Abstract
Background & Aims
Toll-like receptor (TLR)-dependent signaling pathways have been proposed as immunotherapeutic targets against invading pathogens and tumorigenesis. Here we investigated whether TLR5-dependent signaling modulates colonic tumor development in a mouse xenograft model of human colon cancer.
Methods
The expression of MyD88 or TLR5 was stably knocked down in human colon cancer cells (DLD-1). Nude mice were subcutaneously implanted with MyD88-KD, TLR5-KD, or control cells (n=16) to examine the pathophysiology of tumor xenografts. Protein micro-array assessed the differential expression of cytokines in these tumors. Leukocyte infiltration and tumor angiogenesis were assessed by immunohistochemistry with antibodies against neutrophil (Gr-1, 7/4) or macrophage specific antigens (CD68, F4-80), and the vascular endothelial cell marker PECAM-1/CD31, respectively. Tumor xenografts from DLD-1 cells were treated with flagellin (5.0 µg/kg, one injection/every 2 days for 3 weeks) and tumor regression and histopathology of these tumors were examined.
Results
Lack of MyD88 or TLR5 expression dramatically enhanced tumor growth and inhibited tumor necrosis in mouse xenograft of human colon cancer. In contrast, TLR5 activation by peritumoral flagellin treatment substantially increased tumor necrosis, leading to significant tumor regression. Tumors from MyD88- or TLR5-KD cells revealed the reduced production of neutrophil attracting chemokines (ENA-78, MIP3α, and IL-8). Consequently, neutrophil infiltration was dramatically diminished in MyD88 or TLR5 deficient tumor xenografts, while tumor-associated macrophage infiltration or angiogenesis was not changed.
Conclusions
TLR5 engagement by flagellin mediates innate immunity and elicits potent anti-tumor activity, indicating that TLR5-dependent signaling could be a potential immunotherapeutic target to modulate colonic tumors.
Keywords: Toll-like receptor 5, innate immunity, flagellin, colon cancer, anti-tumor activity
The immune defense system detects various foreign antigens to protect and maintain the integrity of the host from invading microorganisms. The immune system also participates in recognizing and modulating tumorigenesis and tumor growth, while tumors are also able to evade immune surveillance by debilitating the host-immune system1, 2. Therefore, molecular mechanisms enhancing host immunity against tumors have been proposed as possible immunotherapeutic approaches against cancer3, 4.
Toll-like receptors recognize microbe-associated molecular patterns (MAMP) such as lipopolysaccharide (LPS), bacterial lipoprotein, bacterial CpG DNA, flagellin and others produced by both pathogenic and commensal microorganisms5, 6, triggering inflammatory and innate immune responses against pathogens7. Innate immune responses initiated by TLR activation not only elicit immediate and non-specific defense responses, but also regulate adaptive immunity characterized with the delayed immune responses (e.g. cytotoxic T lymphocytes and specific antibody production from B cells). Therefore, pathogen recognition in the host by TLRs is a critical step for triggering immune responses.
Large collection of commensal microbiota resides in the human gut and releases various microbial products8, 9. Although Medzhitov et al suggested that TLR4-dependent signaling in the gut regulates intestinal protection from injury10 and MyD88-associated response regulates tumorigenesis in the intestine11, a role of host-commensal interaction by TLRs in the intestinal tumor remains to be investigated. Moreover, among various TLR family members, LPS in moderate concentrations does not induce TLR4-mediated responses at least in several human colonic epithelial cell lines, such as HT-29, Caco-1, and non-transformed colonocytes NCM460 (our unpublished data)12, 13, while a few studies suggested that mouse intestinal crypt epithelial m-ICcl2 cells harbor TLR4 at the Golgi apparatus and consequently are responsive to internalized LPS14, 15. Intestinal epithelial cells highly express TLR5 and are responsive to bacterial flagellin in TLR5 specific manner. In the intestine, TLR5 appears to be localized at the basolateral, not apical, side of the intestinal mucosa16, 17. In addition, among lamina propria cells (LPC) present in submucosa, CD11c-positive cells express TLR5 and produce proinflammatory cytokines in response to bacterial flagellin18, while LPC are anergic to TLR4 activation by LPS18, 19. In the intestine, therefore, TLR5 is an important pattern recognition receptor and plays an important role in host-commensal interaction.
In this study, we investigated whether engagement of TLR5 by bacterial flagellin elicits innate immune responses regulating anti-tumor activity in a mouse xenograft model of human colon cancer. Our data demonstrate that blocking TLR5-dependent signaling substantially inhibits tumor necrosis and promotes tumor growth, whereas activation of TLR5 with bacterial flagellin significantly regresses tumor growth, suggesting that TLR5 engagement with bacterial flagellin elicits potent anti-tumor activity against human colon carcinoma.
Materials and Methods
Mice and reagents
8-week-old female CD-1 nude mice were from Jackson Laboratory (Bar Harbor, ME) and housed in a pathogen free facility. The Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center approved all animal procedures. Purified flagellin from Salmonella typhimurium from InvivoGen (San Diego, CA) was dissolved in LPS-free water (Sigma-Aldrich). The colonic cancer cell line DLD-1 was obtained from ATCC and cultivated as described previously20. Rat anti-mouse 7/4 antibody, rat anti-mouse CD68 antibody, rat anti-mouse F4/80 antibody, and rat anti-mouse Gr-1 antibody were purchased from AbD Serotec (Raleigh, NC). Rat anti-mouse PECAM-1/CD31 was purchased from BD Pharmingen (San Diego, CA).
Generating TLR5 or MyD88 knocked down DLD-1 cells
The silencing vector expressing shRNA targeting human TLR5 or MyD88 and the control vector (psiRNA-GL3Luc) encoding shRNA targeting luciferase gene were obtained from InvivoGen (San Diego, CA)13. These vectors encode GFP fusion protein for tracking the transfected cells. DLD-1 cells were transfected with the construct using Superfect reagent (Qiagen, Valencia, CA) and stably transfected cells were isolated in selection media containing zeocin. Silencing the target molecule was confirmed in isolated colonies, as described before 13.
Xenograft model of human colon cancer
We subcutaneously injected cells into the flanks of 8-week-old female CD-1 nude mice (16 per group), followed by measuring tumors with calipers and calculating volume as (length × width2) × 0.5 20. Tumors were excised from euthanized mice and tumor size and weight were measured. For peritumoral flagellin treatment, two days after injecting cells into the flank of nude mice, we administered flagellin solution (5.0 µg/kg in 150µl) around the tumor site (one injection/every 2 days for 3–4 weeks).
Histology
The tumors were fixed in 10% buffered formalin. Paraffin-embedded sections (6µm thickness) were prepared and stained with Hematoxylin & Eosin. Images were analyzed with Zeiss Axioskop-2 microscope. The area of tumor necrosis was evaluated by Image-J software to calculate the percent of necrotic area in the cross-sectional surface of tumors.
Immunohistochemistry
Dissected tumors were embedded and frozen immediately. Six-micrometer sections were cut and then fixed in acetone for 15 min at 4 °C. After rehydration, sections were blocked in 2% bovine serum albumin (BSA) solution for 10 min, and incubated overnight with primary antibodies [rat anti-mouse 7/4 antibody (, 1:100), rat anti-mouse CD68 (1:100), rat anti-mouse F4/80 (1:200), rat anti-mouse Gr-1 (1:200), and rat anti-mouse PECAM/CD31 (1:100)] diluted in 2% BSA solution with 0.3% Triton X-100 at 4 °C. The negative controls received an equivalent concentration of non-immune rat IgG. After washing with PBS, sections were incubated with biotinylated anti-rat secondary antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 in 2% BSA solution with 0.3% Triton X-100 for 45 min at room temperature. After inactivating endogenous peroxidase, sections were processed for peroxidase immunohistochemistry. 0.2% light green (Sigma) solution was used for counterstaining. Negative control images with isotype control IgG were separately presented in Supplementary Figure 3.
Micro-array analysis
The human cytokine array 5.1 was purchased from Ray Biotech (Norcross, GA). Membranes immobilized with various capture antibodies against 79 different cytokines were used. Briefly, we prepared total protein extracts by homogenizing tumor xenografts (n=4/group) in lysis buffer. Equal amounts of total protein were subjected to human cytokine array, as we previously described12, 13. The expression of each cytokine was visualized in X-ray films and cytokine expression was determined by measuring the density of each spot using Image-J software.
Multi-spectral fluorescence imaging
At 18 days after subcutaneously injecting the cells into nude mice, tumors were visualized in vivo using ‘CRI Maestro multi-spectral fluorescence imager’.
Cytokine measurement, Immunoblotting analysis, and luciferase reporter assays
Statistical analysis
A two-tailed, unpaired Student t-test was used for all statistical analyses.
Results
Blocking TLR5-dependent signaling substantially enhances tumor growth in vivo
Commensal microbiota resides in the intestinal lumen in continuous contact to the intestinal mucosa composed mainly of epithelial cells8, 17. Intestinal epithelial cells highly express TLR5 which is a specific receptor for flagellin12, 13. Oncogenic mutations in epithelial cells transform normal epithelial cells into neoplastic epithelium, resulting in adenocarcinoma in the intestine21, represented by colon cancer. Virtually all colon cancer cell lines such as Caco-2 or HT-29 or DLD-1 and non-transformed colonocytes are strongly responsive to flagellin12, 13, 16. Clinical studies recently showed that imiquimod, TLR7 ligand, suppresses certain types of tumors4, 22. Moreover, Sfondrini et al recently suggested that co-administration of CpG DNA and flagellin suppressed the growth of tumors implanted in mouse with mouse mammary tumor cells, indicating a synergistic anti-tumor activity between flagellin and CpG DNA23. Based on these considerations, we hypothesized that host-microbial interactions by TLR5 elicits anti-tumor activity.
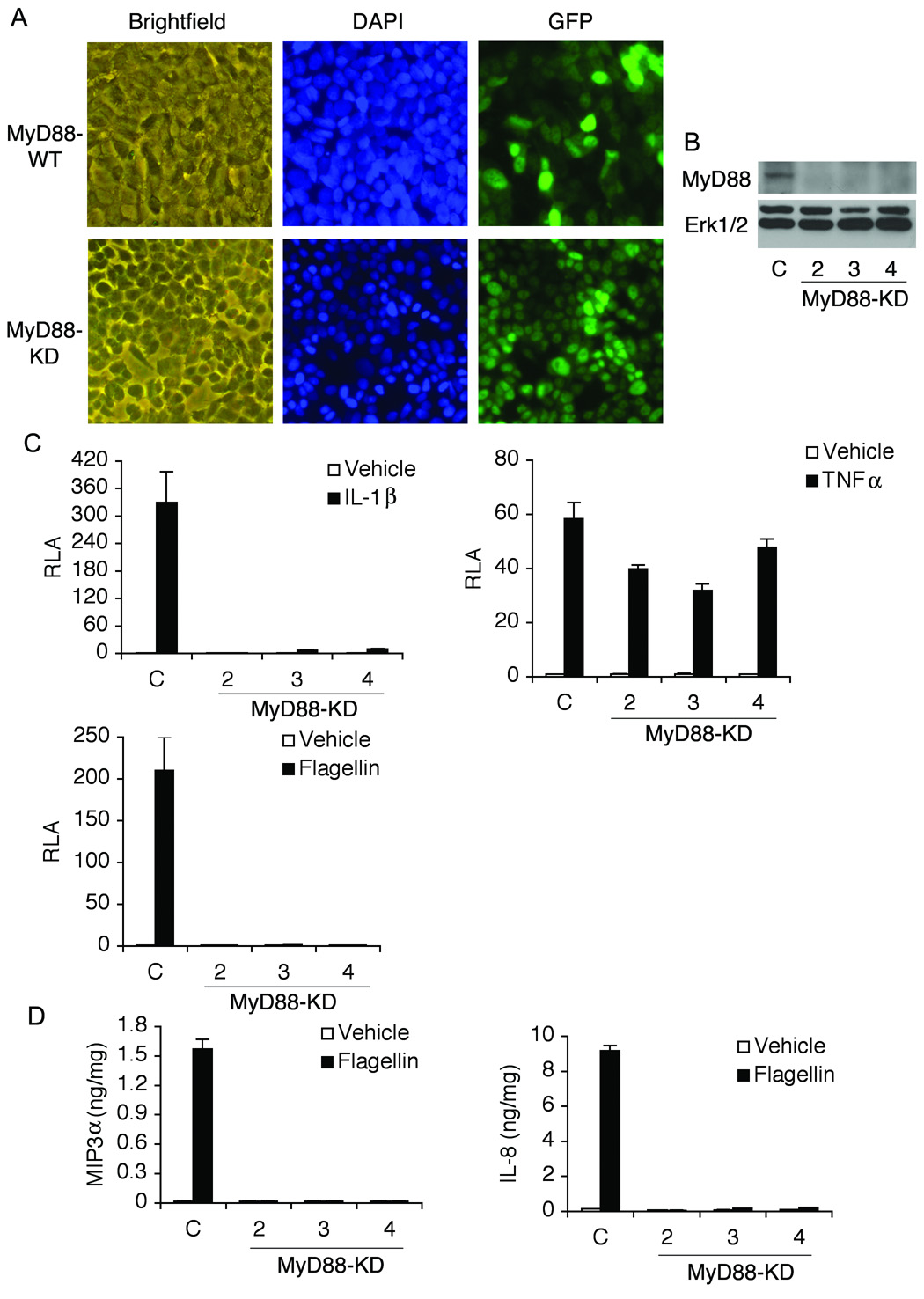
MyD88 is an adaptor molecule mediating signaling pathways from most of TLRs (except TLR3)7. To inhibit the TLR-mediated signaling, we knocked down the expression of MyD88 (MyD88-KD) in DLD-1 colon cancer cells by stably transfecting the vector encoding shRNA for human MyD88. The control vector expressing shRNA targeting luciferase gene was transfected for control DLD-1 cells (MyD88-WT) (Figure 1A and B)13. MyD88-KD cells showed no NFκB activation by IL-1R or TLR5 activation that shares MyD88 as a common adaptor molecule. In contrast, TNFα, mediating MyD88-independent pathways, strongly induced NFκB activation in MyD88-KD cells (Figure 1C). In addition, MyD88-KD cells showed diminished MIP3α and IL-8 expression by flagellin, while MyD88-WT cells strongly produced these cytokines upon flagellin exposure (Figure 1D). We did not observe a difference in cell proliferation of MyD88-KD and MyD88-WT cells (data not shown). These results suggest that endogenous MyD88 expression is specifically knocked down in these cells.
Figure 1. Generating MyD88-KD or MyD88-WT DLD-1 cells.
(A) DLD-1 cells were stably transfected with a construct encoding shRNA against human MyD88 or a control vector. Since these vectors encode GFP fusion protein to confirm stable expression of an exogenous gene, stably transfected cells were identified by fluorescence microscopy. (B) Endogenous MyD88 expression was successfully silenced in several clones (clone number 2, 3, and 4). C, wild type control cells. (C) Lack of MyD88 expression blocked flagellin- or IL-1-induced NFκB-reporter activation, whereas, in wild type control cells, flagellin or IL-1 strongly stimulated NFκB activity. TNFα, stimulating MyD88-independent pathways, is still able to induce NFκB activation in MyD88-KD cells. RLA, relative luciferase activity. (D) Silencing MyD88 expression blocked IL-8 and MIP3α expression in response to flagellin measured by ELISA.
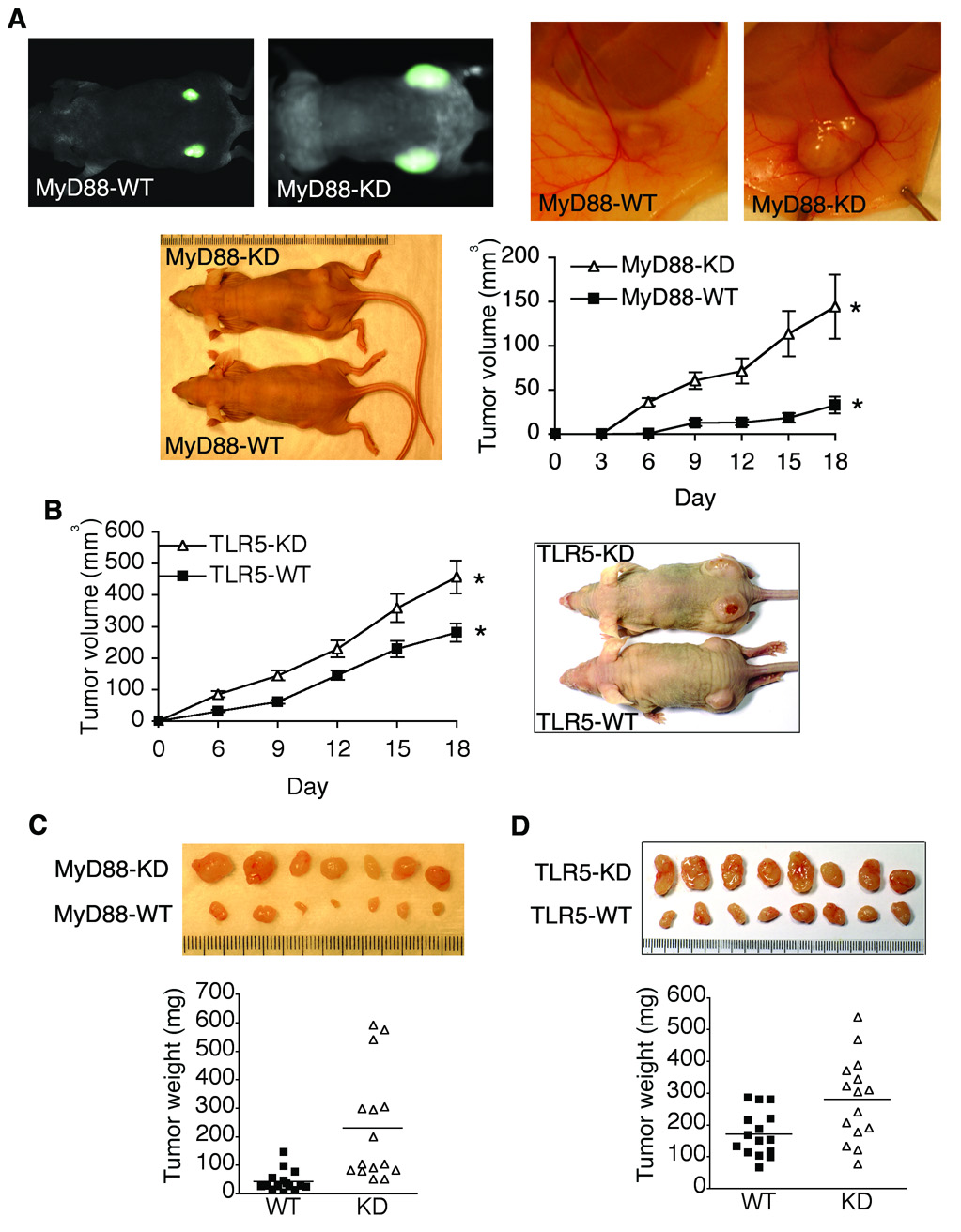
To test our hypothesis, we used a mouse xenograft model of human colon cancer, in which MyD88-KD or MyD88-WT DLD-1 cells were subcutaneously injected into nude mice followed by measuring tumor growth20. Compared to tumors from MyD88-WT cells, tumor volume and size were significantly larger in tumors from MyD88-KD cells (Figure 2A), indicating an important role of TLR-mediated cellular responses in tumor growth in vivo. We also confirmed these results in independent clones of MyD88-KD cells (data not shown), avoiding the possibility of a clone-specific artifact from the stable transfection process.
Figure 2. Blocking TLR5/MyD88-dependent signaling substantially enhanced the tumor growth in mouse xenografts model of human colon cancer.
(A) Multi-spectral fluorescence (upper panel) was used for in vivo imaging of tumor xenografts from MyD88-KD or MyD88-WT DLD-1 cells(1.0 × 106) and gross appearance of xenografts at day 21 was shown. Tumor volume of MyD88-KD and MyD88-WT xenografts was measured. * P = 0.014, n=16. (B) Tumor xenografts from TLR5-KD or TLR5-WT DLD-1 cells (1.6 × 106) at day 21 were shown. Tumor volume of TLR5-KD or TLR5-WT xenografts was measured. * P = 0.006, n=16. (C and D) Tumors from MyD88-KD (C), TLR5-KD (D), or its control DLD-1 cells were excised at day 21 and tumor weight was evaluated. Each index in the ruler represents one mm.
Among various TLR ligands, bacterial flagellin is the most potent microbial product to induce TLR-associated response in colonic epithelial cells12, 17. Moreover, the colon cancer cell, DLD-1, used in this study did not respond to LPS (TLR4 ligand) or Pam3Cys (TLR2 ligand) stimulation (our unpublished data). Therefore, to study the role of TLR engagement in colon cancer, we knocked down the expression of TLR5 in DLD-1 cells (TLR5-KD). As shown in Supplementary Figure 1A and B, endogenous expression of TLR5 was successfully knocked-down in these cells. We did not observe a difference in cell proliferation between TLR5-KD and TLR5-WT cells (data not shown). Since both TLR5 and IL-1R mediate intracellular signaling via the same adaptor molecule MyD88 leading to NFκB activation, we demonstrated that TLR5-KD cells were not responsive to flagellin, but responsive to IL-1 stimulation resulting in NFκB activation (Supplementary Figure 1C), indicating the specific silencing of TLR5 in these cells. Moreover, the functional outcomes of TLR5 engagement such as IL-8 and MIP3α expression were completely blocked in TLR5-KD cells, whereas TLR5-WT cells strongly expressed these cytokines in response to flagellin (Supplementary Figure 1D). These data indicated that TLR5 expression is specifically silenced in TLR5-KD cells.
To test whether TLR5 contributes to tumor development, TLR5-KD or TLR5-WT DLD-1 cells were subcutaneously injected into nude mice. We observed that tumors from TLR5-KD cells grew larger and faster than tumors from TLR5-WT cells, resulting in the substantially increased tumor volume in TLR5-KD tumors, compared to TLR5-WT tumors (Figure 2B). Additionally, at day 21 after implanting tumor cells, the tumor xenograft was excised to examine the tumor size and weight. Our results showed that tumor weight and size were significantly increased in MyD88-KD or TLR5-KD tumors, compared to its control tumors (Figure 2C and D). These results demonstrated that blocking TLR5-dependent signaling substantially promoted tumor growth in mouse xenograft model of human colon cancer.
Both tumor necrosis and leukocyte infiltration are inhibited in MyD88- or TLR5-knocked down tumor xenografts
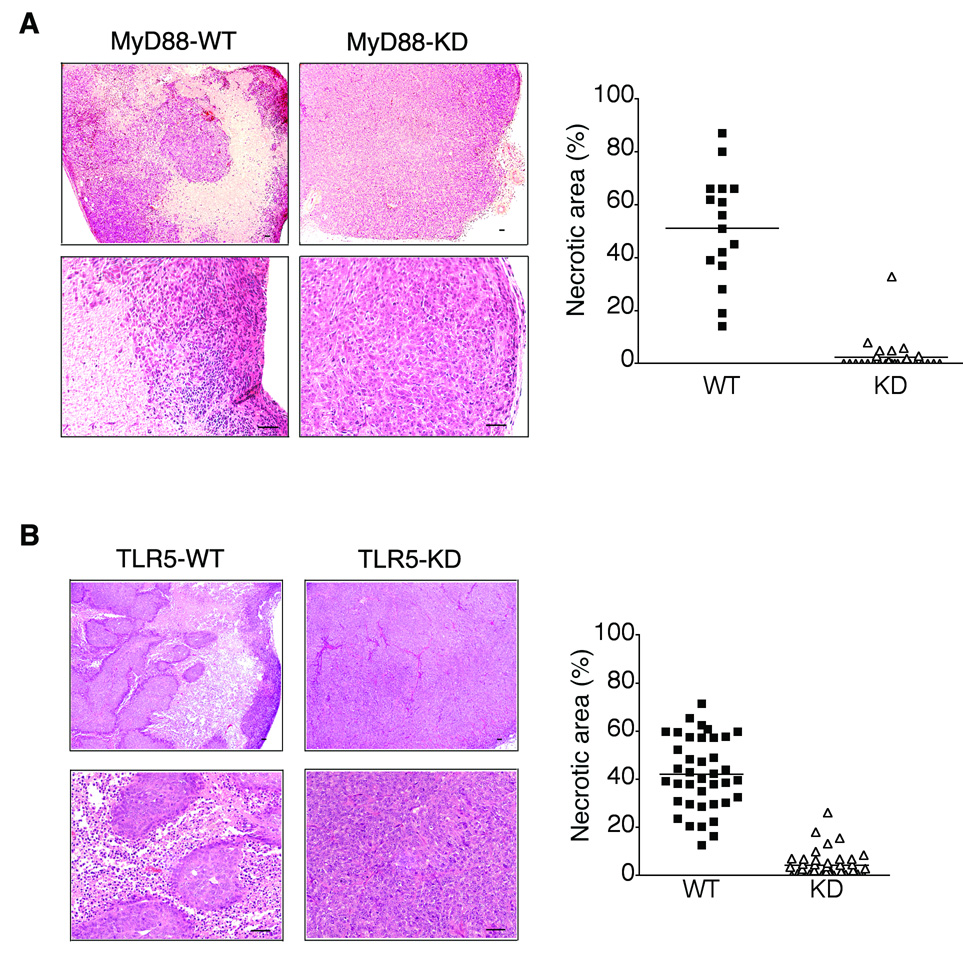
Since tumor necrosis is a critical factor modulating tumor development and growth, we investigated the histopathology of tumor xenografts to determine whether the promoted tumor growth in TLR5- or MyD88-knocked down tumors is associated with the altered tumor necrosis. As shown in Figure 3, examination of the excised tumor revealed that tumor necrosis was dramatically reduced in tumor xenografts developed from MyD88-KD cells, whereas much larger necrotic areas were prevalent in the central region of MyD88-WT xenografts. While MyD88-WT tumors showed multifocal and deeply invading inflammatory infiltrates, tumors developed from MyD88-KD cells revealed relatively minor inflammatory infiltrates that were restricted to the tumor perimeter (Figure 3A). These data showed that blocking MyD88-dependent TLR activation suppresses both tumor necrosis and leukocytes infiltration inside tumors. In addition, examining the tumor histology revealed that tumor xenografts from TLR5-KD cells also showed the dramatically reduced tumor necrosis and leukocyte infiltration, whereas tumor necrosis was prevalent with the increased leukocyte infiltration in tumors from TLR5-WT cells (Figure 3B). These data demonstrated that blocking TLR5-dependent signaling dramatically suppressed the tumor necrosis and leukocyte infiltration in the mouse xenografts of human colon cancer.
Figure 3. Tumor necrosis and leukocytes infiltration were dramatically reduced in MyD88- or TLR5-knocked down tumor xenografts.
(A and B) Tumor sections from MyD88-KD (A), TLR5-KD (B), or its control DLD-1 cells were stained with H&E (upper or lower images represent lower or higher magnification, respectively) to show necrotic or viable non-necrotic area. Percent necrotic surface area was measured using the Image-J software. Scale bar, 50 µm. Horizontal bar in graph represents median.
Expression of neutrophil recruiting cytokines was reduced in MyD88- or TLR5-knocked down tumor xenografts
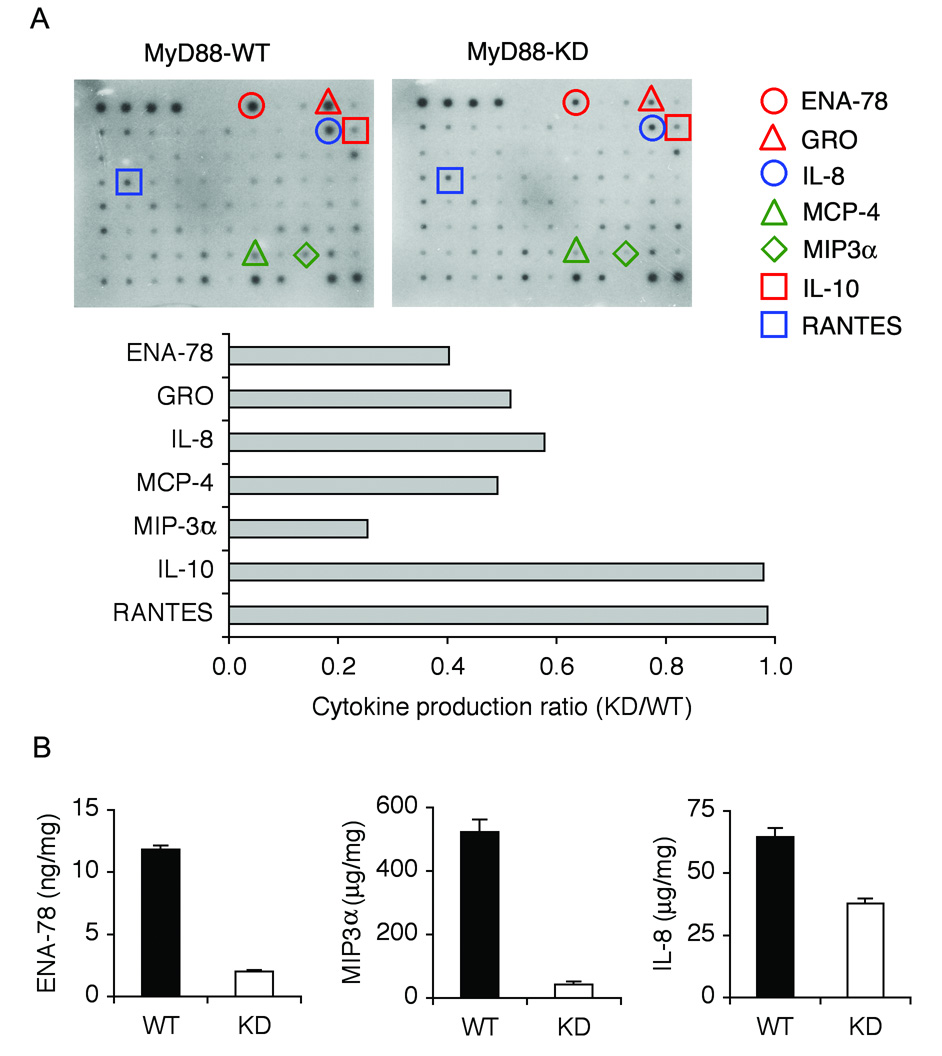
Studies indicate that a distinct profile of cytokines and chemokines is modulated upon TLR-mediated inflammatory and immune responses12. Therefore, to investigate the mechanism by which activation of TLR5/MyD88-associated signaling pathways suppresses tumor development and promotion, we analyzed the differential expression of cytokines/chemokines in tumor xenografts. Tumors from MyD88-KD or MyD88-WT DLD-1 cells (n=4/group) were excised and total protein extracts were used for cytokine micro-array. MyD88-KD tumors revealed the substantially decreased (> 50% reduction) production of several chemokines (e.g. ENA-78, MIP3α, IL-8, MCP-4, or Gro), compared to MyD88-WT tumors, while expression of IL-10 or RANTES was not changed (Figure 4A). These results were also confirmed by ELISA (Figure 4B). Interestingly, the chemokines showing reduced expression in MyD88-KD tumors are mostly involved in recruiting leukocytes24. Investigating the cytokine expression in TLR5-KD tumors using cytokine micro-array and ELISA also confirmed the reduced ENA-78, MIP3α, and IL-8 expression in TLR5-KD tumors, compared to TLR5-WT tumors (Supplementary Figure 2A and B). Therefore, silencing MyD88 or TLR5 expression reduced ENA-78, MIP3α, and IL-8 production in xenografts of colonic tumors.
Figure 4. Neutrophil attracting cytokines were reduced in tumor xenografts from MyD88-KD cells.
(A) MyD88-KD tumors showed the reduced expression of chemokines involved in attracting leukocytes. Equal amounts of total protein extracts (300 µg) from tumor xenografts (n=4/group) were used for human cytokine micro-array analysis (upper panel). Density of each spot was determined to analyze the expression ratio of each cytokine (MyD88-KD to MyD88-WT). Cytokines with significantly altered expression are presented (lower panel). (B) To confirm altered cytokine expression evaluated by micro-array analysis, we measured the level of ENA-78, MIP3α, or IL-8 protein by ELISA.
Silencing TLR5 or MyD88 expression blocks neutrophil infiltration, but does not affect macrophage infiltration and angiogenesis in tumors
MIP3α is a chemokine that attracts lymphocytes and dendritic cells, while IL-8 is a chemoattractant for neutrophils12, 24. ENA-78 is highly homologous to NAP-2, GRO, and IL-8, and induces neutrophil chemotaxis by acting through the same type of receptors as NAP-2, GRO, and IL-825, 26. Due to the modulated expression of these chemokines in TLR5-KD and MyD88-KD tumor xenografts, we speculated that leukocytes infiltration, especially neutrophils, could be influenced in these tumors. Moreover, recent studies demonstrated that neutrophils posses potent anti-tumor activity at least in certain types of cancer27, 28. Neutrophils are able to release reactive oxygen species to kill cancer cells27. Indeed, depleting neutrophils profoundly enhanced tumor growth in mice27, demonstrating an important role of neutrophils in modulating tumor growth.
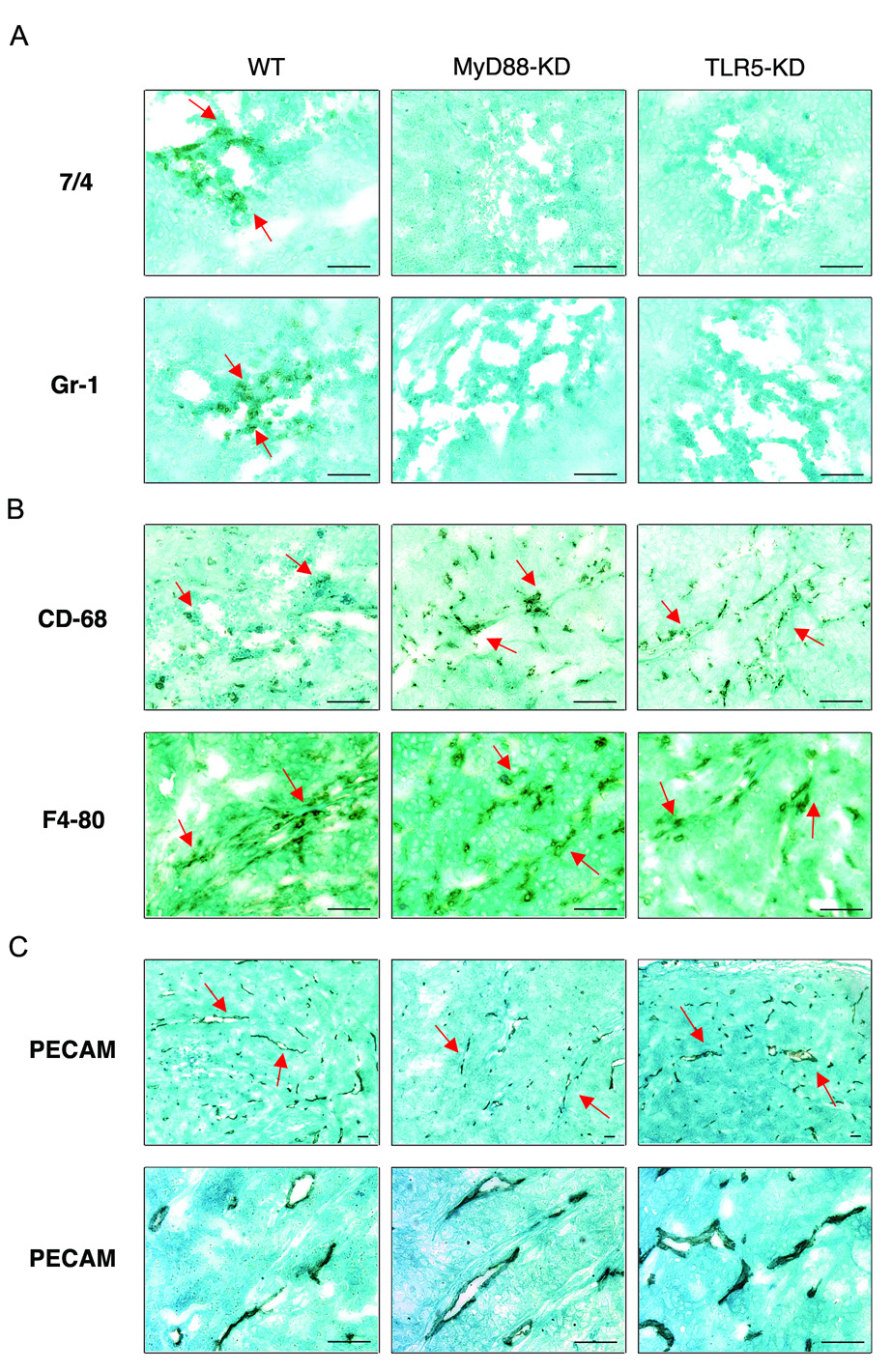
In this context, we investigated whether lack of TLR5 or MyD88 alters neutrophil infiltration in tumor xenografts. To test this, we performed immunohistochemistry with tumor xenografts using antibodies against neutrophil specific markers (Gr-1 and 7/4)28. There were no Gr-1 or 7/4-positive cells in the cross-sectional surface of tumors from MyD88-KD and TLR5-KD cells (Figure 5A). In contrast, Gr-1 and 7/4 positive neutrophils were clearly evident in sections from wild type control tumors (Figure 5A). Interestingly, our immunohistochemistry data showed that infiltrating neutrophils were mainly localized in tumor necrotic areas, but not in non-necrotic viable areas, supporting an anti-tumor effect of neutrophils. Apart from neutrophils, tumor associated macrophages also constitute part of the overall component of leukocytes infiltrated into tumors, although their role in tumor pathophysiology is still controversial. Moreover, angiogenesis in tumors is an important factor required for tumor progression. Therefore, we speculated that the enhanced tumor growth in TLR5- or MyD88-deficient tumors could be attributed to altered macrophage infiltration in tumor xenografts. To address this, we performed immunohistochemistry with antibodies recognizing the macrophage-specific antigens CD68 and F4-8028. Our data showed that macrophages equally infiltrated into necrotic-and non-necrotic areas of tumors generated from MyD88-KD, or TLR5-KD, or control cells, indicating that the impaired TLR5/MyD88 signaling axis does not affect macrophage infiltration in these tumors (Figure 5B and Supplementary Figure 3A and B).
Figure 5. Neutrophil, not macrophage, infiltration was diminished in TLR5 or MyD88 deficient tumors.
(A and B) Immunohistochemistry with antibodies against the neutrophil specific markers 7/4 and Gr-1 (A) or macrophage specific markers CD68 and F4-80 (B) visualized the infiltration of neutrophils or macrophages in tumors. (C) Micro-vessels in tumors were visualized by immunohistochemistry with antibody against PECAM-1/CD31 (Upper or lower panels indicate lower or higher magnification, respectively). Scale bar, 50 µm.
Circulatory vessels represent a main route to supply infiltrating leukocytes into tumors. Moreover, micro-vessel formation is required for tumor promotion by supplying nutrients necessary for proliferating tumor cells. In this context, we next examined the vascularity in xenografts from TLR5-KD, or MyD88-KD, or control DLD-1 cells with immunohistochemistry using an antibody against PECAM-1/CD31, the specific marker of vascular endothelial cell20. There was no difference in micro-vessel formation in theses tumors, indicating that TLR5 or MyD88 deficiency does not affect tumor angiogenesis in xenografts (Figure 5C and Supplementary Figure 3C). These results also imply that the difference in neutrophil infiltration is not due to altered tumor vascularity. These data indicated that the neutrophil infiltration, but not macrophage infiltration nor angiogenesis, could be a key component for TLR5-associated anti-tumor activity.
TLR5 activation by flagellin leads to significant tumor regression in vivo
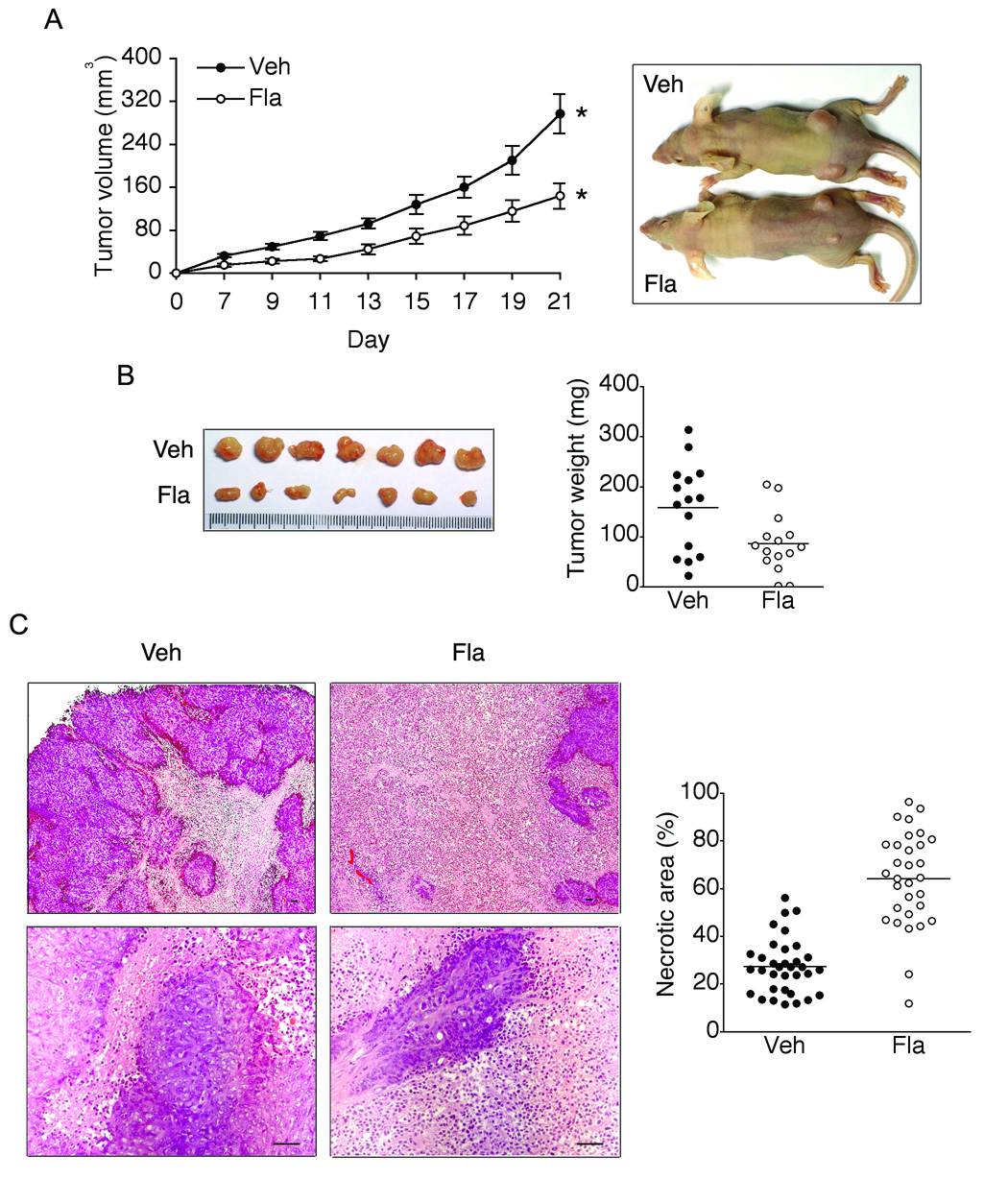
Given the data that blocking TLR5/MyD88-associated signaling resulted in the enhanced tumor growth in the mouse xenograft model of human colon cancer, we hypothesized that activation of TLR5-mediated responses is able to suppress the growth of tumor xenografts. To confirm whether flagellin/TLR5 engagement alters tumorigenesis and tumor growth, we subcutaneously injected DLD-1 cells into nude mice. Two days later, we administered a flagellin solution (5.0 µg/kg in 150µl) around the tumor (peritumoral) site and tumor growth was then measured. As shown in Figure 6A, compared to vehicle treatment, flagellin administration significantly suppressed tumor growth. Tumor size and weight were also significantly reduced in flagellin-treated tumors (Figure 6B). Furthermore, examining the histologic changes in tumors revealed that the necrotic area in the cross-sectional surface of tumors was significantly greater in flagellin-treated xenografts, compared to vehicle treated tumors (Figure 6C). These results imply that flagellin stimulation provoked the anti-tumor activity resulting in the dramatically increased necrosis in tumors and causing the significant tumor regression.
Figure 6. Peritumoral treatment with flagellin suppresses tumor growth.
(A) Two days after DLD-1 cells(1.5 × 106) were subcutaneously injected into nude mice, flagellin (5.0 µg/kg in 150µl) was administered around the tumor site (one injection/every 2 days for 3 weeks) followed by measuring tumor volume. * P = 0.0015, n=16. (B) Tumor xenografts were excised at day 21 and tumor weight was measured. (C) H&E staining of tumor sections showed more prevalent tumor necrosis in flagellin-treated tumors (upper or lower image represents lower or higher magnification, respectively). Scale bar, 50 µm. Horizontal bar in graph represents median. Veh, Vehicle; Fla, Flagellin.
In addition, we subcutaneously implanted DLD-1 cells into nude mice. After two weeks, when solid tumors were established, we administered a flagellin solution or vehicle control around the tumor site for another two weeks and tumor growth was then monitored. In this experimental setting, however, flagellin stimulation did not alter tumor growth (data not shown), indicating that the anti-tumor activity elicited from flagellin/TLR5 engagement may not be sufficient to modulate growth of established tumor xenografts. This result is similar to a previous report indicating that flagellin administration to established solid tumors generated from implanting mouse mammary tumor D2F2 cells did not inhibit tumor growth23. These data indicate that an inhibitory role by flagellin/TLR5 engagement in tumor growth may be effective in the initial step of tumor development, but not sufficient to modulate the established tumor.
While TLR5 recognizes flagellin in the plasma membrane, flagellin introduced into the cytoplasm of macrophages is recognized by Ipaf or Birc1e/Naip5 that are members of the nucleotide binding site leucine-rich repeat (NBS-LRR) superfamily29 30, 31. These TLR5-independent flagellin sensing mediates activation of caspase-1 and interleukin-1β in macrophages infected with pathogenic microbes such as Salmonella or Legionella29 30, 31. Since our results indicate that stimulation of tumor xenografts with flagellin elicits anti-tumor activity via TLR5/MyD88-associated pathways, we tested whether TLR5-independent flagellin signaling is also involved in the tumor regression in response to flagellin. To address this issue, DLD-1-TLR5-KD or MyD88-KD cells were subcutaneously injected into nude mice followed by peritumoral flagellin administration as described in ‘Materials and Methods’. As shown in Supplementary Figure 4, flagellin treatment did not significantly alter the growth of tumor xenografts generated from DLD-1-TLR5-KD or DLD-1-MyD88-KD cells, indicating that TLR5-independent cytosolic signaling is not involved in this flagellin response.
Discussion
Epithelial cells constituting the intestinal epithelial cell lining are in constant contact with the commensal microflora. This host-commensal interaction is known to participate in maintaining intestinal physiology and immunity in various ways. For instance, commensal microbes competitively exclude pathogenic bacteria in the gut and release various antimicrobial factors that interfere with the growth of microbial pathogens32. Additionally, short-chain fatty acids from commensal bacteria lower the luminal pH to limit pathogenic bacteria33.
Since intestinal epithelial cells are able to communicate with microbes in the gut via pattern recognition receptors, the physiological consequences of host-commensal interactions in intestinal epithelial cells are believed to be associated with tumor development and progression in the gut. However, the mechanisms and intracellular responses by which pattern recognition receptor engagement modulates tumorigenesis and tumor growth have been poorly studied. In this study, we demonstrate that impairing TLR5- or MyD88-dependent responses in human colon cancer cells substantially enhanced the size of tumor xenografts, while activating TLR5 by flagellin dramatically regressed the tumor growth. Both tumor necrosis and neutrophil infiltration participating in innate immune responses were significantly diminished in TLR5- or MyD88-deficient tumors, whereas tumor associated macrophages and angiogenesis were not changed. These results imply that bacterial flagellin is able to modulate tumor development and tumor growth via its specific TLR5 receptor. Since virtually all types of intestinal epithelial cells including colon cancer cells and non-transformed intestinal epithelial cells highly express TLR5 and strongly respond to flagellin stimulation, our findings indicate that flagellin/TLR5 engagement may play an important role in tumor immunology in the gut.
Recently, Rakoff-Nahoum et al showed that MyD88-mediated signaling regulates tumorigenesis in the intestine11. According to this study, ApcMin/+ MyD88−/− mice had a reduced number of intestinal polyps, compared to ApcMin/+ MyD88+/+ mice, indicating that MyD88-mediated signaling promotes tumor development in the familial adenomatous polyposis (FAP) model of ApcMin/+ mouse11. This study suggested that enhanced inflammatory responses mediated by MyD88 are associated with colon cancer development in this model. In contrast, our results show that blocking TLR5/MyD88-mediated signaling in human colon cancer mouse xenografts suppresses innate immune responses in this model represented by reduced neutrophil infiltration and tumor necrosis inside the tumors, which is associated with enhanced tumor growth. Our results are consistent with the results reported by Hicks et al, demonstrating that infiltrating leukocytes such as neutrophils, natural killer cells, and macrophages possess potent anti-tumor activities that destroy cancer cells through rapid cytolysis in mice34. In addition, several studies also demonstrated that neutrophils exert potent anti-tumor responses that modulate tumor growth in several types of cancer27, 28. Our data show that TLR5- or MyD88-deficient tumors have reduced production of cytokines that are responsible for recruiting leukocytes (see Figure 4 and Supplementary Figure 2), implying that impaired TLR5/MyD88-associated signaling suppresses innate immunity against tumors.
The opposite finding from these two studies could be due to the different model system. ApcMin/+ mouse has a point mutation at the Apc gene. The mutated Apc protein fails to associate with β-catenin, axin, and GSK3β, resulting in increased β-catenin levels in cytoplasm. Subsequently, β-catenin interacts with the transcription factor Tcf4 to translocate into the nucleus, in which it induces the expression of oncogenes such as c-myc, cycline D1 or c-jun resulting in tumorigenesis35. Despite that the ApcMin/+ mouse model is a promising model of human colorectal cancer harboring the same Apc mutation in FAP tumors35, 36, it does not reflect the genetic aberration in hereditary nonpolyposis colorectal cancer (HNPCC) which is not due to Apc mutations, but to a mutation in a mismatch repair (MMR) gene. In the present study, we used a mouse xenograft model of human colon cancer DLD-1 cells, which has also a mutation at codon 1416 of the Apc gene and loss of the other allele37. In addition, DLD-1 cells harbor oncogenic KRAS mutation (Gly13Asp) which is not detectable in colon tumors of ApcMin/+ mice35. Moreover, DLD-1 cells were originally obtained from a terminal colon cancer patient with invasive adenocarcinoma38. Thus, silencing the expression of TLR5 or MyD88 in this cell line could affect tumor development and growth in a different way compared to tumor growth in ApcMin/+ mice.
A report from Sfondrini et al also showed that flagellin administration at the time of implanting mouse mammary tumor cells resulted in increased tumor growth, rather than growth suppression23. This finding is opposite to our data that tumor growth was regressed in mouse xenograft of human colon cancer cells in response to flagellin (Figure 6). The different results of our study and the results of Sfondrini et al might be related to the use of different cancer cells which have different susceptibility to flagellin stimulation. Mouse mammary cancer cells and colon cancer cells may lead to different immunogenicity in response to innate or adaptive immunity. Indeed, Sfondrini et al showed that while flagellin administration alone failed to suppress tumor growth, co-administration of flagellin and CpG DNA significantly inhibited tumor growth23, indicating that a sufficient level of TLR-dependent response is required to elicit anti-tumor activity in tumors from implanting mouse mammary cancer cells. Our study also showed that flagellin stimulation starting early after colon cancer cell implantation dramatically suppresses tumor growth and enhances tumor necrosis.
Various studies demonstrated that neutrophil infiltration is a key factor eliciting anti-tumor avtivity in several tumor models27, 28. In this study, we determined that TLR5/MyD88-dependent signaling regulates neutrophil infiltration in tumor xenografts. Therefore, the engagement of TLR5 in the gut appears to be crucial to control the innate immune response against tumors, at least in the intestinal epithelium. This notion extends our understanding on the role of commensal microflora to the modulation of tumorigenesis and tumor growth. Our present study shows that TLR5/MyD88-dependent signaling elicits innate immune responses likely to mediate anti-tumor activity in a mouse xenograft model of human colon cancer, indicating that regulating TLR5/MyD88-mediated signaling could be a immunotherapeutic approach against tumors.
Supplementary Material
Generating TLR5-KD DLD-1 cells. (A) Stably transfected cells with psiRNA-hTLR5 were identified by fluorescence microscopy. (B) Silenced TLR5 expression was confirmed by Western blot analysis in several clones (clone number 1, 2, and 3). P, positive control of HEK295 cells transfected with TLR5 expression plasmid. C, wild type control cells. (C) TLR5-KD cells were not responsive to flagellin stimulation, while IL-1 induced NFκB activation in these cells. RLA, relative luciferase activity. (D) Flagellin did not stimulate IL-8 and MIP3α in TLR5-KD cells.
Neutrophil attracting cytokines were reduced in tumor xenografts from TLR5-KD DLD-1 cells. (A) Equal amount of total protein extracts (300 µg) from tumor xenografts (n=4/group) was used for human cytokine micro-array (upper). We measured the density of each spot to analyze the expression ratio of each cytokine (TLR5-KD to TLR5-WT) and cytokines showing a significant change are presented (lower). (B) To confirm the altered cytokine expression examined by microarray, ENA-78, MIP3α, or IL-8 production were measured by ELISA.
Immunohistochemistry with antibodies recognizing the macrophage specific marker CD68 (A) or F4-80 (B) and its isotype control IgG. (C) Micro-vessels in tumors were shown by immunohistochemistry with an antibody against PECAM-1/CD31 and its isotype control IgG (Upper or lower panels indicate lower or higher magnification, respectively). Scale bar, 50 µm.
Flagellin treatment did not alter the growth of tumor xenografts generated from DLD-1-TLR5-KD or DLD-1-MyD88-KD cells. Two days after DLD-1-TLR5-KD (A) or DLD-1-MyD88-KD (B) cells (1.5 × 106) were subcutaneously injected into nude mice, flagellin (5.0 µg/kg in 150µl) or vehicle was administered around the tumor site (one injection/every 2 days) followed by measuring tumor volume. N=8 per group.
Acknowledgement
This work was supported by a Research Fellowship Award (S.H.R.) from the “Crohn’s and Colitis Foundation of America, Inc.”, Young Clinical Scientific Award from Flight Attendant Medical Research Institute, Inc. (S.H.R. and E.I.), and RO-1 DK072471 (C.P.). The authors also thank Dr. Sam Eun Kim for technical assistance in this study.
Abbreviations footnote
- APC
adenomatous polyposis coli
- ENA-78
epithelial cell-derived neutrophil-activating peptide-78
- MCP-1
monocyte chemoattractant protein-1
- MIP3α
macrophage-inflammatory proteinα
- MyD88
myeloid differentiation factor 88
- NAP-2
neutrophil-activating peptide 2
- PECAM
platelet/endothelial cell adhesion molecule
- RANTES/CCL
regulated on activation of normal T cell expressed and secreted
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement:
The authors declare that they have no competing financial interests.
References
- 1.Seliger B, Maeurer MJ, Ferrone S. TAP off--tumors on. Immunol Today. 1997;18:292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 2.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 3.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 4.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SH, Jones BW, Toshchakov V, Vogel SN, Fenton MJ. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J Biol Chem. 2003;278:22506–22512. doi: 10.1074/jbc.M208633200. [DOI] [PubMed] [Google Scholar]
- 7.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 12.Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3alpha gene expression in non-transformed human colonic epithelial cells. J Biol Chem. 2004;279:25179–25188. doi: 10.1074/jbc.M400967200. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SH, Kim H, Moyer MP, Pothoulakis C. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/toll-like receptor 5 engagement in colonic epithelial cells. J Biol Chem. 2006;281:18560–18568. doi: 10.1074/jbc.M513861200. [DOI] [PubMed] [Google Scholar]
- 14.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Aubel RA, Keestra AM, Krooshoop DJ, van Eden W, van Putten JP. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Mol Immunol. 2007;44:3702–3714. doi: 10.1016/j.molimm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz AT, Navas TA, Lyons S, Godowski J, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 17.Rhee SH, Im E, Riegler M, Kokkotou E, O'Brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci U S A. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 19.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 21.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 22.Chang YC, Madkan V, Cook-Norris R, Sra K, Tyring S. Current and potential uses of imiquimod. South Med J. 2005;98:914–920. doi: 10.1097/01.smj.0000176712.01491.98. [DOI] [PubMed] [Google Scholar]
- 23.Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Menard S, Balsari A. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol. 2006;176:6624–6630. doi: 10.4049/jimmunol.176.11.6624. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 25.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 27.Challacombe JM, Suhrbier A, Parsons PG, Jones B, Hampson P, Kavanagh D, Rainger GE, Morris M, Lord JM, Le TT, Hoang-Le D, Ogbourne SM. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 28.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 30.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 31.Coers J, Vance RE, Fontana MF, Dietrich WF. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol. 2007;9:2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 32.Reid G, Howard J, Gan BS. Can bacterial interference prevent infection? Trends Microbiol. 2001;9:424–428. doi: 10.1016/s0966-842x(01)02132-1. [DOI] [PubMed] [Google Scholar]
- 33.Teitelbaum JE, Walker WA. Nutritional impact of pre- and probiotics as protective gastrointestinal organisms. Annu Rev Nutr. 2002;22:107–138. doi: 10.1146/annurev.nutr.22.110901.145412. [DOI] [PubMed] [Google Scholar]
- 34.Hicks AM, Riedlinger G, Willingham MC, Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir HM, Du W, Kim J, Simpson AJ, Old LJ, Cui Z. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci U S A. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada Y, Mori H. Multistep carcinogenesis of the colon in Apc(Min/+) mouse. Cancer Sci. 2007;98:6–10. doi: 10.1111/j.1349-7006.2006.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strate LL, Syngal S. Hereditary colorectal cancer syndromes. Cancer Causes Control. 2005;16:201–213. doi: 10.1007/s10552-004-3488-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761–4766. [PubMed] [Google Scholar]
- 38.Dexter DL, Spremulli EN, Fligiel Z, Barbosa JA, Vogel R, VanVoorhees A, Calabresi P. Heterogeneity of cancer cells from a single human colon carcinoma. Am J Med. 1981;71:949–956. doi: 10.1016/0002-9343(81)90312-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generating TLR5-KD DLD-1 cells. (A) Stably transfected cells with psiRNA-hTLR5 were identified by fluorescence microscopy. (B) Silenced TLR5 expression was confirmed by Western blot analysis in several clones (clone number 1, 2, and 3). P, positive control of HEK295 cells transfected with TLR5 expression plasmid. C, wild type control cells. (C) TLR5-KD cells were not responsive to flagellin stimulation, while IL-1 induced NFκB activation in these cells. RLA, relative luciferase activity. (D) Flagellin did not stimulate IL-8 and MIP3α in TLR5-KD cells.
Neutrophil attracting cytokines were reduced in tumor xenografts from TLR5-KD DLD-1 cells. (A) Equal amount of total protein extracts (300 µg) from tumor xenografts (n=4/group) was used for human cytokine micro-array (upper). We measured the density of each spot to analyze the expression ratio of each cytokine (TLR5-KD to TLR5-WT) and cytokines showing a significant change are presented (lower). (B) To confirm the altered cytokine expression examined by microarray, ENA-78, MIP3α, or IL-8 production were measured by ELISA.
Immunohistochemistry with antibodies recognizing the macrophage specific marker CD68 (A) or F4-80 (B) and its isotype control IgG. (C) Micro-vessels in tumors were shown by immunohistochemistry with an antibody against PECAM-1/CD31 and its isotype control IgG (Upper or lower panels indicate lower or higher magnification, respectively). Scale bar, 50 µm.
Flagellin treatment did not alter the growth of tumor xenografts generated from DLD-1-TLR5-KD or DLD-1-MyD88-KD cells. Two days after DLD-1-TLR5-KD (A) or DLD-1-MyD88-KD (B) cells (1.5 × 106) were subcutaneously injected into nude mice, flagellin (5.0 µg/kg in 150µl) or vehicle was administered around the tumor site (one injection/every 2 days) followed by measuring tumor volume. N=8 per group.