Abstract
Purpose
Cigarette smoking induces CYP1A1/1A2 and is hypothesized to alter erlotinib pharmacokinetics. This study aimed to determine the maximum tolerated dose (MTD) of erlotinib in advanced non–small-cell lung cancer (NSCLC) patients who smoke and compare the pharmacokinetics of erlotinib at the MTD in current smokers with 150 mg.
Patients and Methods
Cohorts of NSCLC patients currently smoking ≥ 10 cigarettes per day for ≥ 1 year received escalating doses of erlotinib for 14 days until dose-limiting toxicity (DLT). A separate cohort of patients was then randomly assigned to erlotinib at either MTD or 150 mg daily with pharmacokinetics assessed at day 14. Erlotinib was continued until progression or intolerable toxicity.
Results
Four dose levels were evaluated in 22 patients: 200, 250, 300, and 350 mg. DLT was observed in one of six patients at 300 mg (rash) and two of five patients at 350 mg (acneiform dermatitis and fatigue/decreased Eastern Cooperative Oncology Group performance status). Thirty-five patients were randomly assigned to 150 mg or 300 mg. Common adverse events (all grades) were: skin toxicity (150 mg, 29%; 300 mg, 67%), diarrhea (150 mg, 18%; 300 mg, 50%), and fatigue (150 mg, 12%; 300 mg, 17%). Erlotinib exposure was dose-proportional within dose range tested. Median steady-state trough erlotinib plasma concentrations were 0.375 and 1.22 μg/mL for 150 mg and 300 mg, respectively.
Conclusion
The MTD of erlotinib in NSCLC patients who smoke was 300 mg. Steady-state trough plasma concentrations and incidence of rash and diarrhea in smokers at 300 mg were similar to those in former or never smokers receiving 150 mg in previous studies. The potential benefit of higher erlotinib doses in current smokers warrants further evaluation.
INTRODUCTION
Erlotinib (Tarceva, OSI Pharmaceuticals Inc, Melville, NY) is an oral epidermal growth factor receptor (EGFR) inhibitor demonstrating statistically significant and clinically meaningful survival benefit, as well as delayed time to deterioration of lung cancer symptoms, in patients with locally advanced or metastatic NSCLC after failure of prior chemotherapy.1–3 While all smoker subgroups benefited from erlotinib therapy compared with placebo, the magnitude of benefit varied with smoking status. Median survival (and hazard ratio [HR]) in the erlotinib arm were: 12.3 months (HR, 0.42) in never-, 5.5 months (HR, 0.84) in former-, and 6.1 months (HR, 0.93) in current smokers, with P = .006 for the interaction between smoking history and treatment benefit.2,4,5 Multiple explanations have been proposed for these observations. The natural history of lung cancer in never-smokers differs from smokers such that never smokers have better outcomes.6,7 Differences in prognostic factors (eg, more females or adenocarcinomas) among never smokers may contribute to this outcome. However, in multivariate analyses, a strong effect from smoking history persisted.8 The observation that former and never smokers experienced more adverse events (eg, rash and diarrhea) than current smokers suggests that variation in erlotinib exposure may also play a role.4,10 Current smokers were found to have as much as a two-fold decrease in erlotinib trough plasma concentrations compared to former or never smokers (mean C24 values of 0.748, 1.26, and 1.45 μg/mL, respectively).4,10
A single-dose study in healthy subjects confirmed that AUC0-inf and C24 were significantly decreased in smokers when compared with nonsmokers suggesting that the reduction could be overcome by doubling the dose from 150 to 300 mg.9,10 Given the known contribution of CYP1A1 and CYP1A2 to erlotinib metabolism, this was consistent with the hypothesis that differences in drug exposure, resulting toxicities and outcome may be due, in part, to induction of CYP enzymes by cigarette smoking.11
This study was performed to determine the MTD of erlotinib in patients with advanced NSCLC who currently smoke cigarettes (part I) and, to compare steady-state pharmacokinetics of erlotinib at the MTD and 150 mg in this patient population (part II).
PATIENTS AND METHODS
Study Design and Treatment Schedule
This was a multicenter, open-label, randomized, study of escalating doses of erlotinib in patients with advanced NSCLC who currently smoke cigarettes. Part I was a 3 + 3 patient dose-escalation study to determine the MTD. Successive cohorts of patients received erlotinib at 200, 250, 300, or 350 mg per day orally for 14 days, and observed for dose-limiting toxicities (DLT) that would necessitate expansion of the cohort up to six patients. Since hematologic toxicities were not expected with single-agent erlotinib, a DLT was defined as any ≥ grade 3 erlotinib-related, nonhematologic toxicity (excluding alopecia or unpremedicated or inadequately treated nausea, vomiting, or diarrhea) occurring within the first 14 days of treatment (considered sufficient time for appearance of common toxicities and ensuring erlotinib had reached steady-state concentrations.). The MTD was defined as the highest dose level at which fewer than two of six patients experienced a DLT. In part II, patients were randomly assigned to receive erlotinib at either the MTD determined in part I or 150 mg to compare steady-state pharmacokinetics.
On completion of 14 days of dosing, patients entered an extended treatment phase, continuing to receive erlotinib until disease progression, intolerable toxicity, request to discontinue therapy, or death. The extended phase erlotinib dose was at investigator's discretion based on tolerability during the initial 14 days. The dose was reduced by 50 mg per day for toxicity higher than National Cancer Institute Common Toxicity Criteria Adverse Event (NCI CTCAE) grade 2 and in cases where patients ceased smoking. In addition, erlotinib dosing was to be interrupted until resolution of toxicity to CTCAE grade 1 or lower. Supportive care for management of rash and/or diarrhea was permitted. Erlotinib 100 and 150 mg tablets were supplied by OSI Pharmaceuticals Inc (Melville NY). This protocol was approved by the appropriate ethical and regulatory bodies before initiation and performed in accordance with Good Clinical Practice (GCP). All patients provided written informed consent before enrollment.
Patients
Eligible patients were current cigarette smokers (ie, smoking ≥ 10 cigarettes per day for ≥ 1 year despite advice and support to quit) with stage IIIB/IV NSCLC after failure of 1 or 2 prior chemotherapy regimens, ECOG PS of 0/1, adequate organ function (bilirubin ≤ 1.5× upper normal limit [ULN], ALT ≤ 2.5× ULN (≤ 5× if liver metastases) and serum creatinine ≤ 1.5× ULN), no prior EGFR inhibitors, no CYP3A4 or other CYP1A2 inducers and/or inhibitors, concurrently or 14 days before study, and no concurrent anticancer therapy.
Study Procedures
Safety was evaluated on day 14 in all patients (an additional assessment was performed on day 7 in part I for identification of early toxicity after dose escalation). Hematology (hemoglobin, WBC, neutrophils, and platelets) and biochemistry (bilirubin, ALT, and creatinine) were assessed at baseline, day 1, and day 14. Toxicity was graded using NCI CTCAE version 3.0. Safety data were summarized separately for the initial 14-day treatment period and extended treatment phase. During the latter, only grade 3 or 4 erlotinib-related adverse events were documented (with serious adverse events and events resulting in study discontinuation, regardless of causality). Smoking status was determined by the COT One Step Cotinine Test (QuitSmoking.com, Cumming, GA); an immunoassay detecting urinary cotinine at ≥ 200 ng/mL; at baseline, day 1, and day 14. Long-term follow-up was collected for patients in part II to obtain estimates of survival.
Pharmacokinetics
Pharmacokinetic samples were collected during part II on day 14 before erlotinib dosing, and 1, 2, 4, 6, 8, and 24 hours after dosing. Erlotinib and its active metabolite OSI-420 were quantified using a validated liquid chromatography/tandem mass spectroscopy assay and plasma pharmacokinetic parameters calculated by noncompartmental methods using WInNonlin (Scientific Consultant, Apex, NC), versio 5.2 (Pharsight Corporation, Mountain View, CA).10 AAG concentrations were determined by a validated turbidimetric method using samples from day 14.12
Statistical Methods
The pharmacokinetic end point required samples to be collected from 44 patients (22 at the MTD for current smokers and 22 patients receiving erlotinib at 150 mg). These sample sizes were to provide sufficient data to characterize the geometric mean ratio of pharmacokinetic parameters for patients at the two doses with 90% CIs no greater than ± 50% and possibly as low as ± 30%. However, an analysis performed after 20 patients had been treated in part II, indicated that the observed variability was substantially less than that estimated to calculate the original sample size, suggesting the objective of part II could be achieved with as few as 10 patients per arm. Accrual was closed when a total of 35 patients had been randomly assigned.
Survival was defined as the time from first study drug administration until death and calculated based on the investigator-selected dose on day 15 (150 mg or escalated [250 or 300 mg]). Patients alive at the time of analysis were censored at the last day known to be alive.
RESULTS
Part I: Dose Escalation Study
Between January and October 2006, 22 patients entered the dose escalation phase. Patient characteristics are listed in Table 1. Five patients were unassessable for DLT assessment; one patient took the incorrect dose and four patients had dose interruptions during the initial 14 days. Three were due to unrelated adverse events (chest infection [two patients] and unrelated hyperkalemia [one patient]). A fourth patient interrupted erlotinib dosing because of grade 1 or 2 dehydration, nausea, vomiting, and diarrhea. While these events resolved, the patient developed unrelated pneumonia which prevented resumption of dosing. None of these events constituted DLTs.
Table 1.
Patient Characteristics: Dose Escalation
| Characteristic | Erlotinib Dose (mg per day) |
||||
|---|---|---|---|---|---|
| 200 (n = 3) | 250 (n = 6) | 300 (n = 8) | 350 (n = 5) | Total (N = 22) | |
| Sex | |||||
| Male | 2 | 3 | 4 | 3 | 12 |
| Female | 1 | 3 | 4 | 2 | 10 |
| Median age, years | 67 | 58 | 61 | 58 | 61 |
| Range | 62-69 | 50-66 | 45-65 | 48-65 | 45-69 |
| Histology | |||||
| Adenocarcinoma | 1 | 2 | 4 | 2 | 9 |
| Squamous | 1 | 1 | 3 | 1 | 6 |
| Other/not specified | 1 | 3 | 1 | 2 | 7 |
| ECOG PS | |||||
| 0 | 0 | 0 | 2 | 0 | 2 |
| 1 | 3 | 6 | 6 | 5 | 20 |
| Prior radiotherapy | 3 | 3 | 5 | 3 | 14 |
| No. of prior chemotherapy regimens | |||||
| 1 | 1 | 4 | 5 | 3 | 13 |
| 2 | 2 | 2 | 3 | 2 | 9 |
| Smoking history | |||||
| Median no. cigarettes per day | 11 | 13 | 20 | 20 | 18 |
| Range | 10-20 | 10-40 | 10-30 | 12-20 | 10-40 |
| Median no. years smoked | 49 | 36 | 41 | 40 | 42 |
| Range | 44-51 | 20-50 | 10-54 | 20-48 | 10-54 |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Toxicity and Dose Modifications
Four dose levels were investigated: 200 mg (n = 3), 250 mg (n = 6), 300 mg (n = 8), and 350 mg (n = 5). Toxicities during the initial 14 days deemed related to erlotinib are listed by cohort in Table 2. An analysis combining the preferred terms of rash, acneiform dermatitis, rash erythematous, and erythema was performed and classified as skin toxicity to provide an indication of overall skin-related adverse events.
Table 2.
Toxicity (adverse events deemed related to erlotinib) Reported in Initial 14 Days: Worst Toxicity Grade Per Patient
| MedDRA Preferred Term | Erlotinib Dose (mg per day) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 200 (n = 3) |
250 (n = 6)* |
300 (n = 8)* |
350 (n = 5) |
|||||
| All | 3/4 | All | 3/4 | All | 3/4 | All | 3/4 | |
| Skin toxicity† | — | — | 4 | — | 6 | 1 | 3 | 1 |
| Rash | — | — | 3 | — | 3 | 1 | 1 | — |
| Dermatitis acneiform | — | — | — | — | 2 | — | 2 | 1 |
| Pruritis | — | — | — | — | 1 | — | 2 | — |
| Dry skin | — | — | — | — | 1 | — | 1 | — |
| Swelling face | — | — | — | — | — | — | 1 | — |
| Diarrhea | — | — | 4 | — | 4 | — | 4 | — |
| Nausea | — | — | 1 | — | — | — | 2 | — |
| Vomiting | — | — | 2 | — | — | — | 1 | — |
| Eye disorders | — | — | — | — | 2 | — | 1 | — |
| Anorexia | — | — | 1 | — | 1 | — | — | — |
| Dehydration | — | — | 1 | — | — | — | — | — |
| Fatigue | — | — | — | — | — | — | 1 | 1 |
| Decreased performance status | — | — | — | — | — | — | 1 | 1 |
NOTE. Events graded according to National Cancer Institute Common Toxicity Criteria Adverse Events version 3.0. Includes those events occurring in > 5% patients or with more than grade 1 severity.
Includes all patients at that dose level.
Since differences in skin rash morphology occurred during erlotinib therapy, an analysis combining the preferred terms of rash, dermatitis acneiform, rash erythematous, and erythema was performed and presented in the tabular summaries as skin toxicity.
No DLTs were reported in either the 200- or 250-mg cohorts. At 300 mg, one of six patients reported grade 3 rash which resolved after interruption of erlotinib and the patient restarted treatment at 250 mg. In the 350-mg cohort, two of five patients experienced DLT. One patient had grade 3 acneiform dermatitis and another had grade 3 fatigue and decreased performance status that resulted in discontinuation of erlotinib. No grade 4 erlotinib-related events were observed during the initial 14 days and 300 mg was determined to be the MTD for this patient population.
Seven patients had dose modifications during the initial 14 days: one patient enrolled at 250 mg took 150 mg in error; three patients (one at 250 mg and two at 300 mg) had interruptions for nonrelated adverse events and three patients (one at 250 mg and two at 350 mg) interrupted or ceased erlotinib dosing for related adverse events, two of which were deemed DLTs as described earlier.
Nineteen patients received erlotinib during the extended treatment phase. The only grade 3 or 4 erlotinib-related event during this period was dermatitis acneiform in a patient receiving 350 mg. Erlotinib was temporarily withheld and restarted at a reduced dose. In addition, a patient on 300 mg discontinued due to grade 1 nausea deemed related to erlotinib (in conjunction with unrelated dizziness). Otherwise the most frequent cause of discontinuation was disease progression (14 of 22; 64%). One patient remained on treatment at 300 mg at the time of data cutoff.
Part II: Randomized Pharmacokinetic Study
Between November 2006 and August 2007, 35 patients were randomly assigned to 150 mg (17 patients) or 300 mg (18 patients). Patient characteristics are listed in Table 3. Three patients were unassessable for pharmacokinetic analyses; two patients due to adverse events before day 14 and one patient had incomplete dosing information. There were no notable differences in disease characteristics between the two cohorts, although more patients in the 300 mg cohort had received two or more prior chemotherapy regimens (44% v 24% for the 150-mg cohort).
Table 3.
Patient Characteristics: Pharmacokinetic
| Characteristic | Erlotinib Dose (mg per day) |
||
|---|---|---|---|
| 150 (n = 17) | 300 (n = 18) | Total (N = 35) | |
| Sex | |||
| Male | 8 | 8 | 16 |
| Female | 9 | 10 | 19 |
| Median age, years | 63 | 61 | 61 |
| Range | 50-78 | 40-75 | 40-78 |
| Histology | |||
| Adenocarcinoma | 6 | 10 | 16 |
| Squamous | 6 | 6 | 12 |
| Undifferentiated large cell | 1 | 1 | 2 |
| Other/not specified | 4 | 1 | 5 |
| ECOG PS | |||
| 0 | 2 | 1 | 3 |
| 1 | 15 | 17 | 32 |
| Prior radiotherapy | 10 | 11 | 21 |
| No. of prior chemotherapy regimens* | |||
| 1 | 13 | 10 | 23 |
| 2 | 3 | 7 | 10 |
| 3 | 1 | 1 | 2 |
| Smoking history | |||
| Median no. cigarettes per day | 18 | 15 | 15 |
| Range | 10-40 | 10-30 | 10-40 |
| Median no. years smoked | 44 | 44 | 44 |
| Range | 2-60 | 20-63 | 2-63 |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Two patients who received three prior regimens were included in the analyses.
Pharmacokinetic Analyses
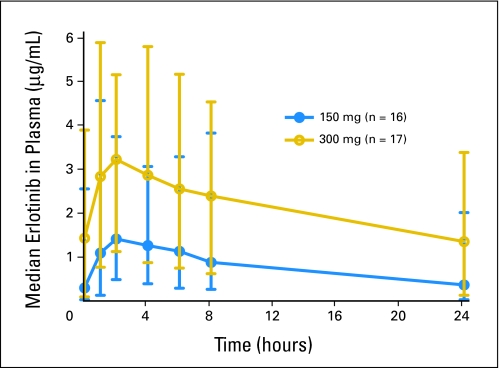
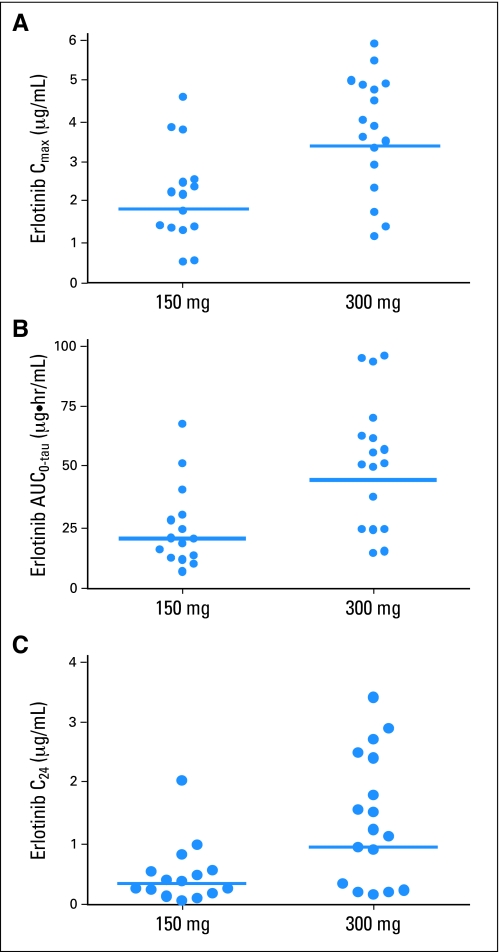
Median steady-state erlotinib plasma concentration versus time plots are shown in Figure 1. After erlotinib on day 14, plasma concentrations of erlotinib peaked at a median Tmax of 2 hours in both dose cohorts. The median peak plasma concentration of erlotinib was 2.16 and 3.86 μg/mL for the 150-mg and 300-mg cohorts, respectively. The corresponding median AUC0-tau values were 19.7 and 51.1 μg × hr/mL. Median steady-state C24 was 0.375 and 1.22 μg/mL for 150 mg and 300 mg, respectively. The relationship between Cmax, AUC0-tau, trough erlotinib plasma concentration (C24), and erlotinib dose are shown in Figure 2.
Fig 1.
Median and range steady state erlotinib plasma concentrations after 150 or 300 mg oral dose of erlotinib in non–small-cell lung cancer patients who are current smokers.
Fig 2.
Comparision of steady state erlotinib plasma pharmacokinetic parameters after 150 or 300 mg oral dose of erlotinib in non–small-cell lung cancer patients who are current smokers. Bars represent geometric mean.
Table 4 summarizes the statistical comparison of the geometric mean ratios (300 mg/150 mg) for maximum plasma concentration (Cmax), C24, and area under the plasma concentration-time curve during the dosing interval (AUC0-tau) of erlotinib and OSI-420. The data demonstrate a significant difference in exposure between the 150-mg and 300-mg cohorts. The percent geometric mean ratios (300 mg/150 mg) for dose-normalized Cmax and AUC0-tau of erlotinib were 93.4 (90% CI, 66.4 to 131; P = .7355) and 113 (90% CI, 76.4 to 166; P = .6042), respectively. These results demonstrate erlotinib exposure was dose-proportional within the dose range tested.
Table 4.
Geometric Means and Geometric Mean Ratios With 90% CIs for Erlotinib and OSI-420 Pharmacokinetic Parameters of 300 mg Patients to 150 mg Patients
| PK Parameter by Analyte | Geometric Mean for Erlotinib by Dose (mg) |
Geometric Mean Ratio |
|||
|---|---|---|---|---|---|
| 150 (n = 15) | 300 (n = 17) | 300 mg/150 mg (%) | 90% CI | P | |
| Erlotinib | |||||
| Cmax | 1.81 | 3.38 | 186.8 | 132.8 to 262.6 | .0041 |
| AUC0-tau | 19.5 | 44.0 | 225.5 | 152.9 to 332.6 | .0013 |
| C24 | 0.324 | 0.935 | 288.3 | 155.7 to 533.9 | .0066 |
| OSI-420 | |||||
| Cmax | 0.192 | 0.385 | 200.5 | 132.8 to 302.8 | .0076 |
| AUC0-tau | 2.11 | 4.99 | 236.5 | 146.9 to 380.9 | .0045 |
| C24 | 0.0356 | 0.0989 | 278.0 | 137.6 to 561.4 | .0195 |
Abbreviations: Cmax, maximum plasma concentration; AUC0-tau, area under the plasma concentration-time curve during the dosing interval; C24, trough erlotinib plasma concentration.
Plasma Cmax and AUC0-tau of the OSI-420 metabolite remained approximately 10% of the erlotinib Cmax and AUC0-tau at both doses (Table 4). While levels of AAG did not differ between cohorts, analysis of both doses combined, identified significant correlations (P < .05) between plasma AAG and dose-normalized erlotinib AUC0-tau and Cmax but not C24 (online-only Appendix).
Toxicity and Dose Modifications
The majority of toxicity reported during the initial 14 days was limited to CTCAE grade 1 and/or 2 (Table 5). Skin toxicity and diarrhea occurred more frequently in the 300-mg arm than the 150-mg arm; 12 patients (67%) versus five patients (29%) and nine patients (50%) versus three patients (18%), respectively.
Table 5.
Toxicity (adverse event deemed related to erlotinib) Reported in Initial 14 Days: Worst Toxicity Grade Per Patient
| MedDRA Preferred Term | Erlotinib Dose (mg per day) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 150 (n = 17) |
300 (n = 18) |
|||||||
| All |
3/4 |
All |
3/4 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Skin toxicity* | 5 | 29 | — | — | 12 | 67 | 1 | 6 |
| Rash | 5 | 29 | — | — | 6 | 33 | 1 | 6 |
| Pruritis | 1 | 6 | — | — | 4 | 22 | — | — |
| Dermatitis acneiform | — | — | — | — | 4 | 22 | — | — |
| Dry skin | 3 | 18 | — | — | 1 | 6 | — | — |
| Rosacea | — | — | — | — | 1 | 6 | — | — |
| Skin fissures | — | — | — | — | 1 | 6 | — | — |
| Diarrhea | 3 | 18 | — | — | 9 | 50 | 1 | 6 |
| Nausea | 3 | 18 | — | — | 3 | 17 | — | — |
| Mouth ulceration | 2 | 12 | — | — | — | — | — | — |
| Stomatitis | 1 | 6 | — | — | 1 | 6 | — | — |
| Vomiting | 1 | 6 | — | — | 1 | 6 | — | — |
| Dysgeusia | 1 | 6 | — | — | — | — | — | — |
| Eye disorders | 2 | 12 | — | — | 4 | 22 | — | — |
| Fatigue | 2 | 12 | — | — | 3 | 17 | — | — |
| Dizziness | 1 | 6 | — | — | 1 | 6 | — | — |
| Headache | — | — | — | — | 1 | 6 | — | — |
| Somnolence | — | — | — | — | 1 | 6 | — | — |
| Anorexia | — | — | — | — | 2 | 11 | — | — |
| Hyperbilirubinema | — | — | — | — | 1 | 6 | — | — |
NOTE. Events graded according to National Cancer Institute Common Toxicity Criteria Adverse Events version 3.0. Includes those events occurring in > 5% patients or with more than grade 1 severity.
Since differences in skin rash morphology occurred during erlotinib therapy, an analysis combining the preferred terms of rash, dermatitis acneiform, rash erythematous, and erythema was performed and presented in the tabular summaries as skin toxicity.
Two patients had dose modifications during the initial 14 days; one patient randomly assigned to 150 mg interrupted dosing due to an unrelated respiratory tract infection and discontinued due to progressive disease while a patient randomly assigned to 300 mg interrupted dosing for a grade 3 skin rash and was subsequently reduced to 250 mg.
Thirty-three patients continued erlotinib during the extended phase. Twelve patients had dose adjustments at day 15: six patients randomly assigned to 150 mg were escalated to 300 mg at investigator discretion, and six patients originally randomly assigned to 300 mg had their dose reduced (four patients reduced to 150 mg and two patients to 250 mg). Reasons for dose reduction included rash (three patients), fatigue (one patient), diarrhea (one patient), and a combination of diarrhea, skin, and eye symptoms (one patient). Grade 3 or 4 erlotinib-related events reported in patients receiving 300 mg during extended treatment were grade 3 fatigue (two patients who both discontinued treatment) and grade 3 diarrhea (one patient who restarted treatment at 150 mg). In addition, one patient receiving 150 mg developed a cataract approximately 4 months after discontinuation of erlotinib. One patient remained on treatment at data cutoff. The most common reason for study discontinuation was disease progression in 24 (71%) of 34 of patients.
Survival
Exploratory survival analyses among part II patients, based on investigator-selected extended phase dose as recorded on day 15, demonstrated a median survival of 5.45 months (95% CI, 3.19 to 10.09) for the 13 patients receiving 150 mg and 9.56 months (95% CI, 6.34 to not reached) for the 20 patients receiving 250 (two patients) or 300 mg (18 patients).
DISCUSSION
This two-part phase I study evaluated the hypothesis that the reduction in erlotinib exposure seen in current smokers may be attributed, in part, to induction of CYP enzymes by tobacco smoking. Part I investigated four dose levels from 200 to 350 mg and determined that the MTD of erlotinib in NSCLC patients who continue to smoke cigarettes was 300 mg per day. This contrasts with the previously accepted MTD of 150 mg per day determined by Hidalgo et al in unselected patients.13
The pharmacokinetic element demonstrated that steady-state trough plasma concentrations in smokers treated with erlotinib at 300 mg in the current study (median, 1.22 μg/mL) were comparable to those in former or never smokers treated at 150 mg in the pivotal phase III study (median, 1.28 or 1.45 μg/mL).10 While skin toxicity and diarrhea occurred more frequently in the 300-mg arm than the 150-mg arm, the majority of adverse events were grade 1 or 2. While six of 18 patients randomly assigned to 300 mg were dose reduced on day 15, only two had reported CTC grade 3 toxicity. In addition, an equal number of patients were escalated from 150 to 300 mg for extended treatment, during which three of 18 patients receiving the higher dose reported toxicity in excess of grade 2. The incidence of adverse events in current smokers who received 300 mg per day in this study was similar to those observed in BR.21 among all patients who received 150 mg per day (eg, rash 67% v 75% and diarrhea 50% v 54%); justifying 300 mg as MTD in this population.2
It is well known that exposure of drugs metabolized by CYP enzymes may be altered by coadministration with medications that inhibit or induce the same specific isoform.11 However, the potential effect of cigarette smoking on pharmacokinetics has been studied for a limited number of drugs.14,15 Our report indicates that exposure and toxicity of an anticancer therapy may be influenced by cigarette smoking, complementing work by van der Bol et al who found that smoking significantly lowered both exposure to irinotecan and treatment-induced neutropenia.15 While smoking cessation clearly remains the optimal route in patient management, the impact of CYP induction by tobacco smoke may warrant dose modification considerations with medications metabolized by similar pathways such as CYP1A1 and 1A2.11,14 However, the financial implications of administering higher dosages need to be considered.
This study focused on the issue of smoking status and did not assess potential patient selection strategies proposed for management of NSCLC patients with EGFR inhibitors.15,16 Previous studies indicated that smoking history was more predictive of survival benefit than EGFR protein expression or other biomarkers.4,5,17,18 As such, the impact of erlotinib dose in relation to smoking history may play an additional role in selection of patients in future trials.4 Subsequent studies should also consider the potential interaction between dose and the role of Kras as a possible marker of insensitivity to EGFR inhibitors, especially as Kras mutations may be more common among smokers.19,20
In BR.21, median survival among all patients treated with 150 mg erlotinib was 6.7 months while in the subgroups of never, former, and current smokers it was 12.3, 5.5, and 6.1 months, respectively.2,4–6 While cross-study comparisons are confounded by inherent differences in study populations, such as performance status and/or number of lines of prior chemotherapy; the median survival of the smokers receiving escalated erlotinib doses (250 or 300 mg) in this trial was 9.56 months (95% CI, 6.34 to not reached) while in patients receiving 150 mg it was 5.45 months (95% CI, 3.19 to 10.09). These findings should be interpreted with caution since the analyses are based on the selected day 15 dose rather than any randomized comparison and are significantly compromised by the small sample size and differences in patient characteristics (eg, sex and histology). Nonetheless, it generates a compelling hypothesis that increasing the erlotinib dose in this population may improve outcome, without a large increase in toxicity.
In conclusion, we determined that the MTD of erlotinib in NSCLC patients who continue to smoke cigarettes was 300 mg per day. Steady-state trough plasma concentrations and incidence of rash and diarrhea in smokers at 300 mg were similar to those in former or never smokers receiving 150 mg in previous studies.2,4,5,10 These findings indicate that higher erlotinib doses of up to 300 mg per day should be further investigated in current smokers in order to confirm any potential improvement in outcome as well as assessing patient safety at the higher dose.
Supplementary Material
Acknowledgment
We thank the patients who consented to take part in this study; the colleagues who referred them; and the following people for their contributions: R. Patel, MD, for patient enrollment; K. Witt, MD, for input to study design and safety analyses; E. Conklin and C. Tucker for bioanalyses of pharmacokinetic samples; D. Zborowski for statistical programming and additional analyses; J. Wheaton, L. Montgomery, N. Graff, H. Davis and investigator-site staff for study coordination.
Appendix
The Acknowledgment and Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
While levels of AAG did not differ between cohorts, exploratory analyses identified statistically significant correlations (P < .05) between the plasma AAG and dose-normalized erlotinib AUC0-tau (r = 0.4144; P = .0184; n = 32) and Cmax (r = 0.6801; P = .0319; n = 32) but not with C24 (r = 0.3120; P = .0822; n = 32). Similarly, statistically significant correlations (P < .05) between the plasma AAG and dose-normalized OSI-420 AUC0-tau (r = 0.3870; P = .0286; n = 32), Cmax (r = 0.3979; P = .0241; n = 32), and C24 (r = 0.3950; P = .0253; n = 32) were observed.
Footnotes
Supported by a grant from OSI Pharmaceuticals Inc.
Presented in part at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2007; WCLC, Seoul, Korea, September 2-6, 2007; and Multidisciplinary Symposium in Thoracic Oncology, Chicago, IL, November 13-15, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00294736.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jonathan B. Chick, OSI Pharmaceuticals (C); Julie Wolf, OSI Pharmaceuticals (C) Consultant or Advisory Role: Marianne Nicolson, Roche (C); Elaine Rankin, Roche (C) Stock Ownership: Jonathan B. Chick, OSI Pharmaceuticals; Ramesh Boinpally, Resticles Stock Unite; Julie Wolf, OSI Pharmaceuticals Honoraria: None Research Funding: W. Jeffrey Petty, OSI Pharmaceuticals; Penella J. Woll, OSI Pharmaceuticals Expert Testimony: None Other Remuneration: Ramesh Boinpally, Employee of OSI Pharmaceuticals when study was conducted.
AUTHOR CONTRIBUTIONS
Conception and design: W. Jeffrey Petty, Jonathan B. Chick, Ramesh Boinpally, Allan Price, Julie Wolf, Andrew N. Hughes
Financial support: Jonathan B. Chick
Administrative support: Jonathan B. Chick
Provision of study materials or patients: Andrew N. Hughes, Mary E.R. O'Brien, W. Jeffrey Petty, Jonathan B. Chick, Elaine Rankin, Penella J. Woll, David Dunlop, Marianne Nicolson, Allan Price
Collection and assembly of data: Andrew N. Hughes, Mary E.R. O'Brien, W. Jeffrey Petty, Jonathan B. Chick, Elaine Rankin, Penella J. Woll, David Dunlop, Marianne Nicolson, Allan Price
Data analysis and interpretation: Mary E.R. O'Brien, W. Jeffrey Petty, Jonathan B. Chick, Elaine Rankin, Penella J. Woll, David Dunlop, Marianne Nicolson, Ramesh Boinpally, Julie Wolf, Allan Price
Manuscript writing: Andrew N. Hughes, Mary E.R. O'Brien, Jonathan B. Chick, Elaine Rankin, Penella J. Woll, David Dunlop, Ramesh Boinpally
Final approval of manuscript: Andrew N. Hughes, Mary E.R. O'Brien, W. Jeffrey Petty, Jonathan B. Chick, Elaine Rankin, Penella J. Woll, David Dunlop, Marianne Nicolson, Julie Wolf, Allan Price
REFERENCES
- 1.Akita RW, Sliwowski MX. Preclinical studies with Erlotinib (Tarceva) Semin Oncol. 2003;30(suppl 7):15–24. [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Bezjak A, Dongsheng Tu, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR. 21. J Clin Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 4.Clark GM, Zborowski DM, Santabarbara P, et al. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group Study BR. 21. Clin Lung Cancer. 2006;7:389–394. doi: 10.3816/clc.2006.n.022. [DOI] [PubMed] [Google Scholar]
- 5.Clark GM. Prognostic factors versus predictive factors:examples from a clinical trial of erlotinib. Mol Oncol. 2008;1:406–412. doi: 10.1016/j.molonc.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126:347–351. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 7.Tsao AS, Liu D, Lee JJ, et al. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106:2428–2436. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005;11:6414–6421. doi: 10.1158/1078-0432.CCR-05-0790. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton M, Wolf JL, Zborowski D, et al. Tarceva™ (erlotinib) exposure/effects (EE) analysis from a phase III study in advanced NSCLC: Effect of smoking on the PK of erlotinib. Proc Am Assoc Cancer Res. 2005;46:1451. abstr 6165. [Google Scholar]
- 10.Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhao M, He P, et al. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13:3731–3737. doi: 10.1158/1078-0432.CCR-07-0088. [DOI] [PubMed] [Google Scholar]
- 12.Indianapolis, IN: Roche Diagnostics Corporation; 2003–2006. Roche: a-1 Acid Glycoprotein: Package insert V4. [Google Scholar]
- 13.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 14.Zevin S, Benowitz NL. Drug interactions with tobacco smoking: An update. Clin Pharmacokinetics. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 15.van der Bol JM, Mathijssen RHJ, Loos WJ, et al. Cigarette smoking and irinotecan treatment: Pharmacokinetic interaction and effects on neutropenia. J Clin Oncol. 2007;25:2719–2726. doi: 10.1200/JCO.2006.09.6115. [DOI] [PubMed] [Google Scholar]
- 16.Sequist LV, Bell Dw, Lynch TJ, et al. Molecular predicters of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 17.Goodin S. Erlotinib: Optimizing therapy with predictors of response? Clin Cancer Res. 2006;12:2961–2963. doi: 10.1158/1078-0432.CCR-06-0426. [DOI] [PubMed] [Google Scholar]
- 18.Clark GM, Zborowski DM, Culbertson JL, et al. Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non-small cell lung cancer for treatment with erlotinib. J Thoracic Oncol. 2006;1:837–846. [PubMed] [Google Scholar]
- 19.Tsao MS, Sakurada A, Cutz J-C, et al. Erlotinib in lung cancer: Molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 20.Massarelli E, Varella-Garcia M, Tang X, et al. Kras mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd FA, Ding K, Sakurada A, et al. Updated molecular analyses of exons 19 and 21 of the epidermal growth factor receptor (Egfr) gene and codons 12 and 13 of the kras gene in non-small cell lung cancer (NSCLC) patients treated with erlotinib in National Cancer Institute of Cancer. J Clin Oncol. 2007;25(suppl):402s. abstr 7571. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.