google Scholar]