Abstract
Purpose
To investigate the prognostic and predictive significance of subtyping node-positive early breast cancer by immunohistochemistry in a clinical trial of a docetaxel-containing regimen.
Methods
Pathologic data from a central laboratory were available for 1,350 patients (91%) from the BCIRG 001 trial of docetaxel, doxorubicin, and cyclophosphamide (TAC) versus fluorouracil, doxorubicin, and cyclophosphamide (FAC) for operable node-positive breast cancer. Patients were classified by tumor characteristics as (1) triple negative (estrogen receptor [ER]–negative, progesterone receptor [PR]–negative, HER2/neu [HER2]–negative), (2) HER2 (HER2-positive, ER-negative, PR-negative), (3) luminal B (ER-positive and/or PR-positive and either HER2-positive and/or Ki67high), and (4) luminal A (ER-positive and/or PR-positive and not HER2-positive or Ki67high), and assessed for prognostic significance and response to adjuvant chemotherapy.
Results
Patients were subdivided into triple negative (14.5%), HER2 (8.5%), luminal B (61.1%), and luminal A (15.9%). Three-year disease-free survival (DFS) rates (P values with luminal B as referent) were 67% (P < .0001), 68% (P = .0008), 82% (referent luminal B), and 91% (P = .0027), respectively, with hazard ratios of 2.22, 2.12, and 0.46. Improved 3-year DFS with TAC was found in the luminal B group (P = .025) and a combined ER-positive/HER2-negative group treated with tamoxifen (P = .041), with a marginal trend in the triple negatives (P = .051) and HER2 (P = .068) subtypes. No DFS advantage was seen in the luminal A population.
Conclusion
A simple immunopanel can divide breast cancers into biologic subtypes with strong prognostic effects. TAC significantly complements endocrine therapy in patients with luminal B subtype and, in the absence of targeted therapy, is effective in the triple-negative population.
INTRODUCTION
There has been a steady evolution in the adjuvant treatment of early breast cancer. In patients with hormone-sensitive tumors, tamoxifen reduces the risk of recurrence and death by more than 30%.1 Subsequently, anthracycline-based chemotherapy1 and, more recently, taxane-containing regimens,2 have been shown to be most efficacious in improving disease-free (DFS) and overall survival (OS) in patients with operable node-positive breast cancer.
Since the study of Perou et al3 using gene expression studies to identify discrete genetic subtypes of breast cancer with distinct prognoses, there has been a paradigm shift in adjuvant therapy decisions, away from risk categories based on stage alone to the determination of tumor responsiveness.4 This molecular advance has been difficult to translate into current clinical practice because a number of highly significant but nonoverlapping gene signatures with prognostic effect have been proposed,5–9 complicating their application, and because the large clinical trials that inform current evidenced-based treatment decisions generally do not have available materials suitable for gene expression profile-based molecular subtyping.
Subsequent work has shown that although the genetic definitions may vary across classification schemes, most of these systems will classify tumors into categories with similar clinical implications.10,11 The primary molecular subdivision is by estrogen receptor (ER) status,3,8,12 with further subdivision of ER-positive tumors into luminal A and a worse-prognosis luminal B category. The ER-negative tumors are subdivided into HER2/neu (HER2)–positive and a basal-like group, the latter being approximated by a triple negative (ER-negative, progesterone receptor [PR]–negative, HER2-negative) phenotype.13 The most significant discriminator in the subset assignment of ER-positive tumors is proliferation.14–16 Recent examination of 357 invasive breast cancers subtyped by gene expression analysis has determined and validated an optimal cut point for a Ki67 proliferation index to distinguish luminal B from luminal A (Cheang et al, manuscript submitted for publication), suggesting that a simple immunopanel of ER, PR, HER2, and Ki67 can serve as a proxy for much of the clinically relevant information generated by genetic subtyping.
In this unplanned, subset analysis of the Breast Cancer International Research Group (BCIRG) 001 trial, immunohistochemically defined subsets of patients were examined to test the prognostic and predictive significance of these subsets in a clinical trial setting.
METHODS
Patients
BCIRG 001 was a multicenter, prospective, randomized, phase III trial comparing docetaxel, doxorubicin, and cyclophosphamide (TAC) with fluorouracil, doxorubicin, and cyclophosphamide (FAC) as adjuvant chemotherapy in 1,491 women with operable, node-positive breast cancer. A representative tumor sample in the form of formalin-fixed paraffin blocks and/or diagnostic histologic slides was available for 1,350 patients (91%). Other data, including baseline characteristics of the patients and clinical outcomes, were extracted from the BCIRG database used for the second interim analysis of survival (median, 55 months of follow-up), results of which have been previously reported.2 All patients provided informed consent. The trial protocol was approved by the research ethics board of the Alberta Cancer Board.
Immunohistochemical and Fluorescent In Situ Hybridization Analysis of Tissue Specimens
Slide review, immunohistochemistry, and fluorescence in situ hybridization (FISH) assays were performed in a central laboratory and read by one pathologist (J.H.). Automated slide processing platforms (Ventana Medical Systems, Tucson, AZ) were used for both immunohistochemical and FISH assays. Representative unstained tumor specimens were stained for ER (clone 6F11, Ventana), PR (clone 636; Dako, Carpinteria, CA), HER2 (clone CB11, Ventana), Ki67 (clone MIB-1, Dako), and p53 (clone 1801; Novocastra Laboratories Ltd, Newcastle upon Tyne, United Kingdom). Immunohistochemical data were recorded as the percentage of positive cells and grade 1 to 3 (greatest) staining intensity. The FISH assay for HER2 was performed according to the manufacturer's instructions (PathVysion; Abbott Molecular Inc, Des Plaines, IL). Tumors were classified as ER- or PR-positive if staining was present in 1% or more of tumor nuclei and p53 positive if more than 10% of nuclei stained. The Ki67 cut point of 13% established by Cheang et al (Cheang et al, manuscript submitted for publication) was used to designate a tumor as high proliferation. For HER2, either intense staining (3+) or a HER2:Cep17 ratio greater than 2.2 were regarded as positive for immunohistochemical analysis or FISH, respectively.17
Statistical Analysis
All data generated during central review were recorded in the BCIRG database. A random sample of 500 cases (hematoxylin-eosin and immunohistochemistry samples) were reviewed by Ian Ellis (Nottingham, United Kingdom). For histologic grade and ER status, there was complete agreement in 69% and 85% of cases, with κ values of 0.56 (moderate) and 0.87 (almost perfect), respectively. The level of agreement for histologic grade was consistent with that of published studies.18,19 In addition, the concordance rate for the FISH results was 97% (29 of 30) with an external laboratory (Michael Press, Los Angeles, CA) on nonstudy cases.
The occurrence of a DFS event, defined as relapse, second primary malignancy, or death, was the primary outcome and was analyzed for each prognostic variable. Univariate and multivariate analyses were performed using the SAS phreg procedure (SAS Institute, Cary, NC) for the Cox regression model for survival. The SAS lifetest procedure was used to calculate Kaplan-Meier probability estimates for DFS and OS. The SAS logistic procedure was used to obtain significance levels with odds ratios (ORs) and 95% CIs for 3-year DFS rates analysis and histologic tumor type, tumor size, extent of vascular invasion, tumor grade, proliferation index, and p53 by biologic subtype relative to the most common subtype in this study cohort, luminal B.
Univariate Cox regression analysis was performed for each prognostic variable. Subsequently, those variables that were not used in the computation of the biologic subtypes and yielded a univariate result of P < .10 were included in the multivariate model. Multivariate Cox regression analysis was performed using the stepwise backward elimination method with model removal set at P > .10. The multivariate model included as primary prognostic variables biologic subtype and chemoendocrine treatment (TAC, FAC, and tamoxifen), with interaction terms for tamoxifen treatment and biologic subtype. Tumor grade, primary tumor size, extent of vascular invasion, and number of positive lymph nodes were added as covariables.
The results are presented in accordance with reporting recommendations for tumor marker prognostic studies criteria.20
RESULTS
The frequency of the clinicopathologic variables in the total population are presented in Table 1. On univariate analysis, a significant prognostic effect was detected for tumor size; histologic subtype; tumor grade; extent of vascular invasion; nodal involvement; hormone receptor status; expression of HER2, Ki67, and p53; chemotherapy; and tamoxifen treatment, with increases in hazard ratios (HRs) for recurrence associated with increases in tumor size and number of positive nodes. No statistically significant prognostic effect was found for age or menopausal status.
Table 1.
Patient Characteristics for the Total Study Population and Biologic Subtypes With Univariate Analysis of Disease-Free Survival in the Total Population
| Characteristic | No. | Total HR | P | Triple Negative |
HER2 |
Luminal B |
Luminal A |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||||
| Age, years | |||||||||||
| < 50 | 711 | 1.00 | — | 111 | 58 | 53 | 47 | 450 | 56 | 84 | 40 |
| 50+ | 639 | 0.88 | .2449 | 81 | 42 | 60 | 53 | 360 | 44 | 127 | 60 |
| Menopausal status | |||||||||||
| Pre | 531 | 1.00 | — | 83 | 43 | 50 | 44 | 293 | 36 | 97 | 46 |
| Post | 819 | 0.90 | .3362 | 109 | 57 | 63 | 56 | 517 | 64 | 114 | 54 |
| Tumor size, cm | |||||||||||
| ≤ 2 | 576 | 1.00 | — | 67 | 35 | 45 | 40 | 347 | 43 | 107 | 51 |
| > 2 | 774 | 1.61 | < .0001 | 125 | 65 | 68 | 60 | 463 | 57 | 104 | 49 |
| Histologic subtype | |||||||||||
| Lobular | 286 | 1.00 | — | 6 | 3 | 3 | 3 | 183 | 23 | 90 | 43 |
| Ductal other | 1064 | 1.72 | .0003 | 186 | 97 | 110 | 97 | 627 | 77 | 121 | 57 |
| Overall histologic grade | |||||||||||
| 1 | 245 | 1.00 | — | 1 | 1 | 1 | 1 | 131 | 16 | 108 | 52 |
| 2 | 603 | 1.96 | .0007 | 26 | 14 | 37 | 33 | 436 | 54 | 96 | 46 |
| 3 | 489 | 3.54 | < .0001 | 163 | 86 | 75 | 66 | 236 | 29 | 4 | 2 |
| Vascular invasion | |||||||||||
| None/minimal | 938 | 1.00 | — | 132 | 70 | 71 | 68 | 557 | 72 | 164 | 85 |
| Extensive | 338 | 1.60 | < .0001 | 56 | 30 | 33 | 32 | 213 | 28 | 29 | 15 |
| Positive lymph nodes | |||||||||||
| 1-3 | 903 | 1.00 | — | 129 | 68 | 74 | 66 | 550 | 69 | 131 | 64 |
| 4-9 | 327 | 1.81 | < .0001 | 50 | 26 | 30 | 27 | 189 | 24 | 54 | 26 |
| 10+ | 103 | 2.79 | < .0001 | 12 | 6 | 8 | 7 | 62 | 8 | 20 | 10 |
| Estrogen receptor | |||||||||||
| Negative | 318 | 1.00 | — | 192 | 100 | 113 | 100 | 3 | 0 | 3 | 1 |
| Positive | 1021 | 0.52 | < .0001 | 0 | 0 | 0 | 0 | 807 | 100 | 208 | 99 |
| Progesterone receptor | |||||||||||
| Negative | 498 | 1.00 | — | 192 | 100 | 113 | 100 | 156 | 19 | 29 | 14 |
| Positive | 841 | 0.50 | < .0001 | 0 | 0 | 0 | 0 | 654 | 81 | 182 | 86 |
| HER2* | |||||||||||
| Negative | 1034 | 1.00 | — | 192 | 100 | 0 | 0 | 626 | 78 | 211 | 100 |
| Positive | 288 | 1.56 | .0002 | 0 | 0 | 113 | 100 | 175 | 22 | 0 | 0 |
| Ki67-1 index | |||||||||||
| Low | 242 | 1.00 | — | 5 | 3 | 5 | 4 | 14 | 2 | 211 | 100 |
| High | 1099 | 1.85 | .0002 | 187 | 97 | 108 | 96 | 796 | 98 | 0 | 0 |
| p53 staining | |||||||||||
| Negative | 1014 | 1.00 | — | 96 | 50 | 56 | 50 | 650 | 81 | 200 | 95 |
| Positive | 320 | 1.52 | .0003 | 96 | 50 | 56 | 50 | 155 | 19 | 11 | 5 |
| Treatment | |||||||||||
| FAC | 663 | 1.00 | — | 90 | 48 | 57 | 50 | 397 | 49 | 107 | 51 |
| TAC | 679 | 0.73 | .0027 | 99 | 52 | 56 | 50 | 408 | 51 | 104 | 49 |
| Tamoxifen | |||||||||||
| No | 442 | 1.00 | — | 167 | 87 | 94 | 83 | 139 | 17 | 30 | 14 |
| Yes | 908 | 0.43 | < .0001 | 25 | 13 | 19 | 17 | 671 | 83 | 181 | 86 |
Abbreviations: HR, hazard ratio; FAC, fluorouracil, doxorubicin, cyclophosphamide; TAC, docetaxel, doxorubicin, cyclophosphamide.
HER2 status was determined using fluorescent in situ hybridization (1,264 patients) or CB11 (53 patients for whom fluorescent in situ hybridization results were not available).
We were able to classify the study population into four breast cancer subtypes (Table 1): triple negative (ER-negative, PR-negative, HER2-negative), HER2 (ER-negative, PR-negative, HER2-positive), luminal B (ER-positive and/or PR-positive and either HER2-positive and/or Ki67high), and luminal A (ER-positive and/or PR-positive and not HER2-positive or Ki67high). These subtypes accounted for 14.5%, 8.5%, 61.1%, and 15.9%, respectively. Twenty-four (1.8%) were unassigned, primarily because of missing HER2 values owing to insufficient tumor material or nonformalin fixation.
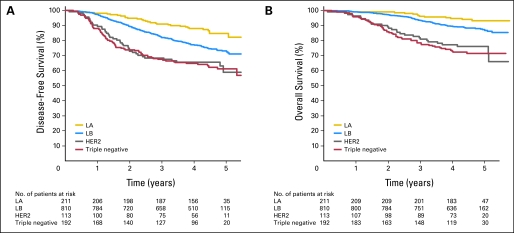
The biologic subtypes were prognostically significant for DFS and OS (Fig 1). The 3-year DFS rates, HR, and P values for pairwise comparison with luminal B were 67% (HR = 2.22; 95% CI, 1.56 to 3.16; P < .0001) for triple negative, 68% (HR = 2.12; 95% CI, 1.37 to 3.29; P = .0008) for HER2, 82% (referent) for luminal B, and 91% (HR = 0.46; 95% CI, 0.28 to 0.77; P = .0027) for luminal A. The triple-negative and HER2 groups had a similarly poor prognosis (P = .788), as has been described.14
Fig 1.
Disease-free and overall survival among patients according to biologic subtype. (A) Disease-free survival and (B) overall survival for patients stratified into triple-negative (estrogen receptor [ER] negative, progesterone receptor [PR] negative, HER2/neu [HER2] negative), HER2 (HER2-positive, ER-negative, PR-negative), luminal B (LB; ER-positive or PR-positive and either HER2-positive or Ki67high) and luminal A (LA; ER-positive or PR-positive and not HER2-positive or Ki67high) groups. Pairwise comparisons between subtypes were statistically significant (see text).
A multivariate analysis, which excluded variables defining the subtypes (ER, PR, HER2, Ki67), identified independent significance for treatment with TAC/FAC, treatment with tamoxifen, size more than 2 cm, grade 3, extensive vascular invasion, number of involved lymph nodes, treatment, and biologic subtypes (Table 2).
Table 2.
Stepwise Multivariate Analysis of Disease-Free Survival for All Cases and for Luminal A and Luminal B
| Characteristic | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| All cases | |||
| TAC treatment | 0.73 | 0.58 to 0.91 | .0051 |
| Tamoxifen treatment | 0.41 | 0.31 to 0.56 | < .0001 |
| Tamoxifen* triple negative | 4.01 | 2.00 to 8.05 | < .0001 |
| Tamoxifen* HER2 | 2.56 | 0.95 to 6.93 | .0633 |
| Tamoxifen* luminal A | 0.56 | 0.34 to 0.93 | .0257 |
| Triple negative | 0.62 | 0.43 to 0.90 | .0124 |
| HER2 | 0.65 | 0.42 to 1.02 | .0602 |
| Tumor size > 2 cm | 1.33 | 1.05 to 1.69 | .0178 |
| Histologic subtype | 1.39 | 0.99 to 1.96 | .0612 |
| Grade 3 | 1.39 | 1.07 to 1.80 | .0137 |
| Vascular invasion | 1.34 | 1.06 to 1.69 | .0141 |
| 4-9 positive nodes | 1.87 | 1.46 to 2.40 | < .0001 |
| 10+ positive nodes | 3.09 | 2.23 to 4.27 | < .0001 |
| Luminal B | |||
| TAC treatment | 0.71 | 0.53 to 0.95 | .022 |
| Tamoxifen treatment | 0.44 | 0.32 to 0.61 | < .0001 |
| Tumor size > 2 cm | 1.49 | 1.10 to 2.02 | .011 |
| Histologic subtype | 1.62 | 1.06 to 2.49 | .027 |
| 1-3 positive nodes | 1.98 | 1.46 to 2.67 | < .0001 |
| 4-9 positive nodes | 1.61 | 1.15 to 2.26 | .005 |
| 10+ positive nodes | 4.33 | 2.93 to 6.41 | < .0001 |
| Luminal A | |||
| TAC treatment | 0.97 | 0.46 to 2.06 | .943 |
| Tamoxifen treatment | 0.15 | 0.07 to 0.33 | < .0001 |
| 4-9 positive nodes | 4.30 | 1.81 to 10.20 | .001 |
| 10+ positive nodes | 5.11 | 1.82 to 14.37 | .002 |
NOTE. Variables involved in the definition of biologic subtypes were not included in this model.
Abbreviation: TAC, docetaxel, doxorubicin, cyclophosphamide.
ORs for the association of biologic subtype with prognostic tumor characteristics are shown in Table 3, with the most common subtype, luminal B, as the referent group. Compared with luminal B, the triple-negative and HER2 subtypes were more likely to have a ductal histogenesis (OR = 9.2; 95% CI, 4.00 to 21.04; P < .0001; and OR = 10.6, 95% CI, 3.33 to 33.85; P < .0001, respectively), whereas luminal A cases were more likely to have nonductal disease (eg, lobular or mixed ductal-lobular histogenesis, OR = 0.4; 95% CI, 0.27 to 0.52; P < .0001). Patients with the luminal A subtype were less likely to have tumors larger than 2 cm (OR = 0.7; 95% CI, 0.54 to 0.99; P < .05), extensive vascular invasion (OR = 0.5; 95% CI, 0.30 to 0.71; P = .0004), grade 3 (OR = 0.05; 95% CI, 0.02 to 0.13; P < .0001), or p53 positivity (OR = 0.2; 95% CI, 0.12 to 0.43; P < .0001). p53 positivity was more frequent in the triple-negative (OR = 4.19; 95% CI, 3.01 to 5.85; P < .0001) and HER2 (OR = 4.19; 95% CI, 2.78 to 6.32; P < .0001) groups, compared with luminal B. Patients with triple-negative tumors had the greatest likelihood of having grade 3 tumors (OR = 14.5; 95% CI, 9.39 to 22.40; P < .0001).
Table 3.
Odds Ratios for Patient and Tumor Characteristics by Biologic Subtype
| Variable Category | Triple Negative |
HER2 |
Luminal B (referent) | Luminal A |
|||
|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | ||
| N1 v N2 | |||||||
| Type, ductal v lobular* | 9.17 | < .0001 | 10.61 | < .0001 | 1.0 | 0.38 | < .0001 |
| Size, > 2 v ≤ 2 cm | 1.40 | .0450 | 1.13 | .5436 | 1.0 | 0.73 | .0408 |
| Grade, 1 v 2 | 0.13 | .0447 | 0.09 | .018 | 1.0 | 3.74 | < .0001 |
| Grade, 3 v 1 + 2 | 14.50 | < .0001 | 4.74 | < .0001 | 1.0 | 0.05 | < .0001 |
| Vascular invasion, positive v negative | 1.11 | .5612 | 1.22 | .3871 | 1.0 | 0.46 | .0004 |
| MIB-1 index, high v low | 1.52 | .4270 | 2.63 | .0683 | 1.0 | NA | |
| p53, positive v negative | 4.19 | < .0001 | 4.19 | < .0001 | 1.0 | 0.23 | < .0001 |
| PR, positive v negative | NA | NA | 1.0 | 1.50 | .0655 | ||
Abbreviations: OR, odds ratio; NA, not applicable; PR, progesterone receptor.
Includes lobular mixed and pure lobular carcinomas.
Compared with patients in the luminal B group, the hazard ratio for a DFS event in patients in the luminal A group was significantly decreased for tumor size more than 2 cm (HR = 0.47; 95% CI, 0.28 to 0.79; P = .0042), moderate (HR = 0.46; 95% CI, 0.23 to 0.91; P = .027) and high (HR = 0.54; 95% CI, 0.33 to 0.87; P = .012) levels of ER expression, as well as in the subgroup with one to three positive nodes (HR = 0.37; 95% CI, 0.19 to 0.70; P = .002).
Nineteen percent and 14% of patients in the luminal B and luminal A groups, respectively, were negative for PR. A multivariate analysis controlling for the presence of PR showed a persistent significant difference between luminal A and luminal B (HR = 0.46; 95% CI, 0.29 to 0.74; P = .0014), excluding differences in PR as the basis for differences between the subtypes in patients treated with tamoxifen.
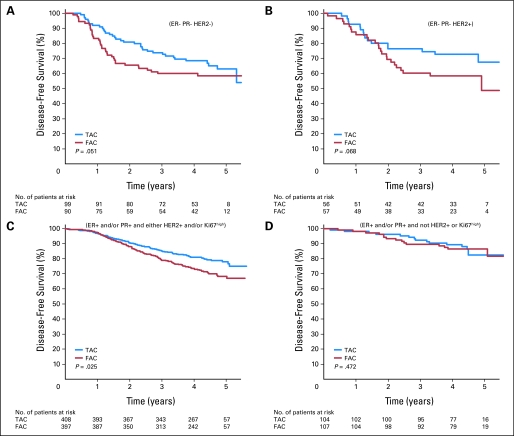
Patients in the luminal B group showed a statistically significant improvement in DFS favoring the taxane arm, with 3-year DFS of 85.2% versus 79% (HR = 0.66; 95% CI, 0.46 to 0.95; P = .025) for TAC and FAC, respectively (Fig 2). There was marginal significance in the triple-negative and HER2 subtypes, with 3-year DFS of 73.5% and 60.0% (HR = 0.50; 95% CI, 0.29 to 1.00; P = .051) and 76.4% and 60.3% (HR = 0.46; 95% CI, 0.20 to 1.06; P = .068) for TAC and FAC in the respective subtypes. There was no difference between chemotherapy regimens in patients in the luminal A group: 92.2% and 89.3%, respectively (HR = 0.70; 95% CI, 0.27 to 1.83; P = .472).
Fig 2.
Disease-free survival (DFS) among patients treated with fluorouracil, doxorubicin, and cyclophosphamide (FAC) or docetaxel, doxorubicin, and cyclophosphamide (TAC) according to biologic subtype. Disease-free survival is shown in patients classified as (A) triple negative, (B) HER2, (C) luminal B, or (D) luminal A treated with FAC or TAC. P values in each panel are logistic regression calculations based on 3-year DFS. ER, estrogen receptor; PR, progesterone receptor.
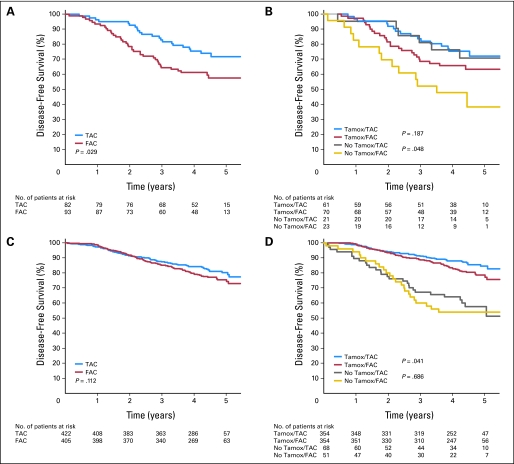
Although the trial protocol specified adjuvant tamoxifen after chemotherapy for all ER-positive patients, 5% did not receive tamoxifen for a variety of reasons and an additional 11% of patients had a negative agreement between the central laboratory and the originating laboratory, such that 17% of patients in the luminal B and 14% of patients in the luminal A groups were not treated with tamoxifen. This magnitude of negative agreement is similar to that reported in the Eastern Cooperative Oncology 2197 trial21 and is a well-described phenomenon in interlaboratory testing.22,23 When tamoxifen treatment was entered into the multivariate Cox and DFS model, significant interactions were found, with biologic subtype indicating that subgroup analysis was appropriate. There were few patients treated with tamoxifen in the triple-negative (13%) and HER2 (17%) subtypes, and there was no significant effect of tamoxifen treatment in these groups (P > .3), so the interaction in these groups is not considered further. The multivariate analysis for luminal A and luminal B subtypes is presented in Table 2. Both the luminal A and luminal B subtypes showed significant responses to tamoxifen, with HR = 0.44 (95% CI, 0.32 to 0.61; P < .0001) for luminal B and HR = 0.15 (95% CI, 0.07 to 0.33; P < .0001) for luminal A. As in the unadjusted comparisons, when patients were treated with tamoxifen, TAC was superior to FAC in patients in the luminal B group, with a 3-year DFS of 89.4% and 82%, respectively (HR = 0.71; 95% CI, 0.53 to 0.95; P = .02, multivariate model) and not in patients in the luminal A group, with a 3-year DFS of 93% and 96.6% (HR = 0.97; 95% CI, 0.46 to 2.06; P = .90, multivariate model; Fig 3).
Fig 3.
Forest plot of covariate adjusted hazard ratios (HR) for disease-free survival in patients in the luminal A and luminal B groups by treatment (hazard ratios and 95% CIs using fluorouracil, doxorubicin, and cyclophosphamide and no tamoxifen as baseline treatments). Values less than 1 show an advantage to docetaxel, doxorubicin, and cyclophosphamide (TAC) or tamoxifen.
We performed an exploratory analysis similar to a recently published24 examination of the response of ER-positive/HER2-positive and ER-positive/HER2-negative patients to adjuvant taxane chemotherapy. As in that study, log-rank P values are provided as a measure of discordance and should be viewed as descriptive, not inferential. We found that TAC was associated with improved DFS among the ER-positive/HER2-positive patients (Fig 4A), with similar trends seen in both the tamoxifen-treated and untreated patients (Fig 4B). Contrary to that study, in the ER-positive/HER2-negative patients who had received tamoxifen, there was a benefit to the taxane-containing chemotherapy arm (Fig 4D). This advantage was not seen in those patients who did not receive tamoxifen (Fig 4D), nor in the unsegregated population (Fig 4C).
Fig 4.
Disease-free survival among estrogen receptor positive patients treated with fluorouracil, doxorubicin, and cyclophosphamide (FAC) or docetaxel, doxorubicin, and cyclophosphamide (TAC) according to HER2 status and tamoxifen (Tamox) treatment. Disease-free survival for estrogen receptor (central lab)–positive patients, (A) positive or (C) negative for HER2, by treatment arm. (B, D) Subdivision of these patients by tamoxifen treatment. Log-rank P values are descriptive only.
DISCUSSION
It is now accepted that breast cancer is heterogeneous at a molecular level.4 There is increasing evidence that these molecular subtypes differ in their response to therapeutic agents.14,16,25 Our results build on these findings and indicate that patients with ER-negative tumors (both triple-negative and HER2) show a better response to TAC than to FAC, with the difference in response to the taxane regimen being marginally statistically significant. Despite this response, these subtypes have a worse DFS and OS, with the majority of events occurring early. This is consistent with neoadjuvant reports that document increased chemosensitivity in these subtypes, but poor outcome as a result of higher and faster relapse among those with residual disease.16,25 It is expected that if trastuzumab had been included in this early protocol, the 3-year recurrence risk would have been halved in the HER2 population.26
The triple-negative phenotype used in this study only approximates the basal-like subtype,27 although the profile of the DFS14,27 and phenotypic characteristics (eg, predominantly ductal [97%], grade 3 [86%], p53 abnormal [50%] with a high proliferation index [97%]) are in keeping with characteristics of the basal subtype.28,29 The basal-like subtype itself is heterogeneous and encompasses the majority of BRCA1-related carcinomas, medullary carcinomas, and metaplastic carcinomas13 and is overrepresented in the aggressive tumors seen in premenopausal African Americans.30,31 Although this heterogeneity can theoretically alter chemosensitivity,31 we were not able to further define subgroups within this subtype to investigate that possibility.
The use of a simple proliferation index results in a highly effective separation of ER-positive patients into two intrinsically different populations, luminal A and luminal B (Cheang et al, manuscript submitted for publication).11,14–16,32 These subtypes have a different outcome when compared across tumor size, nodal status, and level of estrogen or progesterone receptor positivity. This underscores the importance of proliferation and supports suggestions that incorporation of a proliferation score into therapy decisions may complement11 or even supplant16,33 histologic grade. Assignment of biologic subtype without consideration of histologic type resulted in significant separation, with the triple-negative and HER2 groups being almost exclusively of ductal origin. Approximately one half of the luminal A subtype consisted of lobular and mixed ductal-lobular carcinomas, consistent with evidence supporting a close genetic relationship between low-grade ductal carcinoma and lobular carcinomas.34,35
In patients treated with tamoxifen, patients in the luminal B but not luminal A group show a significant benefit of TAC over FAC chemotherapy. The benefit of taxanes in hormone receptor–positive tumors is controversial.36 Analysis of first-generation taxane trials in adjuvant therapy including the Grupo Espanol de Investigacion del Cancer de Mama 990637 and a pooled analysis of this present trial and PACS0138 found no statistically significant interaction between hormone receptor status and taxane response, with benefit in both the ER-positive and ER-negative patients. However, subset analyses of two other taxane trials, the E219739 and the Cancer and Leukemia Group B (CALGB),24,40 as well as neoadjuvant data,16,41 suggest that the response to taxanes is less in ER-positive compared with ER-negative tumors. Our results show that the benefit of the addition of taxanes to tamoxifen is restricted to the luminal B population, and because this group comprises the majority of ER-positive patients in this study, this benefit is probably responsible for the positive effect of TAC in the tamoxifen-treated ER-positive/HER2-negative (Fig 4D) and in the ungrouped ER-positive patients.38 The patients in the luminal A group show no benefit to docetaxel when added to tamoxifen (Fig 3), a finding that is not surprising given that taxanes' stabilization of microtubules and mitotic arrest42 would be expected to preferentially target rapidly dividing cells. Of note, subset analysis of the PACS 01 trial, presented in abstract form, also found that a “luminal” subtype, defined as ER-positive and basal-like subtype parameters–negative, showed no difference in response to the addition of docetaxel.43
These findings parallel the experience in node-negative patients. Currently, large cooperative trials in North America (Trial Assigning Individualized Options for Treatment)44 and Europe (Microarray in Node-Negative Disease May Avoid Chemotherapy45 and Node Negative Breast Cancer46) are studying the integration of genomic or biochemical profiles into decisions concerning the necessity of adding chemotherapy to hormonal therapy in selected groups of patients with hormone receptor–positive disease.
In the ER-positive/HER2-negative patients treated with tamoxifen, there was a significant benefit to the taxane regimen (Fig 4D). This differs from the analysis of a similar subset in the CALGB 9344/INTO148 trial of patients with node-positive breast cancer treated with doxorubicin plus cyclophosphamide, followed by paclitaxel or observation.24 In that report, there was no benefit to the addition of paclitaxel in a similar group of patients. Cross-trial comparisons are difficult, but possible reasons for this difference include superior performance of docetaxel to the paclitaxel dosing protocol used in that trial,47,48 or alternatively, there may have been a greater admixture of patients with low-proliferative luminal A disease in the CALGB subset, thus diluting the effect of the taxane in the unsegregated ER-positive population.
The high risk of recurrence in ER-positive tumors not treated with tamoxifen is similar to that reported in the subset analysis by Berry et al40 and is in keeping with the meta-analysis results1 showing that the major benefit in adjuvant therapy in the ER-positive tumors is due to tamoxifen. This suggests that the prognostic effect seen with molecular subdivision is due in large part to its ability to predict response to treatments optimized to each subtype, rather than differing metastatic potential between the subtypes. In this regard, our results show that TAC significantly complements endocrine therapy in tamoxifen-treated patients in the luminal B group, analogous to the improved outcomes in HER2-positive patients treated with doxorubicin and cyclophosphamide followed by docetaxel with trastuzumab. In the absence of specific targeted therapy, TAC is also effective in the triple-negative population. Because this is a retrospective, unplanned subset analysis without adjustment for multiple comparisons, these findings, although consistent with existing literature, should be regarded as hypothesis generating.
Acknowledgment
We thank the patients and pathologists who shared their material and made this study possible; and Todd Chaba, MD, and Blake Gilks, MD, for their support and guidance.
Footnotes
Supported by Grants No. P50-CA58223 and U01 CA114722-01 (to C.M.P.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Emmanuelle Magherini, Sanofi-aventis (C) Consultant or Advisory Role: Judith Hugh, Sanofi-Aventis (U); John Mackey, Sanofi-Aventis (C); Miguel Martin, Sanofi-Aventis (C) Stock Ownership: Charles M. Perou, University Genomics; Emmanuelle Magherini, Sanofi-Aventis Honoraria: John Mackey, Sanofi-Aventis; Miguel Martin, Sanofi-Aventis; Charles Vogel, Sanofi-Aventis Research Funding: Judith Hugh, Aventis; Torsten O. Nielsen, Sanofi-Aventis; Charles Dumontet, aventis; John Reed, Aventis; Charles Vogel, Sanofi-Aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Judith Hugh, Maggie Chon U. Cheang, Torsten O. Nielsen, Charles M. Perou, Charles Dumontet, Miguel Martin, Charles Vogel
Administrative support: Judith Hugh, John Hanson, John Mackey
Provision of study materials or patients: Matthieu Rupin, John Mackey, Miguel Martin, Charles Vogel
Collection and assembly of data: Judith Hugh, Charles Dumontet, John Reed, Maryla Krajewska, Isabelle Treilleux, Matthieu Rupin, Emmanuelle Magherini
Data analysis and interpretation: Judith Hugh, John Hanson, Torsten O. Nielsen, Charles M. Perou, Charles Dumontet, John Reed, Maryla Krajewska, Matthieu Rupin, Emmanuelle Magherini, John Mackey, Miguel Martin, Charles Vogel
Manuscript writing: Judith Hugh, John Hanson, Torsten O. Nielsen, Charles M. Perou, Charles Dumontet, John Reed, Matthieu Rupin, Emmanuelle Magherini, John Mackey, Miguel Martin, Charles Vogel
Final approval of manuscript: Judith Hugh, John Hanson, Maggie Chon U. Cheang, Torsten O. Nielsen, Charles M. Perou, Charles Dumontet, John Reed, Maryla Krajewska, Isabelle Treilleux, Matthieu Rupin, Emmanuelle Magherini, John Mackey, Miguel Martin, Charles Vogel
REFERENCES
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Dinh P, Sotiriou C, Piccart MJ. The evolution of treatment strategies: Aiming at the target. Breast. 2007;16(suppl 2):S10–S16. doi: 10.1016/j.breast.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Nuyten DS, Sneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 10.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 11.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 12.Gruvberger S, Ringner M, Chen Y, et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61:5979–5984. [PubMed] [Google Scholar]
- 13.Kreike B, van Kouwenhove M, Horlings H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 15.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med. 2007;131:18. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 18.Dalton LW, Page DL, Dupont WD. Histologic grading of breast carcinoma: A reproducibility study. Cancer. 1994;73:2765–2770. doi: 10.1002/1097-0142(19940601)73:11<2765::aid-cncr2820731119>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Frierson HF, Jr, Wolber RA, Berean KW, et al. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol. 1995;103:195–198. doi: 10.1093/ajcp/103.2.195. [DOI] [PubMed] [Google Scholar]
- 20.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 21.Badve SS, Baehner FL, Gray RP, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 22.Francis GD, Dimech M, Giles L, et al. Frequency and reliability of oestrogen receptor, progesterone receptor and HER2 in breast carcinoma determined by immunohistochemistry in Australasia: Results of the RCPA Quality Assurance Program. J Clin Pathol. 2007;60:1277–1283. doi: 10.1136/jcp.2006.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes A, Jasani B, Barnes DM, et al. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: Interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 25.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, Perez EA, Pienkowski T, et al. Adjuvant trastuzumab: A milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11(suppl 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 27.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 28.Jones C, Ford E, Gillett C, et al. Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res. 2004;10:5988–5997. doi: 10.1158/1078-0432.CCR-03-0731. [DOI] [PubMed] [Google Scholar]
- 29.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 30.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 31.Di Leo A, Claudino WM, Pestrin M, et al. Using specific cytotoxics with a targeted mind. Breast. 2007;16(suppl 2):S120–S126. doi: 10.1016/j.breast.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Haibe-Kains B, Desmedt C, Sotiriou C, et al. A comparative study of survival models for breast cancer prognostication based on microarray data: Does a single gene beat them all? Bioinformatics. 2008;24:2200–2208. doi: 10.1093/bioinformatics/btn374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer JS, Alvarez C, Milikowski C, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: Reproducibility of grade and advantages of proliferation index. Mod Pathol. 2005;18:1067–1078. doi: 10.1038/modpathol.3800388. [DOI] [PubMed] [Google Scholar]
- 34.Simpson PT, Reis-Filho JS, Gale T, et al. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 35.Buerger H, Simon R, Schafer KL, et al. Genetic relation of lobular carcinoma in situ, ductal carcinoma in situ, and associated invasive carcinoma of the breast. Mol Pathol. 2000;53:118–121. doi: 10.1136/mp.53.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin M, Mackey J, Vogel C. Benefit from adjuvant taxanes and endocrine responsiveness in breast cancer. Breast. 2007;16(suppl 2):S127–S131. doi: 10.1016/j.breast.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Martín M, Rodriguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 38.Andre F, Broglio K, Roche H, et al. Estrogen receptor expression and efficacy of docetaxel-containing adjuvant chemotherapy in patients with node-positive breast cancer: Results from a pooled analysis. J Clin Oncol. 2008;26:2636–2643. doi: 10.1200/JCO.2007.14.9146. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein LJ, O'Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J Clin Oncol. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Minckwitz G, Sinn HP, Raab G, et al. Clinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breast. Breast Cancer Res. 2008;10:R30. doi: 10.1186/bcr1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller ML, Ojima I. Chemistry and chemical biology of taxane anticancer agents. Chem Rec. 2001;1:195–211. doi: 10.1002/tcr.1008. [DOI] [PubMed] [Google Scholar]
- 43.Jacquemier J, Penault-Llorca F, Mnif H, et al. Identification of a basal-like subtype and comparative effect of epirubicin-based chemotherapy and sequential epirubicin followed by docetaxel chemotherapy in the PACS 01 breast cancer trial: 33 markers studied on tissue-microarrays (TMA) J Clin Oncol. 2006;24(suppl):5s. abstr 509. [Google Scholar]
- 44.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 45.Cardoso F, Van't Veer L, Rutgers E, et al. Clinical application of the 70-gene profile: The MINDACT trial. J Clin Oncol. 2008;26:729–735. doi: 10.1200/JCO.2007.14.3222. [DOI] [PubMed] [Google Scholar]
- 46.Thomssen C, Vetter M, Guerts A, et al. Is tumor-biological risk-assessment realistic in node-negative breast cancer? A status report of the ongoing clinical multicenter trial NNBC 3-Europe: SABCS 2006. Breast Cancer Res Treat. 2006;100(suppl 1):S114. abstr 2100. [Google Scholar]
- 47.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 48.Hudis C, McArthur H, Dang C. Current status of the taxanes as adjuvant therapy for breast cancer. Breast. 2007;16(suppl 2):S132–S135. doi: 10.1016/j.breast.2007.07.022. [DOI] [PubMed] [Google Scholar]