Abstract
Purpose
To assess rates and causes of mortality in patients with Wilms tumor (WT).
Methods
Through 2002, 6,185 patients enrolled onto the National Wilms Tumor Study between 1969 and 1995 were actively observed. Deaths were classified on the basis of medical records as the result of original disease, late effects (including second malignant neoplasms [SMNs], cardiac causes, pulmonary disease, and renal failure), or other causes. Standardized mortality ratios (SMRs) and Cox regression were used to assess the effects of sex, age, and calendar period of diagnosis on mortality.
Results
Within 5 years of WT diagnosis, 819 deaths occurred, and 159 deaths occurred among 4,972 known 5-year survivors. The SMR was 24.3 (95% CI, 22.6 to 26.0) for the first 5 years, was 12.6 (95% CI, 10.0 to 15.7) for the next 5 years, and remained greater than 3.0 thereafter. For deaths in the first 5 years, the mortality risk decreased by 5-year calendar period of diagnosis (rate ratio [RR] = 0.78 per period). No such trend occurred for later deaths. Among 5-year survivors, 62 deaths were attributed to late effects of treatment or disease, including 27 to SMNs. A trend of decreased risk with calendar period of diagnosis was observed for late-effects mortality (RR = 0.86; 95% CI, 0.67 to 1.10) and for SMN mortality (RR = 0.82; 95% CI, 0.55 to 1.21).
Conclusion
Although the survival outlook for WT patients has improved greatly over time, survivors remain at elevated risk for death many years after their original diagnosis.
INTRODUCTION
The treatment of Wilms tumor (WT) is one of the great success stories in childhood cancer. Thanks, in part, to therapeutic advances assessed in clinical trials of the National Wilms Tumor Study (NWTS), the 5-year relative survival percentage for children diagnosed with WT in the United States increased from approximately 70% in 1970 to 1973, to 92% in 1989 to 1996.1 For children diagnosed after 1990 with completely resected, localized disease of favorable histology (FH), 8-year survival was 95%.2
Several studies have documented increased risk of chronic health conditions and mortality in childhood cancer survivors.3–6 Mertens et al3 assessed overall and cause-specific mortality in a cohort of 20,227 5-year survivors from the Childhood Cancer Survivor Study (CCSS). Patients were diagnosed from 1970 to 1986 with a variety of malignant diseases. Relative to the US population, they experienced an almost 11-fold increase in mortality (standardized mortality ratio [SMR] = 10.8; 95% CI, 10.3 to 11.3). Among the 1,617 WT survivors in the CCSS cohort, 65 deaths were observed, yielding an SMR of 6.2 (95% CI, 4.8 to 7.9). In a recent update, the SMR for all childhood cancers was 8.2 (95% CI, 7.9 to 8.5).7 Causes of death were determined through proxy interviews and death certificates (DCs). The number of deaths as a result of subsequent cancers (SMR = 15.0), cardiac toxicity (SMR = 6.9), and pulmonary complications (SMR = 8.7) were all elevated.
The NWTS is uniquely positioned for additional examination of the long-term mortality experience of WT survivors. The NWTS cohort reported here includes more than 6,000 patients, 52% of whom were diagnosed after 1985. The NWTS maintains active follow-up starting at diagnosis, so information is available on deaths occurring before and after the 5-year point that typically defines entry into survivor cohorts. Because medical records are requested in all deaths, causes of death can be determined independently of and for comparison with DC information. Knowledge of long-term mortality risk is critically important to WT survivors, parents, and health care providers, so they can seek appropriate care and manage potential complications of the original cancer treatment.
METHODS
Study Population
Between 1969 and 1995, medical institutions in the United States enrolled 6,185 children age 15 years and younger with renal neoplasms onto one of four NWTS protocol studies.8–11 Approximately 90% of enrollees had WT of FH or anaplastic histology; the remainder had clear cell sarcoma of the kidney (CCSK), rhabdoid tumor of the kidney (RTK), or other rare histologic variants.12 The NWTS actively pursues follow-up for all surviving enrollees as part of a late-effects study.
The NWTS Late Effects Study protocol was approved by the institutional review board of the Fred Hutchinson Cancer Research Center (Seattle, WA), and the clinical trial protocols were approved by the institutional review board of each participating institution. Each patient's parent/guardian provided informed consent at enrollment. At age 18 years, each patient was contacted and asked to provide informed consent for continued participation as an adult.
Determination of Date and Cause of Death
In June 2003, as part of a methodologic study, identifying information for 984 known decedents and 3,406 patients whose vital status as of January 1, 2002, was unknown was submitted to the National Death Index (NDI). Results reported elsewhere have suggested that the NDI had substantially under-ascertained deaths among patients lost to follow-up by the NWTS, and thus the NDI could not be used to reliably fill in missing follow-up data.13 Therefore, in the current investigation, each patient's vital status and date of death, if applicable, as of January 1, 2002, were ascertained in April 2005 based only on NWTS follow-up records. Patients lost to follow-up before 2002 were censored in the analyses.
For deceased patients, the underlying cause of death was determined in two ways. First, NWTS investigators studying medical records carefully classified each death to one of three groups: original disease, in which deaths directly resulted from the original WT diagnosis, including acute effects of treatment (within 6 months) and recurrence of WT; late effects, in which deaths resulted from nonacute effects of treatment, including second malignant neoplasms (SMNs), congestive heart failure and other cardiac conditions, restrictive and other pulmonary disease, end-stage renal disease (ESRD), and other late conditions; and non–treatment-related events, in which deaths resulted from other medical conditions or external causes.
Second, cause of death was coded using International Classification of Diseases, Ninth Revision (ICD-9), codes on the basis of DC information. For patients with an NDI match, the underlying cause of death supplied by the NDI was used. For patients without an NDI match, DCs were requested directly from the states, and the underlying cause of death was coded by a trained nosologist. Deaths were grouped as follows: WT (ICD, 189.0), secondary or subsequent cancer (ICD, 140 to 239, excluding 189.0), cardiac causes (ICD, 390 to 398, 402, 404, and 410 to 429), pulmonary causes (ICD, 460 to 519), external causes (ICD, 800 to 999), and all other causes.
Statistical Analysis
SMRs comparing observed with expected numbers of deaths were used to quantify the relative risk of death from all causes in the first 5 years after diagnosis, and then separately among 5-year survivors. Patients entered these cohorts at WT diagnosis or 5 years after the date of diagnosis, respectively, and exited at the earliest of three dates—date of death, date of last follow-up, or January 1, 2002—or for the initial cohort, the date 5 years after diagnosis. Person-years at risk were calculated using the Lexis program14 available in the R statistical package (R software; R Foundation for Statistical Computing, Vienna, Austria).15 Expected numbers of deaths were computed using annual age-specific (5-year groups) and sex-specific US mortality rates obtained from the National Center for Health Statistics.16 SMRs were computed by sex, age at diagnosis (0 to 4, 5 to 9, and 10 to 15 years), calendar period of diagnosis (1969 to 1974, 1975 to 1979, 1980 to 1984, 1985 to 1989, and 1990 to 1995), time since diagnosis (0 to 4, 5 to 9, 10 to 14, 15 to 19, and 20 or more years), and histology (FH, anaplasia, CCSK, RTK, and other/unknown). CIs for the SMRs were computed using Byar's approximation.17 When SMRs were plotted, a locally fit second-degree polynomial curve was added.18
Cox proportional hazards regression, with date of WT diagnosis as time zero, was also used to quantify the relative risk of death. Multiple regression models with the factors listed in the previous paragraph were fit to examine the effects of each factor adjusted for the others, with baseline risk estimated internally from the data, rather than externally from population rates.17 To detect trends in death rates with regard to calendar period and age at diagnosis, additional models were fit treating these as grouped linear variables. For the cohort of 5-year survivors, additional models for cause-specific mortality were fit, with the date 5 years after WT diagnosis as time zero. The assumption of proportional hazards was tested using the cox.zph function in R, which computes the correlation between the Schoenfeld residuals and time for each covariate.19
RESULTS
All-Cause Mortality
Of the 6,185 patients included in this analysis, 4,972 (80%) were observed for 5 or more years after their date of diagnosis. There were 819 deaths observed in the first 5 years of follow-up, and 159 deaths observed after that. Of the remaining 5-year survivors, 2,870 (59.6%) were known to be alive on January 1, 2002. Dates of last contact were within the preceding periods of 0 to 5 and 5 to 10 years for 1,321 (27.4%) and 423 (8.8%) patients, respectively. The remaining 199 (4.1%) patients had been out of contact for more than 10 years. The median age at last contact was 17.6 years, with a range of 5.3 to 43.1 years.
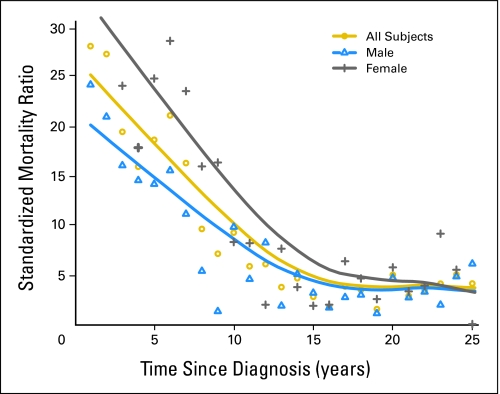
Numbers of observed and expected deaths and SMRs for all-cause mortality are listed in Table 1 for the first 5 years, and in Table 2 for the 5-year survivors. The SMR was 24.3 (95% CI, 22.6 to 26.0) for the first 5 years, and 12.6 (95% CI, 10.0 to 15.7) for years 5 to 10. Although the SMR continued to decline, it remained above 3.0 thereafter (Table 2; Fig 1). The SMR among 5-year survivors was higher for females, for those diagnosed at an older age, and for those diagnosed with anaplastic WT, CCSK, or RTK.
Table 1.
Relative Risk of Death From All Causes in First 5 Years After Wilms Tumor Diagnosis
| Characteristic | No. of Patients | Person-Years | No. of Deaths | No. of Expected Deaths | SMR* | 95% CI | RR† | 95% CI |
|---|---|---|---|---|---|---|---|---|
| All patients | 6,185 | 27,238 | 819 | 33.8 | 24.3 | 22.6 to 26.0 | ||
| Sex | ||||||||
| Male | 2,958 | 13,034 | 382 | 19.1 | 20.0 | 18.0 to 22.1 | 1.0 | |
| Female | 3,227 | 14,204 | 437 | 14.6 | 29.8 | 27.1 to 32.8 | 1.02 | 0.88 to 1.17 |
| Age at diagnosis, years | ||||||||
| 0-4 | 4,720 | 21,056 | 548 | 32.1 | 17.1 | 15.7 to 18.6 | 1.0 | |
| 5-9 | 1,279 | 5,409 | 226 | 1.4 | 165.1 | 144.3 to 188.1 | 1.62 | 1.38 to 1.90 |
| 10-15 | 186 | 774 | 45 | 0.3 | 131.8 | 96.1 to 176.4 | 2.14 | 1.57 to 2.91 |
| Year of diagnosis | ||||||||
| 1969-1974 | 557 | 2,336 | 112 | 4.4 | 25.7 | 21.1 to 30.9 | 1.0 | |
| 1975-1979 | 908 | 3,862 | 166 | 6.0 | 27.7 | 23.7 to 32.3 | 0.77 | 0.61 to 0.98 |
| 1980-1984 | 1,475 | 6,489 | 204 | 8.4 | 24.2 | 21.0 to 27.8 | 0.59 | 0.47 to 0.75 |
| 1985-1989 | 1,560 | 7,022 | 180 | 8.1 | 22.3 | 19.2 to 25.8 | 0.49 | 0.38 to 0.62 |
| 1990-1995 | 1,685 | 7,530 | 157 | 6.9 | 22.7 | 19.3 to 26.5 | 0.37 | 0.29 to 0.47 |
| Histology | ||||||||
| Favorable | 5,199 | 23,759 | 458 | 29.7 | 15.4 | 14.0 to 16.9 | 1.0 | |
| Anaplasia | 380 | 1,217 | 175 | 1.0 | 183.2 | 157.0 to 212.4 | 6.72 | 5.63 to 8.04 |
| CCSK | 200 | 844 | 56 | 1.3 | 43.9 | 33.1 to 57.0 | 3.44 | 2.60 to 4.56 |
| RTK | 105 | 176 | 80 | 0.3 | 229.6 | 182.1 to 285.8 | 23.07 | 18.04 to 29.48 |
| Other/unknown | 301 | 1,242 | 50 | 1.5 | 33.5 | 24.9 to 44.2 | 1.73 | 1.29 to 2.32 |
Abbreviations: SMR, standardized mortality ratio; RR, rate ratio; CCSK, clear cell sarcoma of the kidney; RTK, rhabdoid tumor of the kidney.
Estimated SMRs are univariate.
RRs come from a joint Cox proportional hazards model including all covariates shown.
Table 2.
Relative Risks of Death From All Causes Among 5-Year Survivors of Wilms Tumor
| Characteristic | No. of Patients | Person-Years | No. of Deaths | Expected Deaths | SMR* | 95% CI | RR† | 95% CI |
|---|---|---|---|---|---|---|---|---|
| All patients | 4,972 | 49,928 | 159 | 26.7 | 6.0 | 5.1 to 7.0 | ||
| Sex | ||||||||
| Male | 2,372 | 23,726 | 83 | 17.9 | 4.6 | 3.7 to 5.8 | 1.0 | |
| Female | 2,600 | 26,202 | 76 | 8.8 | 8.6 | 6.8 to 10.8 | 0.79 | 0.58 to 1.08 |
| Age at diagnosis, years | ||||||||
| 0-4 | 3874 | 39,223 | 98 | 19.1 | 5.1 | 4.2 to 6.3 | 1.0 | |
| 5-9 | 971 | 9,503 | 46 | 6.4 | 7.2 | 5.2 to 9.6 | 1.96 | 1.38 to 2.80 |
| 10-15 | 127 | 1,202 | 15 | 1.2 | 12.7 | 7.1 to 20.9 | 5.01 | 2.90 to 8.66 |
| Year of diagnosis | ||||||||
| 1969-1974 | 436 | 8,845 | 31 | 7.0 | 4.4 | 3.0 to 6.3 | 1.0 | |
| 1975-1979 | 700 | 11,081 | 37 | 7.3 | 5.1 | 3.6 to 7.0 | 1.05 | 0.63 to 1.76 |
| 1980-1984 | 1,203 | 14,423 | 42 | 7.5 | 5.6 | 4.0 to 7.5 | 1.04 | 0.61 to 1.76 |
| 1985-1989 | 1,296 | 10,214 | 28 | 3.6 | 7.8 | 5.2 to 11.3 | 0.93 | 0.51 to 1.67 |
| 1990-1995 | 1,337 | 5,366 | 21 | 1.3 | 16.6 | 10.3 to 25.4 | 1.11 | 0.58 to 2.12 |
| Survival after diagnosis, years | ||||||||
| 5-9 | 4,972 | 22,302 | 79 | 6.3 | 12.6 | 10.0 to 15.7 | ||
| 10-14 | 3,694 | 14,609 | 36 | 8.0 | 4.5 | 3.2 to 6.2 | ||
| 15-19 | 2,190 | 8,113 | 22 | 7.3 | 3.0 | 1.9 to 4.6 | ||
| 20 or more | 1,092 | 4,905 | 22 | 5.2 | 4.3 | 2.7 to 6.5 | ||
| Histology | ||||||||
| Favorable | 4409 | 44,213 | 129 | 23.6 | 5.5 | 4.6 to 6.5 | 1.0 | |
| Anaplasia | 192 | 1739 | 9 | 0.9 | 10.4 | 4.7 to 19.7 | 1.57 | 0.80 to 3.11 |
| CCSK | 137 | 1297 | 8 | 0.7 | 11.4 | 4.9 to 22.4 | 2.26 | 1.10 to 4.65 |
| RTK | 24 | 226 | 1 | 0.1 | 10.1 | 0.1 to 55.9 | 1.91 | 0.27 to 13.71 |
| Other/unknown | 210 | 2453 | 12 | 1.4 | 8.7 | 4.5 to 15.2 | 1.61 | 0.89 to 2.92 |
Abbreviations: SMR, standardized mortality ratio; RR, rate ratio; CCSK, clear cell sarcoma of the kidney; RTK, rhabdoid tumor of the kidney.
Estimated SMRs are univariate.
RRs come from a joint Cox proportional hazards model including all covariates shown.
Fig 1.
Standardized mortality ratios (SMRs) for all-cause mortality by time since diagnosis. The points represent yearly SMR estimates, and the lines are best-fit polynomial curves for all patients, males, and females.
The joint Cox regression did not suggest an increased number of deaths among males; however, an increase was seen among patients diagnosed at an older age. Estimated rate ratios (RRs) decreased with calendar period of diagnosis for deaths occurring in years 0 to 5 (Table 1), but not for later deaths (Table 2). RRs with calendar period of diagnosis as a grouped linear variable were 0.78 (95% CI, 0.74 to 0.82) for years 0 to 5, and 1.00 (95% CI, 0.87 to 1.16) thereafter. The fitted baseline hazard (for males diagnosed with FH WT at age 0 to 4 years in 1969 to 1974) indicated an annual death rate of approximately 5% in years 0 to 2, 1.5% in years 3 to 4, and 0.2% in years 5 to 20.
Cause-Specific Mortality
Table 3 compares causes of death coded by the NWTS with those coded on the basis of DCs. Of the 887 deaths categorized by both methods, 744 (83.9%) agreed on cause. Among 5-year survivors, the rate of agreement was 70% (100 of 143). Frequently, however, deaths considered a result of the original disease by the NWTS were categorized differently on DCs. Of 17 deaths attributed to pulmonary causes on DCs, 13 were classified by the NWTS as resulting from acute toxicity or infection in WT treatment. Of 37 deaths attributed by the NWTS to WT and on the DC to other cancers, 21 had ICD codes for malignant neoplasms without specification of site, or of unspecific nature.
Table 3.
Comparison of Cause of Death Groupings Based on NWTS Coding and Death Certificate Information for All Subjects
| NWTS-Coded Cause of Death | Cause of Death Grouped on the Basis of Death Certificate ICD Underlying Cause Codes (No. of Deaths) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Wilms Tumor | Other Cancers | Cardiac Causes | Pulmonary Causes | External Causes | All Other Causes | Missing | Total | |
| Original disease | 672 | 37 | 4 | 16 | 2 | 33 | 71 | 835 |
| Late effects | ||||||||
| SMN | 4 | 31 | 0 | 0 | 0 | 1 | 2 | 38 |
| CHF | 10 | 0 | 7 | 1 | 0 | 0 | 2 | 20 |
| ESRD | 9 | 0 | 2 | 0 | 0 | 7 | 0 | 18 |
| Pulmonary | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Other late effects | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 5 |
| Non–treatment-related cause | ||||||||
| External | 1 | 0 | 1 | 0 | 20 | 0 | 1 | 23 |
| Medical | 1 | 0 | 3 | 0 | 3 | 14 | 8 | 29 |
| Unknown | 3 | 0 | 0 | 0 | 2 | 2 | 0 | 7 |
| Total | 703 | 69 | 17 | 17 | 27 | 61 | 84 | 978 |
Abbreviations: NWTS, National Wilms Tumor Study; ICD, International Classification of Diseases; SMN, second malignant neoplasm; CHF, congestive heart failure; ESRD, end-stage renal disease.
Table 4 lists frequencies of NWTS-coded specific causes of death. Of 978 deaths, 835 (85.4%) were attributed to original disease, 84 (8.6%) to late effects of treatment, and 52 (5.3%) to non–treatment-related causes. For seven deaths (0.7%), cause of death could not be determined. Most deaths (819, or 91.0%) occurred during the first 5 years, including 771 attributed to original disease, 11 to SMNs, six to cardiac causes, five to ESRD, seven to external causes, and one to an unknown cause. Of the 159 deaths that occurred among 5-year survivors, a much lower proportion were attributed to original disease (64, or 40.2%), whereas the proportion of deaths attributed to late effects of treatment (62, or 39.0%), non–treatment-related causes (27, or 17.0%), and unknown causes (six, or 3.8%) increased. Common sites for fatal SMNs among 5-year survivors included the brain and other parts of the nervous system, the digestive organs and peritoneum, and the lymphatic and hematopoietic systems.
Table 4.
Specific Causes of Death As Determined by the NWTS
| NWTS-Coded Cause of Death | No. of Deaths |
|
|---|---|---|
| First 5 Years | 5-Year Survivors | |
| Original disease | 771 | 64 |
| Late effects | ||
| Subsequent malignant neoplasms | 11 | 27 |
| Bone and articular cartilage | 0 | 4 |
| Brain and other parts of nervous system | 3 | 5 |
| Breast | 0 | 1 |
| Connective and other soft tissue | 0 | 1 |
| Digestive organs and peritoneum | 0 | 10 |
| Genitourinary organs | 0 | 1 |
| Lymphatic and hematopoietic | 8 | 5 |
| Cardiac | 6 | 14 |
| Cardiomyopathy | 3 | 8 |
| CHF | 3 | 6 |
| ESRD | 5 | 13 |
| Pulmonary | 0 | 3 |
| Pulmonary fibrosis | 0 | 2 |
| Other pulmonary | 0 | 1 |
| Other late effects | 0 | 5 |
| Non–treatment-related cause | ||
| External | 7 | 16 |
| Motor vehicle accident | 2 | 10 |
| Homicide | 1 | 4 |
| Suicide | 0 | 1 |
| Other accident | 4 | 1 |
| Medical | 18 | 11 |
| HIV | 0 | 1 |
| Pneumonia | 1 | 0 |
| Other bacterial/viral infection | 3 | 2 |
| Heart disease | 5 | 3 |
| Cerebrovascular disease | 1 | 1 |
| Other medical condition | 8 | 4 |
| Unknown | 1 | 6 |
| Total* | 819 | 159 |
Abbreviations: NWTS, National Wilms Tumor Study; CHF, congestive heart failure; ESRD, end-stage renal disease.
Totals do not reflect the sum of all data in the column. Some deaths are counted once by their primary type and once by their subtype.
Table 5 lists observed and expected numbers of deaths and RRs for all-cause, late-effect, and SMN mortalities among 5-year survivors. The SMN mortality among females was less than half that among males (RR = 0.43; 95% CI, 0.19 to 0.96). For all three cause categories, there was a downward trend in adjusted risk with calendar period of diagnosis, and an upward trend with age at diagnosis, but only the latter was statistically significant. When period of diagnosis was included in the model as a grouped linear variable, the estimated RRs were 1.00 (95% CI, 0.87 to 1.16) per 5-year period for all-cause mortality, 0.86 (95% CI, 0.67 to 1.10) for late-effects mortality, and 0.82 (95% CI, 0.55 to 1.21) for SMN mortality. When age at diagnosis was included in the model as a grouped linear variable, the estimated RRs were 2.14 (95% CI, 1.68 to 2.72) per period for all-cause mortality, 2.00 (95% CI, 1.35 to 2.97) for late-effects mortality, and 2.56 (95% CI, 1.48 to 4.44) for SMN mortality. None of the global tests of proportionality for all covariates in a given model were statistically significant. Specific tests for interaction between time and age at diagnosis and between time and calendar period of diagnosis were not statistically significant. Hence there is no evidence to suggest that the RRs presented in Table 5 vary over the length of follow-up.
Table 5.
Relative Rates of Mortality From All Causes, Late Effects, and SMNs Among 5-Year Survivors
| Characteristic | No. of Patients | Cause of Death |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Causes |
Effects of Treatment |
SMNs |
||||||||
| No. of Deaths | RR | 95% CI | No. of Deaths | RR | 95% CI | No. of Deaths | RR | 95% CI | ||
| Total | 4,972 | 159 | 62 | 27 | ||||||
| Sex | ||||||||||
| Male | 2,372 | 83 | 1.00 | 30 | 1.00 | 18 | 1.00 | |||
| Female | 2,600 | 76 | 0.79 | 0.58 to 1.08 | 32 | 0.93 | 0.57 to 1.54 | 9 | 0.43 | 0.19 to 0.96 |
| Age at diagnosis, years | ||||||||||
| 0-4 | 3,874 | 98 | 1.00 | 38 | 1.00 | 14 | 1.00 | |||
| 5-9 | 971 | 46 | 1.97 | 1.39 to 2.80 | 20 | 2.23 | 1.30 to 3.84 | 11 | 3.46 | 1.57 to 7.64 |
| 10-15 | 127 | 15 | 5.03 | 2.91 to 8.67 | 4 | 3.38 | 1.20 to 9.50 | 2 | 4.57 | 1.03 to 20.17 |
| Year of diagnosis | ||||||||||
| 1969-1974 | 436 | 21 | 1.00 | 18 | 1.00 | 9 | 1.00 | |||
| 1975-1979 | 700 | 37 | 1.08 | 0.65 to 1.81 | 18 | 1.07 | 0.52 to 2.21 | 8 | 0.79 | 0.29 to 2.19 |
| 1980-1984 | 1,203 | 42 | 1.05 | 0.62 to 1.77 | 15 | 0.83 | 0.37 to 1.86 | 6 | 0.59 | 0.19 to 1.84 |
| 1985-1989 | 1,296 | 28 | 0.93 | 0.52 to 1.68 | 7 | 0.57 | 0.21 to 1.56 | 2 | 0.36 | 0.07 to 1.90 |
| 1990-1995 | 1,337 | 21 | 1.13 | 0.59 to 2.15 | 4 | 0.65 | 0.19 to 2.22 | 2 | 0.76 | 0.13 to 4.49 |
Abbreviations: SMN, second malignant neoplasm; RR, rate ratio.
DISCUSSION
This study was based on the cohort of NWTS patients diagnosed in the United States between 1969 and 1995. Because the NWTS strives to maintain active follow-up with all participants, and independently determines cause of death through medical records, it is well suited to assess the mortality experience of WT. In the first 5 years after diagnosis, children with WT experienced a 24-fold higher risk of death than did the general population. The excess risk persisted among 5-year survivors at a level almost 13-fold the level among the general population for the next 5 years, and at a level three- to four-fold higher for 15 years after that. The overall SMR for 5-year survivors was 6.0 (95% CI, 5.1 to 7.0), consistent with the CCSS estimate of 6.2 (95% CI, 4.8 to 7.9).3
The estimated SMRs listed in Tables 1 and 2 were univariate and based on external standardization, whereas the RRs came from a multiple regression model using internal standardization.17 The two sets of results are best interpreted together. Among 5-year survivors, the SMR for females was almost double that of males. However, the RR of 0.79 suggests that after adjustment for other variables, females have lower death rates than do males. The SMR for females was higher because standard death rates for males are nearly double those for females in the teenage and early adult years, in which most of this cohort's follow-up occurred.
In general, patients diagnosed in early childhood fared better than did those diagnosed later. The number of deaths (32.1) expected in the 5 years after diagnosis for those diagnosed at age 0 to 4 years is likely inflated, because standard rates for this group included a large number of deaths occurring before age 12 months. Because most WT diagnoses occur after this age, the SMR of 17.1 is likely an underestimate. This explains the discrepancy between the ratios of SMRs by age at diagnosis and the RRs.
In the first 5 years of follow-up, SMRs and RRs for all-cause mortality showed a downward trend with year of diagnosis; however, a similar trend was not seen among 5-year survivors. This confirms that advances in treatment over the course of the NTWS have led to increased survival overall, but additional advances may be necessary to improve long-term survival rates among those who are cured. The SMR of 16.6 observed for the 5-year survivors diagnosed in 1990 to 1995 is likely inflated, because available follow-up was concentrated in the period immediately after the 5-year point after diagnosis.20
Underlying cause of death was determined using two methods. The first was based on careful evaluation of family reports, physician reports, and medical records. The second was based on ICD causes of death coded on the basis of DCs. Previous studies have found a high prevalence of errors in the causes of death listed on DCs.21–24 The only category with reasonably good agreement between these two methods was external causes of death. Because 69 deaths attributed to other cancers were identified in classification on the basis of DCs—many more than the 38 identified by the NWTS—the use of DC data alone would have grossly inflated the SMR for SMNs. However, virtually all of the discrepancies were concentrated in the first 5 years. Nonetheless, this level of discrepancy should serve as a warning for studies that rely solely on DCs to determine underlying cause of death.
The great majority of patients who survived fewer than 5 years died as a result of progressive disease or acute effects of treatment. However, 22 late-effects deaths were observed in this period: 11 caused by SMNs (eight leukemias), six caused by congestive heart failure, and five caused by ESRD, three of which occurred in patients with Denys-Drash syndrome.
A final analysis based on the 5-year survivors suggests that the risk of mortality caused by late effects of treatment and SMNs may be lower for patients diagnosed in recent years. Although the trends were not statistically significant, they provide preliminary evidence in support of the NWTS philosophy of reducing use of radiation and chemotherapy over the course of the four clinical trials to the minimal levels needed for cure. The analysis was limited by relatively small numbers of cause-specific deaths, and the fact that the length of follow-up for patients diagnosed in recent periods was short. The estimated RRs for recently diagnosed participants were based on deaths that occurred relatively early in follow-up. For those diagnosed in 1990 to 1995, for example, only deaths that occurred within 5 to 12 years of WT diagnosis contributed to the RR comparing their mortality with that of participants diagnosed earlier. The rates for late effects or SMNs with a long latency period cannot yet be compared with calendar period of diagnosis. Additional studies and continued follow-up of the cohort will be needed to confirm that currently observed trends persist.
Survivors of WT have more favorable long-term survival prospects than do survivors of many other types of childhood cancer. However, their risk of death, in particular, as a result of late effects of treatment including SMNs, remains elevated even 20 years after diagnosis. It is important for survivors and their health care providers to understand these risks. It can be anticipated that with additional advances in treatment for both newly diagnosed WT and late effects of therapy, long-term survival outcomes will continue to improve.
Acknowledgment
We thank the investigators of the Pediatric Oncology Group and the Children's Cancer Group, and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children enrolled onto the National Wilms Tumor Studies.
We acknowledge the vital record offices of Alabama, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming, and Washington, DC, for providing death certificates.
In addition, we acknowledge the following disclaimer: these data were supplied by the Health Statistics Section of the Colorado Department of Public Health and Environment, which specifically disclaims responsibility for any analyses, interpretations, or conclusions it has not provided.
Footnotes
Supported by National Institutes of Health Grant No. 2RO1CA54498.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Norman E. Breslow
Financial support: Norman E. Breslow
Administrative support: Norman E. Breslow
Collection and assembly of data: Cecilia A. Cotton, Susan Peterson, Patricia A. Norkool, Janice Takashima, Yevgeny Grigoriev
Data analysis and interpretation: Cecilia A. Cotton, Susan Peterson, Patricia A. Norkool, Janice Takashima, Norman E. Breslow
Manuscript writing: Cecilia A. Cotton, Norman E. Breslow
Final approval of manuscript: Cecilia A. Cotton, Susan Peterson, Patricia A. Norkool, Janice Takashima, Yevgeny Grigoriev, Norman E. Breslow
REFERENCES
- 1.Ries LA. Cancer rates. In: Harras A, Edwards BK, Blot WJ, et al., editors. Cancer: Rates and Risks. Bethesda, MD: National Cancer Institute; 1996. pp. 9–54. [Google Scholar]
- 2.Green DM. The treatment of stages I-IV favorable histology Wilms tumor. J Clin Oncol. 2004;22:1366–1372. doi: 10.1200/JCO.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 4.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 5.Cardous-Ubbink MC, Heinen RC, Langeveld NE, et al. Long-term cause-specific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004;42:563–573. doi: 10.1002/pbc.20028. [DOI] [PubMed] [Google Scholar]
- 6.MacArthur AC, Spinelli JJ, Rogers PC, et al. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48:460–467. doi: 10.1002/pbc.20922. [DOI] [PubMed] [Google Scholar]
- 7.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007;48:723–726. doi: 10.1002/pbc.21114. [DOI] [PubMed] [Google Scholar]
- 8.D'Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms tumor: Results of the National Wilms Tumor Study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.D'Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms tumor: Results of the Second National Wilms Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.D'Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms tumor: Results of the Third National Wilms Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms tumor. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 12.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: Results from the First National Wilms Tumor Study. Cancer. 1978;41:1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Cotton CA, Peterson S, Norkool PA, et al. Mortality ascertainment of participants in the National Wilms Tumor Study using the National Death Index: Comparison of active and passive follow-up results. Epidemiol Perpect Innov. 2007;4:5. doi: 10.1186/1742-5573-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Public Health, University of Copenhagen. Epi package for epidemiological analysis in R. http://staff.pubhealth.ku.dk/∼bxc/Epi/
- 15.The R Foundation for Statistical Computing. http://www.r-project.org/
- 16.National Center for Health Statistics, Centers for Disease Control and Prevention. Faststats on deaths/mortality. http://www.cdc.gov/nchs/fastats/deaths.htm.
- 17.Breslow NE, Day NE. Lyon, France: International Agency for Research on Cancer; 1987. Statistical Methods in Cancer Research, Volume 2: The Design and Analysis of Cohort Studies. pp. 69–72.pp. 103–106. [PubMed] [Google Scholar]
- 18.Cleveland WS, Grosse E, Shyu WM. Local regression models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Pacific Grove, CA: Wadsworth & Brooks/Cole; 1992. pp. 309–376. [Google Scholar]
- 19.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 20.Yasui Y, Whitton J. Problems in using age-stratum-specific reference rates for indirect standardization. J Clin Epidemiol. 1999;52:393–398. doi: 10.1016/s0895-4356(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 21.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;161:277–284. doi: 10.1001/archinte.161.2.277. [DOI] [PubMed] [Google Scholar]
- 22.Percy C, Stanek E, III, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 31:242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sington JD, Cottrell BJ. Analysis of the sensitivity of death certificates in 440 hospital deaths: A comparison with necropsy findings. J Clin Pathol. 2002;55:499–502. doi: 10.1136/jcp.55.7.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritt BS, Hardin NJ, Richmond JA, et al. Death certification errors at an academic institution. Arch Pathol Lab Med. 2005;129:1476–1479. doi: 10.5858/2005-129-1476-DCEAAA. [DOI] [PubMed] [Google Scholar]