Abstract
Purpose
Approximately 50% of glioblastomas (GBMs) are characterized by overexpression of the epidermal growth factor receptor (EGFR) and EGFR gene amplification. In approximately 25% of instances, constitutively activated EGFR mutants are present. These observations make EGFR-inhibiting drugs a logical approach for trials in recurrent GBM.
Patients and Methods
In a randomized, controlled, phase II trial, 110 patients with progressive GBM after prior radiotherapy were randomly assigned to either erlotinib or a control arm that received treatment with either temozolomide or carmustine (BCNU). The primary end point was 6-month progression-free survival (PFS). Tumor specimens obtained at first surgery were investigated for EGFR expression; EGFRvIII mutants; EGFR amplification; EGFR mutations in exons 18, 19, and 21; and pAkt. These results were correlated with outcome. Pharmacokinetic analysis was part of the study.
Results
Treatment was well tolerated in general; skin toxicity was the most frequent adverse effect of erlotinib. The 6-month PFS rate in the erlotinib arm was 11.4% (95% CI, 4.6% to 21.5%), and it was 24% in the control arm. Of all explored biomarkers, only low pAkt expression appeared to be of borderline significance to an improved outcome. None of the eight patients who had tumors with EGFRvIII mutant presence and PTEN expression had 6-month PFS. The use of enzyme-inducing anticonvulsants significantly increased erlotinib clearance, but pharmacokinetic findings were not related to outcome.
Conclusion
Erlotinib has insufficient single-agent activity in unselected GBM. No clear biomarker associated with improved outcome to erlotinib was identified.
INTRODUCTION
Despite the recent advances that were obtained by the introduction of chemoradiotherapy with temozolomide (TMZ), the overall outcome of patients with glioblastoma multiforme (GBM) remains dismal.1 Especially for patients with recurrent disease, options are limited, and novel treatments are needed. One potential target is the epidermal growth factor receptor (EGFR) signaling pathway. Expression of high levels of EGFR protein and EGFR amplification are present in 40% to 60% of GBMs.2,3 Forty percent of GBMs with EGFR amplification also have EGFR mutations, most commonly the EGFRvIII variant that lacks exons 2 to 7 through intragenic deletion rearrangements and that has a constitutively phosphorylated tyrosine kinase domain.2,4 The activated EGFR stimulates the RAS/RAF/MAPK and PI3-K/Akt pathways, which results in increased cell proliferation and survival.
Erlotinib is a EGFR–tyrosine kinase inhibitor (TKI), which binds the phosphorylation site of the receptor and prohibit autoactivation of the wild-type EGFR as well as the truncated EGFRvIII mutant. EGFR-TKIs have shown clinical activity in lung carcinoma, in particular in tumors that have mutations in the adenosine triphosphate binding pocket of the tyrosine kinase domain of the EGFR gene (exons 19 to 21). In GBM, mutations in this domain are virtually absent.5–9 In a phase I study of erlotinib with or without TMZ, in which 57 assessable patients experienced eight responses, six of the responding patients had only received erlotinib.10 In that study, six patients were free from progression at 6 months. Another study showed greater than 20% 6-month progression-free survival (PFS) in recurrent GBM after erlotinib treatment.11 Another study noted erlotinib activity in particular in GBM with combined presence of the EGFRvIII mutant and PTEN expression.9 Because of these initial results, the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group investigated the activity of erlotinib in recurrent GBM in a randomized, controlled, phase II study.
PATIENTS AND METHODS
This randomized, phase II study initially assigned patients randomly to erlotinib or TMZ. After the acceptance of chemoradiotherapy with TMZ as the standard of care for the newly diagnosed GBM patients, the trial was amended to allow patients who were previously treated with TMZ chemoradiotherapy.1
Main Eligibility Criteria
Patients were eligible if they had a histologically proven GBM recurrent disease after previous radiation therapy documented by magnetic resonance imaging; no prior chemotherapy for recurrent disease or a maximum of only one prior chemotherapy regimen given as adjuvant treatment; completion of all prior chemotherapy at least 4 weeks (or 6 weeks if nitrosourea treatment) before registration into the study; no receipt of radiotherapy in the past 3 months; at least one bidimensionally measurable target lesion with one diameter of at least 2 cm; a Karnofsky performance status ≥ 70; and adequate bone marrow, renal, and hepatic function.
Treatment
Erlotinib was started at 150 mg daily, with dose escalation to 200 mg daily if no or minimal toxicity was experienced, in patients who were not on enzyme-inducing anticonvulsants (EIAEDs), and at 300 mg daily, with dose escalation in 50-mg increments up to 500 mg daily if no or minimal toxicity, for patients on EIAEDs. Four weeks of erlotinib treatment comprised one cycle. Patients randomly assigned to the control arm received either TMZ—or carmustine (BCNU) if TMZ was part of initial treatment. TMZ was started at 200 mg/m2 on days 1 to 5 every 4 weeks in chemotherapy-naïve patients or at 150 mg/m2 on days 1 to 5 every 4 weeks after prior adjuvant chemotherapy, with dose escalation to 200 mg/m2 in the absence of significant toxicity (Common Terminology Criteria of Adverse Events < 2) in cycle 1. BCNU was given initially at a dose level of 80 mg/m2 on days 1 to 3 every 8 weeks for a maximum of five cycles. This dose level had been observed previously to be safe.12 Because of the BCNU-induced myelosuppression observed after chemoradiotherapy with TMZ, the dose was reduced to 60 mg/m2 on days 1 to 3 every 8 weeks.
End Point and End Point Assessment
The sample size was determined according to Fleming design, and the PFS rate at 6 months was the primary end point. P0 was set at 15%, and P1 was set at 30%; α was set at 20%, and β was set at 5%. Fifty patients were needed in each treatment group. Erlotinib would be considered sufficiently active to warrant additional investigation if greater than nine patients were alive and free of progression 6 months after the start of treatment. Secondary end points were response, survival, and toxicity. All analyses were planned on the intent-to-treat population. Patients were randomly assigned centrally at the EORTC Data Center in Brussels, either by internet or by phone. A minimization technique was stratification by institution.13 The trial design was approved by the EORTC protocol review committee and by national and institutional review boards of the participating centers according to European, national, and local regulations. All patients provided written informed consent. The database was organized, maintained, and analyzed by EORTC statisticians.
Response was assessed by using bidimensional criteria by Macdonald et al.14 Magnetic resonance imaging scans of all patients in whom a response or a 6-month PFS was reported were centrally reviewed. Toxicity was assessed by using the Common Terminology Criteria for Adverse Events version 3.0. Pathology review was part of the study.
Translational Research
Fluorescent in situ hybridization and data analysis were performed. Probes to EGFR (BAC RPCI 11-148p17; provided by A. Perry) and centromere 7 (CEP7; P7t1) were labeled with digoxigenin-16-dUTP (148p17; Roche Diagnostics, Mannheim, Germany) or Spectrum Green (P7t1; Vysis Inc, Downers Grove, IL). Slide preparation and scoring were done as previously described.15 Sixty nonoverlapping nuclei were counted, and ratios were calculated by dividing the number of signals of EGFR by the number of signals of the reference (CEP7). A ratio of EGFR/CEP7 greater than 2 was considered EGFR amplification.
Immunohistochemistry of Antihuman EGFR, EGFRvIII, pAKT, and PTEN and Data Analysis
Sections stained with anti-EGFR antibodies were processed without additional antigen retrieval. Other slides were submitted to antigen retrieval in a microwave that contained 10 mmol/L Tris-EDTA (Klinipath, Duiven, the Netherlands) during 20 minutes and were allowed to cool down to room temperature. Endogeneous peroxidase activity was blocked with 3% H2O2 in methanol for 20 minutes and subsequently was washed in tris-bufferered saline with Tween. Specific binding sites were blocked by a 10-minute incubation with Dako block solution (Dako, Glostrup, Denmark) at room temperature. Primary antibodies to EGFR (1:500, clone E30; Dako), EGFRvIII (1 μg/mL, L8A4; provided by D. Bigner), pAKT (1:50, clone 736E11; Cell Signaling Technology, Beverly, MA) or PTEN (1:100, clone 138G6; Cell Signaling Technology) were diluted in normal antibody dilutent (Dako) and were incubated for 1 hour at room temperature (EGFR) or overnight (EGFRvIII, PTEN) and for 48 hours (pAKT) at 4°C. Slides then were washed in tris-bufferered saline with Tween. The enzyme-conjugated polymer (EnVision; Dako) and 3,3′-diaminobenzidine (Dako) were used according to manufacturer recommendations as the visualization system and the chromogen, respectively. For EGFR, EGFRvIII, and pAkt, the slides were evaluated as described by Allred et al16 For evaluation of PTEN staining, only the samples with clear positive staining of endothelial cells (ie, internal positive control) were included.17 Only the intensity of PTEN stained cells was recorded (as no, faint, and clear), as generally all cells stained with similar intensity in each sample. Faint and clear stainings were considered positive.
Mutational Analysis
Mutational analysis was performed in the erlotinib-treated patients who experienced 6-month PFS for EGFR gene exons 18, 19, and 21 on DNA retrieved from sections of routine formalin-fixed and paraffin-embedded tissues; primers were designed as described elsewhere.18 Tissue areas were selected for a high percentage of tumor cells to increase the EGFR mutation detection sensitivity.
Pharmacokinetic Analysis
For pharmacokinetic analysis, a 3-mL blood sample was collected from all erlotinib-treated patients before erlotinib dosing on day 1 of cycles 1, 2, 3, 4, 5, and 6. The impact of the use of EIAEDs was assessed in a subset of 12 patients (six in the EIAED and six in the non-EIAED group) from selected centers. In addition to the predose samples taken on day 1 of cycles 2, 3, 4, 5, and 6, serial blood samples were collected on both days 1 and 8 of cycle 1 at 0 (predose); 30 minutes; and 1, 2, 4, 6, 8, and 24 hours postdose. For each patient, maximum concentration (Cmax) and area under the concentration-time curve (AUC(tau)) were evaluated for any apparent relationship to clinical toxicity and/or efficacy. Analysis was performed as described elsewhere.19 Plasma concentrations for erlotinib (ie, OSI-774) and its metabolite OSI-420 (as the sum of the metabolites OSI-420 and OSI-413) were measured.
RESULTS
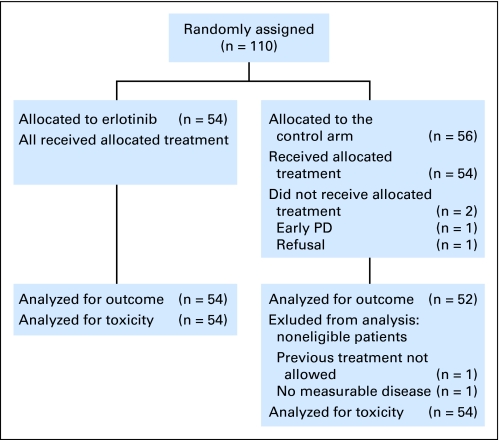
One hundred ten patients were randomly assigned; 54 were assigned to erlotinib, and 56 were assigned to the control arm. In the control arm, 29 were treated with BCNU, and 27 were treated with TMZ. Table 1 lists the clinical characteristics of the patients (see CONSORT diagram, Fig 1). No differences were observed between the two groups with respect to known prognostic factors.
Table 1.
Baseline Patient Characteristics
| Characteristic | Treatment Arm |
|||
|---|---|---|---|---|
| TMZ/BCNU (n = 56) |
Erlotinib (n = 54) |
|||
| No. | % | No. | % | |
| Age, years | ||||
| Median | 54.2 | 54.7 | ||
| Range | 19.5-78.8 | 18.7-71.4 | ||
| Sex | ||||
| Male | 37 | 66.1 | 35 | 64.8 |
| Female | 19 | 33.9 | 19 | 35.2 |
| Karnofsky performance status | ||||
| 70-80 | 26 | 46.4 | 24 | 44.4 |
| 90-100 | 30 | 53.6 | 30 | 55.6 |
| Prior adjuvant chemotherapy | ||||
| No | 20 | 35.7 | 19 | 35.2 |
| Yes | 36 | 64.3 | 35 | 64.8 |
| Antiepileptic treatment | ||||
| No | 23 | 41.1 | 18 | 33.3 |
| EIAED | 14 | 25.0 | 9 | 16.7 |
| Non-EIAED | 19 | 33.9 | 27 | 50.0 |
Abbreviations: TMZ, temozolomide; BCNU, carmustine; EIAED, enzyme-inducing anticonvulsant.
Fig 1.
CONSORT diagram. PD, disease progression.
Two of the patients allocated to TMZ/BCNU did not start treatment (early progression, n = 1; patient refusal, n = 1). Two additional control-arm patients were found ineligible at review (no measurable lesion, n = 1; prior treatment not allowed, n = 1). Tumor tissue was available from 101 patients (92%) for additional research. After progression, 46% in the control arm and 67% in the erlotinib arm received additional treatment. (In the erlotinib arm, this was mostly chemotherapy—in particular, TMZ, BCNU, and fotemustine). At the time of this report, 84 patients (76%) have died. The median number of cycles in the erlotinib arm was two (range, one to 30); in the TMZ-treated patients, the median number was four (range, one to 12); and in the BCNU-treated patients, the median number was one (range, zero to six).
Toxicity
In general, treatment with erlotinib was well tolerated. Related grades 3 and 4 toxicities in the erlotinib arm were predominantly to the skin (Appendix Table A1, online only). Grades 3 and 4 toxicities in the control arm were mainly hematologic (TMZ, n = 4, mainly thrombocytopenia; BCNU, n = 13, both leukopenia and thrombocytopenia; Appendix Table A1). Three patients in the erlotinib arm and four in the control arm discontinued treatment because of toxicity.
Outcome
Table 2 lists the outcome. With a 6-month PFS of 11.4% (95% CI, 4.6% to 21.5%) the trial failed to meet the preset efficacy end point in the erlotinib arm; in the control arm, 6-month PFS was 24.1%. No complete response was observed. A partial response was observed in two (3.7%) of 54 patients in the erlotinib arm and in five (9.6%) of 52 patients in the control arm. Stable disease was observed in nine patients (16.7%) in the erlotinib arm and in 18 patients (34.6%) in the control arm. Overall survival (OS) in the two arms was similar; the median OS rates were 7.7 months in the erlotinib arm and 7.3 months in the control arm. In erlotinib-treated patients, prolonged PFS and OS were observed in patients that developed skin toxicity of grade 2 or greater during treatment (P = .011 for both).
Table 2.
PFS and OS Summary Statistics
| Survival Data | Treatment Arm |
|
|---|---|---|
| Erlotinib | BCNU/TMZ | |
| Median PFS, months | 1.8 | 2.4 |
| 6-month PFS | ||
| % | 11.4 | 24.1 |
| 95% CI* | 4.6 to 21.5 | |
| 1-year PFS, % | 5.7 | 4.0 |
| Median OS, months | 7.7 | 7.3 |
| 6-month OS, % | 57.6 | 58.5 |
| 1-year OS, % | 21.9 | 26.7 |
Abbreviations: PFS, progression-free survival; OS, overall survival; BCNU, carmustine; TMZ, temozolomide.
Molecular Studies
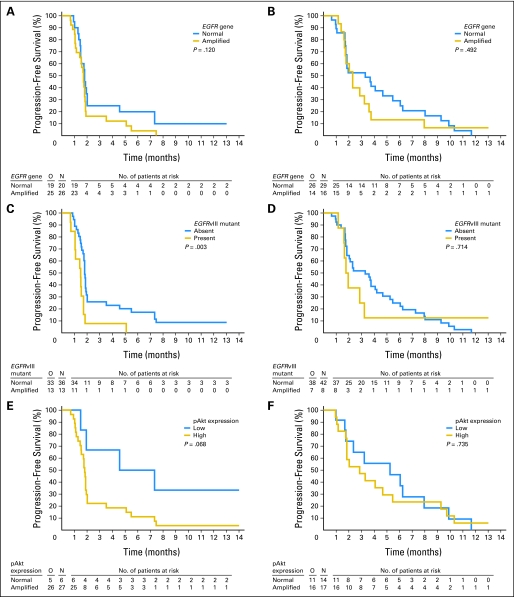
Table 3 lists the molecular findings in each arm and the correlation with 6-month PFS. In 82 patient cases, PTEN expression could be determined, in 64 patients, blocks were available for pAkt determination. EGFR overexpression, the presence of the EGFRvIII mutant, and EGFR gene amplification were strongly correlated. Low pAkt expression was more frequent in the control arm. For PFS, the presence of EGFRvIII mutant was correlated with poor survival in the erlotinib arm (P = .003) but not in the control arm (P = .714). EGFR gene amplification was borderline significant for poor outcome in the entire study group (P = .048) but in none of the individual arms. In eight patients, both EGFRvIII expression and PTEN expression were present, but none had 6-month PFS or an objective response. Of the six patients in the erlotinib arm who had low pAkt expression, three were free from progression and alive at 6 months (P = .068), whereas no correlation was observed between PFS and pAkt expression in the control arm (P = .735). Figure 2 summarizes the correlation with outcome of PFS and EGFR amplification, EGFRvIII mutant, and pAkt expression. No mutations were detected in the EGFR gene exons 19 to 21 of any of the six erlotinib-treated patients who experienced 6-month PFS.
Table 3.
Molecular Parameters in Both Treatment Arms
| Parameter | Treatment Arm |
|||||||
|---|---|---|---|---|---|---|---|---|
| Erlotinib |
BCNU/TMZ |
|||||||
| Positive |
All (No.) | 6-month PFS |
Positive |
All (No.) | ||||
| No. | % | No. | % | No. | % | |||
| EGFR overexpression | 31 | 63 | 49 | 3 | 10 | 26 | 52 | 50 |
| EGFRvIII present | 13 | 27 | 49 | 0 | 0 | 8 | 16 | 50 |
| EGFR amplification | 26 | 57 | 46 | 1 | 4 | 16 | 36 | 45 |
| PTEN expressed | 23 | 51 | 45 | 2 | 9 | 19 | 51 | 37 |
| Low pAkt expression | 6 | 18* | 33 | 3 | 50 | 14 | 45 | 31 |
NOTE. Positive indicates the number of samples that had molecular characteristics; all indicates the number of samples that were analyzed.
Abbreviations: BCNU, carmustine; TMZ, temozolomide; PFS, progression-free survival; EGFR, epidermal growth factor receptor.
P = .03.
Fig 2.
Correlation between progression-free survival and (A) epidermal growth factor receptor (EGFR) amplification in the erlotinib arm; (B) EGFR amplification in the control arm; (C) the presence of EGFRvIII mutant in the erlotinib arm; (D) EGFRvIII mutant in the control arm; and (E) pAkt expression in the erlotinib arm.
Fig 3.
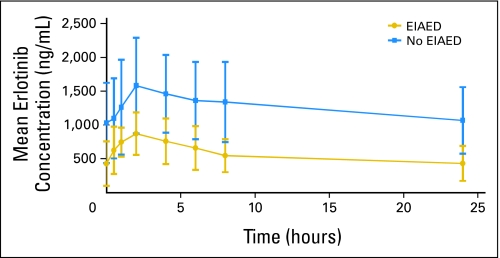
Mean (± standard deviation) plasma concentration versus time profile for erlotinib (OSI-774) after oral administration of erlotinib in cycle 1, day 8: erlotinib 150 mg without enzyme-inducing anticonvulsants (EIAEDs) and erlotinib 300 mg with EIAEDs.
Pharmacokinetic Analysis
Table 4 lists the exposure (area under the plasma concentration-time curve over the dosing interval [AUCtau]) to erlotinib and to OSI-420 in patients without and with EIAEDs. Nine (17%) of the 54 erlotinib-treated patients received EIAEDs. On day 1, exposure to erlotinib was similar in patients who received erlotinib 150 mg without EIAEDs and in those who received erlotinib 300 mg with concomitant EIAEDs. On cycle 1 day 8, however, AUCtau was much lower in patients who were taking concomitant EIAEDs, although they received double the dose of those without EIAED treatment (Fig 2). The ratio of the metabolite to the parent drug was approximately twice as high in the group that received EIAEDs. The use of EIAEDs did not have any significant effect on PFS or OS (data not shown).
Table 4.
Exposure to Erlotinib and OSI-420 in Patients Who Did or Did Not Receive EIAEDs With Erlotinib
| Drug or Metabolite Exposure | Treatment Arm |
|
|---|---|---|
| Erlotinib 150 mg + No EIAED | Erlotinib 300 mg + EIAED | |
| Erlotinib AUCtau, (ng × h)/mL | ||
| Cycle 1 day 1 | 9,790 ± 3,660 | 9,200 ± 4,880 |
| Cycle 1 day 8 | 30,200 ± 13,000 | 13,500 ± 4530 |
| OSI-420 AUC, (ng × h)/mL | ||
| Cycle 1 day 1 | 673 ± 312 | 1,200 ± 1,080 |
| Cycle 1 day 8 | 2,680 ± 1,380 | 1,810 ± 712 |
| Mean OSI-420/erlotinib ratio | ||
| Cycle 1 day 1 | 0.069 | 0.130 |
| Cycle 1 day 8 | 0.089 | 0.134 |
NOTE. OSI-420 is the main metabolite of erlotinib.
Abbreviations: EIAED, enzyme-inducing anticonvulsant; AUCtau, area under the plasma concentration-time curve over the dosing interval.
DISCUSSION
After initial studies suggested activity of erlotinib in recurrent GBM, this study was set up as a randomized, controlled, phase II study to control for random biases that may occur in uncontrolled studies in this disease. However, no significant activity of erlotinib was observed. With a 6-month PFS rate of 11% in the erlotinib arm, the study failed to reach its objective end point, whereas more than 20% of patients in the control arm were still free from progression at 6 months. Appendix Table A2 (online only) lists the findings in other studies on EGFR-inhibiting TKIs, which in general also show disappointing results. Similar to studies in lung cancer, outcome to erlotinib treatment was improved in patients who developed skin toxicity in this study.20 There is no clear explanation for this; pharmacokinetic parameters were not related to outcome.
Although erlotinib may show insufficient activity in unselected patients with GBM, the drug might still be valuable for a selected group of patients with GBM if a biomarker would allow the identification of erlotinib-responsive patients. Much interest was raised by a study that observed a favorable outcome to EGFR-TKIs in 60% to 80% of patients with tumors that were characterized by the presence of both the EGFRvIII mutation and PTEN expression.9 In contrast, a similar study that seemed to include, at least in part, the same patients found high levels of EGFR expression and low levels of phosphorylated PKB/Akt related to response to erlotinib treatment, and it found no clear association with outcome and the presence of EGFRvIII mutants.3 The use of a control arm in this trial allows the distinction between prognostic and predictive markers for outcome; relations with survival observed in single-arm studies can simply reflect prognostic significance.21 In this study, none of the eight patients who had combined expression of EGFRvIII mutants and PTEN had 6-month PFS. Both PFS and OS were actually worse in the patients with expression of the EGFRvIII mutant in the erlotinib arm but not in the control arm (Figs 2C and 2D). Thus, at present, there is no indication that erlotinib is particularly active in this subset of tumors. We only observed a borderline relation between low pAkt expression and outcome to erlotinib, which was not present in the control arm. An additional confirmation of the relation with low pAkt expression is necessary, because the P value was just greater than 0.05 and should be corrected for multiple testing. Other studies failed to find a correlation between response and expression of EGFR expression or the presence of EGFRvIII mutants.11,22 A major shortcoming of all these analyses is that they investigated tumor samples obtained at the time of first surgery, which do not necessarily have the same molecular characteristics as the recurrent tumor. Making biopsies mandatory before study entry is, unfortunately, not feasible in patients with recurrent GBM. However, this possible change in molecular characteristics with time clearly poses a considerable challenge in the development of targeted treatments for glioma.
The much lower exposure to erlotinib in patients who received EIAEDs, although they received twice the erlotinib dose, is consistent with the expected CYP3A4 induction via EIAEDs. Despite the lower AUC, the outcome in the few patients who received EIAEDs with erlotinib was similar compared with patients who did not receive EIAEDs. It is clear, though, that attempts to achieve similar exposure in patients who receive EIAEDs or agents metabolized through the CYP450 3A4 (CYP3A4) system by increasing the dosage of the investigational compound do not yield predictable results. At present, the preferred approach in trials on CYP3A4-metabolized agents is to exclude patients who are receiving EIAEDs and to switch them to noninducing agents before study entry. The clinical problem, however, is limited; at present, only a minority of patients with glioma use EIAEDs.
The obvious question, of course, is why the trials with EGFR-TKIs failed to produce clinically meaningful results in GBM, despite the presence of activated EGFR signaling pathways in approximately half of these tumors. The potential reasons are many, and they range from insufficient penetration into the tumor and insufficient target inhibition to a limited dependence on EGFR pathway signaling for cell survival and proliferation, even in GBM with EGFR gene amplification or increased expressions of other growth factor receptors on the GBM cell surface that activate downstream targets of the EGFR pathway.23 If the latter assumption is correct, EGFR-inhibiting agents could still play a role in combination treatments. Indeed, support for multitarget inhibition comes from several laboratory models in which activity was observed if multiple targets were simultaneously inhibited, either in multiple pathways (ie, horizontal inhibition) or in one pathway (ie, vertical inhibition).9,23 Obviously, the validity of this concept needs to be demonstrated in clinical trials. Unfortunately, no reliable biomarker has been identified so far that may help to select patients who may benefit from EGFR-inhibiting agents as part of a multitargeted strategy. Also, before additional clinical studies in GBM with EGFR-inhibiting agents are considered, it should be established first whether the investigational agent achieves adequate tumor penetration and target inhibition.
In conclusion, this study does not show clinically significant activity of erlotinib in unselected patients with a recurrent GBM. In the molecular side studies, an interesting relation was observed between low pAkt expression and PFS, but no clear biomarker profile of good outcome to erlotinib was identified. In particular, no favorable outcome was observed in the subgroup of patients with coexpression of the EGFRvIII mutant and PTEN at the time of first surgery.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Table A1.
Grade 3 or 4 Hematologic and Biochemistry Toxicities per Treatment Arm That Were Considered Treatment Related by the Local Investigator
| Toxicity | Treatment Arm |
||
|---|---|---|---|
| Erlotinib (n = 54) | TMZ (n = 27) | BCNU (n = 27) | |
| WBC | 1 | 1 | 8 |
| ANC | 1 | 10 | |
| Platelets | 4 | 11 | |
| Hemoglobin | 1 | ||
| Any hematotoxicity | 1 | 4 | 13 |
| Creatinine | 1 | 2 | |
| Bilirubin | 2 | 1 | 2 |
| ALT | 1 | 2 | |
| Hyperkalemia | 1 | ||
| Hypokalemia | 2 | ||
| Rash/dermatologic | 6 | ||
| Diarrhea | 1 | ||
| Febrile neutropenia | 1 | ||
| Pain | 1 | ||
Abbreviations: TMZ, temozolomide; BCNU, carmustine; ANC, absolute neutrophil count.
Table A2.
Outcome to Treatment With EGFR Tyrosine Kinase Inhibitors Erlotinib and Gefitinib in Glioblastoma
| Study by Treatment Agent | No. of Patients | Outcome Measure |
||
|---|---|---|---|---|
| Response Rate (%) | MTP (weeks) | 6-Month PFS (%) | ||
| Gefitinib | ||||
| Rich22 | 53 | NA | 8.1 | 13 |
| Lieberman* | 38 | 13 | 8 | 9 |
| Erlotinib | ||||
| Vogelbaum11 | 31 | 6 | NS | 26 |
| Raizer† | 31 | 0 | 12 | 0 |
| Cloughesy‡ | 48 | 8 | NS | 17 |
Abbreviations: EGFR, epidermal growth factor receptor; MTP, median time to progression; PFS, progression-free survival; NA, not applicable; NS, not stated.
Lieberman FS, Cloughesy T, Malkin MG: J Clin Oncol 22:105, 2003 (abstr 421).
Raizer JJ, Abrey LE, Wen P, et al: J Clin Oncol 40:107, 2004 (abstr 1502).
Cloughesy T, Yung A, Vrendenberg J, et al: J Clin Oncol 41:115s, 2005 (abstr 1507).
Footnotes
Supported in part by Hoffman-la Roche Ltd, Basel, Switzerland; by Grants No. 5U10 CA11488-34 through 2U10 CA11488-36 and 5U10 CA11488-38 from the National Cancer Institute; the European Organisation for Treatment of Cancer headquarters is supported by Fonds Cancer.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00086879.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Heidemarie Kletzl, Hoffman la Roche (C); Barbara Klughammer, Hoffman la Roche (C) Consultant or Advisory Role: Martin J. van den Bent, Hoffman la Roche (C) Stock Ownership: Barbara Klughammer, Hoffman la Roche Honoraria: Martin J. van den Bent, Hoffman la Roche Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Martin J. van den Bent, Alba A. Brandes, Barbara Klughammer, Denis Lacombe, Thierry Gorlia
Administrative support: Martin J. van den Bent, Denis Lacombe
Provision of study materials or patients: Martin J. van den Bent, Alba A. Brandes, Roy Rampling, Johan M. Kros, Antoine Carpentier, Paul M. Clement, Marc Frenay, Mario Campone, Jean Francois Baurain, Jean Paul Armand, Martin Taphoorn, Alicia Tosoni, Denis Lacombe
Collection and assembly of data: Martin J. van den Bent, Alba A. Brandes, Roy Rampling, Mathilde C.M. Kouwenhoven, Paul M. Clement, Jean Paul Armand, Denis Lacombe, Thierry Gorlia
Data analysis and interpretation: Martin J. van den Bent, Roy Rampling, Mathilde C.M. Kouwenhoven, Heidemarie Kletzl, Barbara Klughammer, Denis Lacombe, Thierry Gorlia
Manuscript writing: Martin J. van den Bent, Alba A. Brandes, Mathilde C.M. Kouwenhoven, Paul M. Clement, Martin Taphoorn, Heidemarie Kletzl, Barbara Klughammer, Thierry Gorlia
Final approval of manuscript: Martin J. van den Bent, Alba A. Brandes, Roy Rampling, Mathilde C.M. Kouwenhoven, Johan M. Kros, Antoine Carpentier, Paul M. Clement, Marc Frenay, Mario Campone, Jean Francois Baurain, Jean Paul Armand, Martin Taphoorn, Heidemarie Kletzl, Barbara Klughammer, Denis Lacombe, Thierry Gorlia
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 3.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 4.Frederick L, Eley G, Wang XY, et al. Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro Oncol. 2000;2:159–163. doi: 10.1093/neuonc/2.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Marie Y, Carpentier AF, Omuro AM, et al. EGFR tyrosine kinase domain mutations in human gliomas. Neurology. 2005;64:1444–1445. doi: 10.1212/01.WNL.0000158654.07080.B0. [DOI] [PubMed] [Google Scholar]
- 8.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: Tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 9.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 10.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelbaum MA, Peereboom D, Stevens GHJ, et al. Response rate to single agent therapy with the EGFR tyrosine kinase inhibitor erlotinib in recurrent glioblastoma multiforme: Results of a phase II study. Neuro Oncol. 2004;6:384. abstr TA-59. [Google Scholar]
- 12.Brandes AA, Tosoni A, Amista P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63:1281–1284. doi: 10.1212/01.wnl.0000140495.33615.ca. [DOI] [PubMed] [Google Scholar]
- 13.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 14.Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 15.Kouwenhoven MC, Kros JM, French PJ, et al. 1p/19q loss within oligodendroglioma is predictive for response to first line temozolomide but not to salvage treatment. Eur J Cancer. 2006;42:2499–2503. doi: 10.1016/j.ejca.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 17.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 18.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 19.Ling J, Fettner S, Lum BL, et al. Effect of food on the pharmacokinetics of erlotinib, an orally active epidermal growth factor receptor tyrosine-kinase inhibitor, in healthy individuals. Anticancer Drugs. 2008;19:209–216. doi: 10.1097/CAD.0b013e3282f2d8e4. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer. 2006;8(suppl 1):S7–S14. doi: 10.3816/clc.2006.s.008. [DOI] [PubMed] [Google Scholar]
- 21.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12:3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 22.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 23.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]