Abstract
Objectives. We tested the effect of aerobic exercise on autonomic regulation of the heart in healthy young adults.
Methods. Healthy, sedentary young adults (n = 149; age = 30.4 ± 7.53 years) were randomized to receive 12 weeks of either aerobic conditioning or strength training. Primary outcomes were heart rate and RR interval variability (RRV) measured before and after training and after 4 weeks of sedentary deconditioning. RRV, a noninvasive index of cardiac autonomic regulation, reflects variability in the intervals between consecutive R waves of the electrocardiogram.
Results. Aerobic conditioning but not strength training led to a significant increase in aerobic capacity (3.11 mL/kg/min), a decrease in heart rate (−3.49 beats per minute), and an increase in high-frequency RRV (0.25 natural log msec2), each of which returned to pretraining levels after deconditioning. Significant 3-way interactions, however, revealed autonomic effects only in men.
Conclusions. In sedentary, healthy young adults, aerobic conditioning but not strength training enhances autonomic control of the heart, but post hoc analyses suggested that gender plays a significant role in this exercise-related cardioprotection.
The public health benefits of physical exercise, especially for cardioprotection, are widely accepted, and consensus panels consistently have recommended exercise across the age spectrum as a central activity of a healthy life.1–3 Among the many biological mechanisms proposed to account for this risk-reducing effect is autonomic nervous system regulation of the heart.
The role of the autonomic nervous system in cardioprotection is well established. Autonomic regulation of the heart may be measured noninvasively as variation in the time series of intervals between consecutive R waves (the representation of depolarization of the ventricles) in the electrocardiogram. RR interval variability (RRV) at high frequencies (0.15–0.50 Hz) reflects cardiac parasympathetic modulation, and variability at lower frequencies (0.04–0.15 Hz) reflects both sympathetic and parasympathetic influences in the heart. RRV is a strong prognostic indicator for the development of cardiovascular disease and death in community-dwelling nonclinical populations4,5 and for progression of heart disease in patients,6 suggesting a role for the autonomic nervous system in the pathophysiology of coronary artery disease. Low levels of RRV also predict death after acute myocardial infarction7,8 and heart failure,9 consistent with the hypothesis that increased cardiac parasympathetic nervous system regulation protects against arrhythmic death.10
Most studies report that aerobic conditioning enhances autonomic control of the heart, as indicated by training-induced reductions in heart rate or increases in RRV, but the evidence is only partially consistent with training-induced autonomic benefits. Many studies report no effect of training or no difference between trained and sedentary participants in heart rate11–13 or RRV.14–17 Cross-sectional studies often contrast highly trained athletes with sedentary controls, raising the possibility of self-selection biases. Longitudinal studies of training often have only a small number of participants15,18 or lack a control group,19 and many include men only.18,20–24
To address these concerns, we contrasted the cardiac autonomic effect of aerobic conditioning with that of strength training in a large sample of healthy young men and women. We hypothesized that cardiac autonomic regulation would be improved by aerobic conditioning but not strength training.
METHODS
We conducted a randomized controlled trial of the effect of aerobic conditioning versus strength training on RRV. We sought healthy, sedentary young adults, aged 18 to 45 years.
Recruitment and Eligibility
Participants were recruited by flyers posted on the campuses of Columbia and St John's universities. Respondents were eligible if they did not exercise regularly and if they did not exceed American Heart Association standards for average fitness (VO2max [maximum oxygen uptake] ≤ 43 for men and ≤ 37 mL/kg/min for women). Exclusion criteria included current symptoms of affective disorder, psychosis, or substance abuse; current use of psychotropic medication; and any medical condition that affected the autonomic nervous system or cardiovascular system. (A protocol flow diagram is available as a supplement to the online version of this article at http://www.ajph.org.)
We received 1195 phone calls in response to the recruitment flyers. Written informed consent was provided by 412 respondents; 263 were disqualified because they had scheduling difficulties, exceeded VO2max criteria, or had resting systolic blood pressure higher than 140 mm Hg or frequent premature ventricular contractions (> 6/min) during VO2max testing. The remaining 149 participants were randomized to either the aerobic conditioning (n = 74) or strength training (n = 75) group. Data collection began in December 1998 and ended in January 2003.
Experimental Protocol
After phone screening to determine eligibility, we assessed maximum aerobic fitness (VO2max) by a graded exercise test on an Ergoline 800S electronic-braked cycle ergometer (SensorMedics Corp, Anaheim, CA). Each participant began exercising at 30 watts for 2 minutes, and the work rate was increased by 30 watts every 2 minutes until maximum exercise was achieved (respiratory quotient ≥ 1.1, increases in ventilation without concomitant increases in VO2, maximum age-predicted heart rate, or volitional fatigue). Minute ventilation was measured by a pneumotachometer connected to a FLO-1 volume transducer module (PHYSIO-DYNE Instrument Corp, Quogue, NY). We measured the percentage of expired oxygen and carbon dioxide with paramagnetic oxygen and infrared carbon dioxide analyzers connected to a computerized system (MAX-1, PHYSIO-DYNE Instrument Corp) and calibrated them against known medical-grade gases. The highest VO2 value attained during the graded exercise test was considered VO2max.25
Respondents meeting inclusion criteria were randomized to an aerobic conditioning or strength training group. Both programs lasted 12 weeks, and before training, all participants met individually with a trainer to review their exercise regimens. After that, they exercised on their own, 3 to 4 times per week, in designated facilities. They were permitted to construct individualized exercise programs if they met the study's criteria. Adherence to training programs was documented by weekly logs and computerized attendance records. During weekly phone contacts, participants’ training progress was monitored, and they received additional supervision as needed and motivational support to adhere to their regimen.
After completion of training, participants returned for posttraining VO2max and laboratory testing, then began 4 weeks of sedentary deconditioning, during which they were instructed to abstain completely from any form of exercise. After deconditioning, they returned for a final testing session. Data collection staff were blinded to training group assignment. Each participant received a 6-month membership in a fitness facility and $300 for participation in the study.
Conditioning Programs
Aerobic conditioning.
Participants chose from a selection of activities, such as cycling on a stationary ergometer, running on a treadmill, or using a stair-climbing machine. They were instructed to exercise at 70% of their maximum heart rate (220 − age for men, 226 − age for women). They were given an initial goal of at least 20 minutes of aerobic exercise per session, and they increased duration gradually over 2 to 3 weeks, up to 45 to 60 minutes. A trainer helped participants choose a starting workload setting for each machine, and participants were instructed to increase the workload over time when they felt able, all the while maintaining their heart rate at 70% of maximum throughout the session. Participants measured their heart rates manually by palpation.
Strength training.
At the initial session, participants established a level of effort that permitted them to complete 3 sets of 10 repetitions for each of the following exercises: bench presses, shoulder presses, quadriceps extensions, biceps curls, lateral pulls, triceps presses, and hamstring curls. They were instructed to increase the difficulty of these exercises by adding 5 pounds every 2 weeks.
Measurements
During laboratory testing, 10 minutes of continuous electrocardiogram and respiration were recorded during quiet rest in both the seated and supine positions, as well as in response to laboratory challenges. Challenge data will be reported in another paper. We placed electrodes on the right shoulder, on the left anterior axillary line at the 10th intercostal space, and in the right lower quadrant. Analog electrocardiogram signals were digitized at 500 Hz by a National Instruments (Austin, TX) 16-bit analog-to-digital conversion board and passed to a microcomputer. We then submitted the electrocardiogram waveform to an R wave detection routine implemented by custom-written event detection software, resulting in an RR interval series. Errors in marking of R waves were corrected by visual inspection. Ectopic beats were corrected by interpolation.
We computed mean heart rate, the standard deviation of the RR interval (SDRR), and spectral power in the low-frequency (0.04–0.15 Hz) and high-frequency (0.15–0.50 Hz) bands. We calculated spectra on 300-second epochs with an interval method for computing Fourier transforms similar to that described by DeBoer et al.26 Prior to computing Fourier transforms, we subtracted the mean of the RR interval series from each value in the series, then filtered the series with a Hanning window,27 and summed the power (i.e., variance, in msec2) over the low-frequency and high-frequency bands. Estimates of spectral power were adjusted to account for attenuation produced by this filter.27
We collected thoracic and abdominal respiration signals with a Respitrace monitor (Ambulatory Monitoring, Ardsley, NY). We submitted signals to a specially written respiration-scoring program that produced minute-by-minute means of respiratory rate.
Statistical Analysis
For both the seated and supine 10-minute resting periods, we averaged data from each 300-second epoch of analysis to create a single value. All indices of RRV were log transformed prior to statistical analysis.
Statistical power calculations initially specified that 126 participants would be required to detect an effect size of 0.50 SDs, with power of 0.80 and an α level of .05, assuming a 20% dropout rate during active training. However, because of a higher-than-expected dropout rate early in the study, we increased the enrollment target to 150 participants.
We analyzed data according to intention-to-treat principles. Thus, all participants randomized to either treatment condition were included in the statistical analysis regardless of whether they completed the study. Data from study dropouts were treated as missing at random. We used random-effects models to determine the association between the group assignment and the outcome variables, after correcting for important covariates. We used an unstructured covariance matrix to model the correlation among repeated measures. We selected this matrix according to the Akaike criterion.28
We fitted measures of heart rate and RRV independently in separate models. We chose physical position (seated or supine), training group assignment, measurement session (baseline, training, deconditioning), and the group × session interaction as the primary predictors. Other covariates included gender, age, and body mass index. Analyses of the effect of the training interventions were based on the covariate-adjusted full model. We predicted that the group × session interaction would be significant for all indices of RRV, indicating that the aerobic conditioning but not the strength training would enhance cardiac autonomic regulation.
For all main effects, we considered an α level of less than .05 to be statistically significant. As suggested by Fleiss, an α level of less than .10 was accepted as statistically significant for interactions.29
RESULTS
We randomized and tested 149 healthy, nonsmoking adults (58 men and 91 women) before training. The mean age was 30.4 years (range = 18–45 years; SD = 7.53). Demographic and physical characteristics of participants prior to training are presented in Table 1. A total of 102 participants completed training, and 88 completed deconditioning. Dropouts were significantly younger (27.9 versus 32.2 years; P < .001) and had marginally greater baseline aerobic capacity (34.7 versus 32.8 mL/kg/min; P = .067) than did those who completed the study. Those who completed deconditioning and dropouts did not differ in other demographic and physical characteristics.
TABLE 1.
Baseline Demographic and Physical Characteristics of Study Participants (N = 149)
| Aerobic-Conditioning Group |
Strength-Training Group |
|||
| Men, Mean (SD) | Women, Mean (SD) | Men, Mean (SD) | Women, Mean (SD) | |
| Age, y | 30.43 (6.27) | 29.48 (7.63) | 30.89 (7.62) | 31.04 (8.23) |
| Weight, lb | 177.77 (29.57) | 141.14 (25.61) | 183.14 (33.21) | 138.74 (25.96) |
| Height, in | 69.73 (2.53) | 64.57 (3.01) | 69.07 (3.27) | 63.94 (3.55) |
| BMI, kg/m2 | 25.69 (3.48) | 23.86 (3.82) | 27.02 (4.25) | 23.87 (3.61) |
| Hear rate, bpm | 72.53 (9.06) | 69.50 (8.76) | 69.21 (9.34) | 72.82 (8.54) |
| VO2max (ml/kg/min) | 37.14 (6.47) | 31.94 (5.26) | 35.70 (5.08) | 31.48 (5.21) |
Note. BMI = body mass index; VO2max = maximum oxygen uptake. In the aerobic-conditioning group, the sample size for men was n = 30 and for women was n = 44. In the strength-training group, the sample size for men was n = 28 and for women was n = 47.
Effect of Training
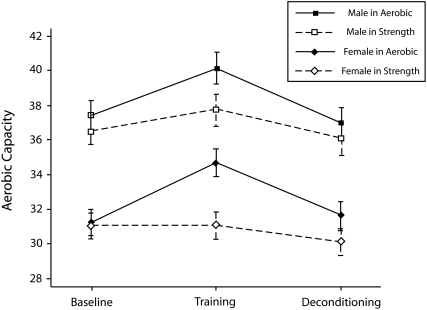
Analysis revealed significant effects of group assignment (F1,503 = 3.83; P < .001), testing session (F2,503 = 68.95; P < .001), gender (F1,503 = 46.16; P < .001), body mass index (F1,503 = 15.20; P = .001), and age (F1,503 = 8.84; P < .001) on aerobic capacity. Women had lower VO2max than did men, which was consistent with the gender differences in the Heart Association fitness standards used as inclusion criteria. Body mass index and age were inversely related to VO2max. Most importantly, the group × session interaction was highly significant (F2,503 = 26.80; P < .001). Aerobic capacity increased after training and decreased after deconditioning only in the aerobic-conditioning group (Figure 1).
FIGURE 1.
The impact of aerobic conditioning versus strength training on aerobic capacity tested at baseline, immediately after training, and after sedentary deconditioning.
Note. VO2max = maximum oxygen uptake. Data were derived from adjusted means from regression models.
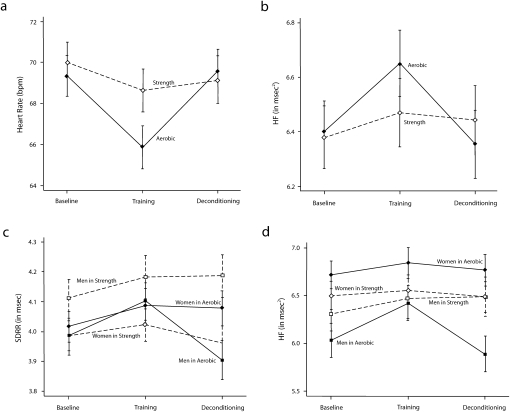
We observed a significant training group × session interaction for heart rate and high-frequency power (Table 2). In the aerobic-conditioning group, heart rate fell 3.49 beats per minute (bpm; 95% confidence interval [CI] = −4.74, −2.24; Figure 2) and high-frequency power rose 0.25 natural log (ln) msec2 (95% CI = 0.09, 0.41; Figure 2) from baseline to training and then returned to baseline levels after sedentary deconditioning (heart rate = 3.69 bpm; 95% CI = 2.23, 5.04; high frequency = −0.30 ln msec2; 95% CI = −0.48, −0.12). The group × session interactions for all other variables failed to achieve statistical significance. We controlled for respiratory rate, which slightly attenuated the group × session interaction for high-frequency power.
TABLE 2.
F Values From Regression Analyses of Gender and Training Group Interactions
| Heart Rate, bpm | ln SDRR | ln Low-Frequency Power | ln High-Frequency Power | |
| Predictors of heart rate and RRVa | ||||
| Group | 0.55 | 0.24 | 2.03 | 0.06 |
| Position | 42.56† | 18.59† | 2.43 | 0.00 |
| Session | 15.22† | 5.84*** | 3.57** | 5.15*** |
| Gender | 0.80 | 0.66 | 3.90** | 6.05** |
| BMI | 0.25 | 0.32 | 1.55 | 0.02 |
| Age | 0.41 | 39.54† | 37.01† | 36.46† |
| Group × session | 5.52*** | 1.06 | 1.25 | 2.32* |
| Predictors of heart rate and RRV, including 3-way interactionb | ||||
| Group | 0.02 | 0.78 | 3.21* | 0.02 |
| Position | 43.26† | 18.96† | 2.46 | 0.00 |
| Session | 18.23† | 6.12*** | 3.94** | 5.52*** |
| Gender | 1.61 | 0.85 | 4.12** | 5.24** |
| BMI | 0.07 | 0.12 | 1.04 | 0.00 |
| Age | 0.10 | 36.76† | 34.41† | 33.76† |
| Group × session | 4.45** | 1.31 | 1.46 | 2.52* |
| Group × gender | 5.08** | 4.64** | 4.88** | 3.26* |
| Session × gender | 7.30† | 0.88 | 0.81 | 1.50 |
| Session × group × gender | 0.06 | 4.29** | 1.72 | 2.44* |
Note. bpm = beats per minute; ln = natural log; SDRR = standard deviation of the RR interval; RRV = RR interval variability; BMI = body mass index.
For group, position, gender, BMI, age, df = 1, 513; for session, group × session, df = 2, 513.
For group, position, gender, BMI, age, and group × gender, df = 1, 509; for session, session × group, session × gender and 3-way interactions, df = 2, 509.
*P < .10; **P < .05; ***P < .01; †P < .001.
FIGURE 2.
The effect of aerobic conditioning versus strength training measured at baseline, immediately after training, and after sedentary deconditioning, on changes in (a) heart rate, (b) high-frequency RR interval variability, (c) SDRR, and (d) high-frequency RR interval variability.
Note. HR = heart rate; HF = high-frequency power; SDRR = standard deviation of the RR interval. Data were derived from adjusted means from regression models.
Effect of Gender
Because women have been substantially underrepresented in studies of the autonomic effects of aerobic exercise, we looked for gender differences by adding the group × session × gender interaction to the model and repeating the analyses. Table 2 shows a significant 3-way interaction for SDRR and high-frequency power. In men, SDRR (0.12 ln msec; 95% CI = 0.04, 0.20) and high-frequency power (0.39 ln msec2; 95% CI = 0.15, 0.63) increased after training and decreased after deconditioning (SDRR = −0.20 ln msec; 95% CI = −0.30, −0.10; high-frequency power = −0.54 ln msec2; 95% CI = −0.79, −0.29) in the aerobic-conditioning but not in the strength-training group (Figure 2cd). Women showed no significant change after training and deconditioning in either group.
The absence of an effect of aerobic conditioning on RRV in women raised questions about the apparent lack of a gender difference in the training-related reduction in heart rate suggested by the nonsignificant 3-way interaction (Table 2). To explore this issue, we conducted post hoc contrasts of the within-group heart rate changes from baseline to training. In the aerobic-conditioning group, heart rate fell by 5.50 bpm (95% CI = −7.28, −3.72) in men and 1.65 bpm (95% CI = −3.39, 0.09) in women, suggesting that in women, the absence of an exercise-induced increase in cardiac vagal modulation is paralleled by the lack of a change in heart rate.
Analysis of Dropouts
We considered the possibility that differential dropout rates might account for our findings. With the exception of men in the aerobic-conditioning group (10%), dropout rates did not differ appreciably among groups: 39% among women in the aerobic-conditioning group; 39% and 34%, respectively, among men and women in the strength-training group. To determine whether differences in dropout rate influenced our findings, we conducted a sensitivity analysis by comparing an analysis of only participants who did not drop out with the intention-to-treat analysis. The results were highly similar, suggesting that the data were missing at random.
We also performed a pattern-mixture analysis. Small sample size made some of the pattern-mixture models invalid, but data from those models that were valid yielded no indication that the missing data were informative. Together, these supplementary analyses suggested that our findings were likely not affected by participants’ attrition.
DISCUSSION
Exercise is a central characteristic of a healthy lifestyle, but the cardioprotective mechanisms underlying its effects are unclear. We conducted a randomized controlled trial contrasting the autonomic effects of aerobic conditioning and strength training in healthy, sedentary young men and women and found that VO2max increased significantly, as expected, after aerobic conditioning but not strength training. Heart rate fell and high-frequency power rose after aerobic conditioning but not strength training, and sedentary deconditioning reversed these changes. These data suggest that aerobic conditioning produced small but expected autonomic effects.
However, our findings were complicated by an effect of gender. As shown in Figure 2, the autonomic-enhancing effect of aerobic conditioning appeared only in men. Strength training had no such effect in men or women.
Although studies routinely report that exercise training leads to increased cardiac autonomic regulation,24,30–32 the evidence about the autonomic benefits of exercise is less clear than is commonly assumed. First, many of these studies were cross-sectional comparisons of groups that differed substantially from one another. Typically, well-conditioned participants were contrasted to sedentary controls.13,33–43 Some of these studies showed the expected heart rate or RRV effects and others did not, but it is difficult to attribute their findings to differences in physical conditioning and not to other factors associated with self-selection.
Other studies tested the effect of training regimens, but many lacked the control groups required to adequately assess the effect of training.19,30,44–47 Like their cross-sectional counterparts, some of these studies observed the expected heart rate and RRV changes and others did not. Many studies that randomly assigned participants to training and control groups reported training-related changes in heart rate or RRV,18,24,31,33,48–51 but others did not.11,52–54 None of these studies, however, conducted intention-to-treat analyses.
Finally, women have been substantially underrepresented in research on the autonomic effects of exercise. For example, cross-sectional examinations of 5535 and 14455 men reported that RRV was greater in trained than in untrained participants. In 2 randomized trials of aerobic conditioning, one showing an increase in RRV24 and the other no increase,53 55 and 140 men, respectively, were recruited. By contrast, the Heritage Family Study enrolled 507 participants, 292 of whom were women. Aerobic conditioning led to a small but significant reduction in heart rate (3.2 bpm), approximately equivalent to the heart rate reduction we found, with no gender difference in the heart rate response to training.19 However, this study had no control group and no discussion of dropouts, and analyses did not conform to intention-to-treat principles.
The gender effects on cardiac autonomic regulation in our findings might have been attributable to differences in the aerobic effects of training. However, as shown in Figure 1, men and women did not differ in their increased aerobic capacity after training and decreased capacity after deconditioning.
Prior to training, women had greater high-frequency FHFhhRRV than did men, raising the possibility that a ceiling effect accounted for the absence of a cardiac autonomic effect. However, baseline SDRR was identical in men and women, and it, too, increased only in men after aerobic conditioning, suggesting that a ceiling effect is unlikely to account for these findings.
The absence of an RRV effect of aerobic conditioning in young, premenopausal women, along with the greatly diminished heart rate response, suggests a possible role for estrogen in moderating the autonomic response to exercise training. Considerable evidence reveals an effect of estrogen on cardiac autonomic regulation. RRV varies depending on the phase of the menstrual cycle, with greater levels during the follicular phase.56 Hormone replacement therapy has been shown to enhance autonomic regulation of the heart in women who experienced natural menopause and in those who had a hysterectomy with oophorectomy.57–60 In animals, exogenous administration of estrogen increases parasympathetic cardiac autonomic regulation.61
Moreover, a recent report, in combination with our findings, implicates estrogen in the diminished autonomic response to exercise training. Earnest et al. demonstrated that, by contrast to the absence of an RRV response in our younger women, postmenopausal (aged 45–75 years) sedentary women who were overweight or obese showed an increase in RRV in a 6-month exercise training program.62 However, data from other studies raise questions about the role of estrogen. In another study of postmenopausal sedentary, overweight women, exercise training led to increased RRV, even in participants receiving hormone replacement therapy.49 In addition, rats that underwent oophorectomy or a “sham” surgery showed equivalent reductions in heart rate after exercise training.63 Thus, although it is clear that estrogen influences autonomic regulation of the heart, it is not yet known whether it plays a role in moderating the autonomic response to aerobic conditioning.
Limitations
Our study was designed only to assess the effect of aerobic conditioning and strength training on cardiac autonomic modulation. It was not designed to examine gender effects and was not powered to detect such differences; our results should lead to new hypotheses and further research rather than to specific conclusions about a gender effect.
Another potential limitation was our reliance on self-reported measures of adherence to exercise regimens. After the initial training session, participants exercised on their own, although they received weekly calls to provide additional supervision and support. Because the study lacked independent indices of adherence, we could not be certain that the 2 groups exercised as intended. However, because the groups differed precisely as predicted on aerobic capacity after training and deconditioning, it appeared that participants exercised as instructed.
A related concern was the dropout rate. In our study, 32% of randomized participants dropped out of the training programs between baseline and posttraining testing. Few recent reports on dropout rates in training studies of young adults exist, but older estimates of 50% have been reported,64 suggesting that our dropout rate was not unusually high. Supplementary analyses suggested that dropouts did not account for our findings.
Finally, because we studied healthy young adults, who are at low risk of heart disease, it could be argued that our findings lack public health significance. Such a view is inconsistent with evidence that atherosclerosis begins very early in life65–67; risk factors for adult coronary heart disease also characterize atherosclerosis in childhood.65 As Strong et al. wrote, “True primary prevention of atherosclerosis, as contrasted with primary prevention of clinically manifest atherosclerotic disease, must begin in childhood or adolescence.”65(p734)
Conclusions
Our analyses suggest that although there is evidence for cardioprotection through increased autonomic activity generated by aerobic exercise in our overall sample, this was primarily attributable to effects in men but not in women. Given the wealth of evidence of the health benefits of physical activity, no single study can justify modifying clinical and public health recommendations to engage in exercise. However, our findings, combined with evidence demonstrating a cardioprotective effect of RRV and the childhood origin of atherosclerosis, support the hypothesis that aerobic conditioning, as measured by RRV, confers greater cardioprotection in men than in women.
In a randomized trial of the effect of aerobic conditioning versus strength training on autonomic regulation of the heart in a sample of 149 healthy young adults, we found that aerobic conditioning, but not strength training, led to reductions in heart rate and increases in high-frequency RRV. Sedentary deconditioning reversed these changes. These findings are consistent with the hypothesis that aerobic conditioning increases cardiac vagal modulation. However, a post hoc analysis suggested a gender effect on training-induced changes in RRV: in men but not women, RRV increased after aerobic conditioning but not after strength training. Further study is required to test this hypothesis and examine the operative mechanisms.
Acknowledgments
This study was supported in part by the National Institute of Mental Health (Independent Scientist Award K02 MH01491), the National Heart Lung and Blood Institute (grant R01 HL61287), the General Clinical Research Centers Program of the National Institutes of Health (grant M01-RR00645), and the Nathaniel Wharton Fund.
We are grateful for the assistance of Timothy Church.
Human Participant Protection
The institutional review boards of Columbia University Medical Center and St John's University approved this study. All participants provided informed consent.
References
- 1.Fletcher GF. How to implement physical activity in primary and secondary prevention. A statement for healthcare professionals from the Task Force on Risk-Reduction, American Heart Association. Circulation 1997;96:355–357 [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Bazzarre TL, Daniels SR, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation 2003;107(4):645–651 [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002;106(3):388–391 [DOI] [PubMed] [Google Scholar]
- 4.Liao D, Cai J, Rosamond WD, et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol 1997;145(8):696–706 [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–2855 [DOI] [PubMed] [Google Scholar]
- 6.Huikuri HV, Jokinen V, Syvanne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19:1979–1985 [DOI] [PubMed] [Google Scholar]
- 7.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992;85:164–171 [DOI] [PubMed] [Google Scholar]
- 8.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998;351:478–484 [DOI] [PubMed] [Google Scholar]
- 9.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003;107(4):565–570 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J 1998;19(suppl F):F72–F80 [PubMed] [Google Scholar]
- 11.Pigozzi F, Alabiso A, Parisi A, et al. Effects of aerobic exercise training on 24 hr profile of heart rate variability in female athletes. J Sports Med Phys Fitness 2001;41:101–107 [PubMed] [Google Scholar]
- 12.Madden KM, Levy WC, Stratton JK. Exercise training and heart rate variability in older adult female subjects. Clin Invest Med 2006;29(1):20–28 [PubMed] [Google Scholar]
- 13.Ueno LM, Moritani T. Effects of long-term exercise training on cardiac autonomic nervous activities and baroreflex sensitivity. Eur J Appl Physiol 2003;89(2):109–114 [DOI] [PubMed] [Google Scholar]
- 14.Sacknoff DM, Gleim GW, Stachenfeld N, Coplan NL. Effect of athletic training on heart rate variability. Am Heart J 1994;127:1275–1278 [DOI] [PubMed] [Google Scholar]
- 15.De Meersman RE. Respiratory sinus arrhythmia alteration following training in endurance athletes. Eur J Appl Physiol Occup Physiol 1992;64:434–436 [DOI] [PubMed] [Google Scholar]
- 16.Loimaala A, Huikuri H, Oja P, Pasanen M, Vuori I. Controlled 5-mo aerobic training improves heart rate but not heart rate variability or baroreflex sensitivity. J Appl Physiol 2000;89(5):1825–1829 [DOI] [PubMed] [Google Scholar]
- 17.Boutcher SH, Stein P. Association between heart rate variability and training response in sedentary middle-aged men. Eur J Appl Physiol Occup Physiol 1995;70:75–80 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Miyachi M, Saitoh T, Yoshioka A, Onodera S. Effects of endurance training on resting and post-exercise cardiac autonomic control. Med Sci Sports Exerc 2001;33(9):1496–1502 [DOI] [PubMed] [Google Scholar]
- 19.Wilmore JH, Stanforth PR, Gagnon J, et al. Heart rate and blood pressure changes with endurance training: the HERITAGE Family Study. Med Sci Sports Exerc 2001;33(1):107–116 [DOI] [PubMed] [Google Scholar]
- 20.Bonaduce D, Petretta M, Cavallaro V, et al. Intensive training and cardiac autonomic control in high level athletes. Med Sci Sports Exerc 1998;30(5):691–696 [DOI] [PubMed] [Google Scholar]
- 21.Hautala AJ, Mäkikallio TH, Kiviniemi A, et al. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 2003;285(4):H1747–H1752 [DOI] [PubMed] [Google Scholar]
- 22.Sedgwick AW, Craig RJ, Crouch R, Dowling B. The effects of physical training on the day and night long-term heart-rates of middle-aged men. Eur J Appl Physiol Occup Physiol 1974;33(4):307–314 [DOI] [PubMed] [Google Scholar]
- 23.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Jpn J Physiol 1995;45:1053–1069 [DOI] [PubMed] [Google Scholar]
- 24.Tulppo MP, Hautala AJ, Makikallio TH, et al. Effects of aerobic training on heart rate dynamics in sedentary subjects. J Appl Physiol 2003;95(1):364–372 [DOI] [PubMed] [Google Scholar]
- 25.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp B. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol 1983;55:1558–1564 [DOI] [PubMed] [Google Scholar]
- 26.DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability spectra. IEEE Trans Biomed Eng 1984;31:384–387 [DOI] [PubMed] [Google Scholar]
- 27.Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc IEEE 1978;66:51–83 [Google Scholar]
- 28.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control 1974;AC19:716–723 [Google Scholar]
- 29.Fleiss JL. The Design and Analysis of Clinical Experiments New York, NY: Wiley; 1986 [Google Scholar]
- 30.Carter JB, Banister EW, Blaber AP. The effect of age and gender on heart rate variability after endurance training. Med Sci Sports Exerc 2003;35(8):1333–1340 [DOI] [PubMed] [Google Scholar]
- 31.Hautala AJ, Makikallio TH, Kiviniemi A, et al. Heart rate dynamics after controlled training followed by a home-based exercise program. Eur J Appl Physiol 2004;92(3):289–297 [DOI] [PubMed] [Google Scholar]
- 32.Okazaki K, Iwasaki K-I, Prasad A, et al. Dose–response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol 2005;99(3):1041–1049 [DOI] [PubMed] [Google Scholar]
- 33.Boutcher SH, Nurhayati Y, McLaren PF. Cardiovascular response of trained and untrained old men to mental challenge. Med Sci Sports Exerc 2001;33(4):659–664 [DOI] [PubMed] [Google Scholar]
- 34.Buchheit M, Simon C, Piquard F, Ehrhart J, Brandenberger G. Effects of increased training load on vagal-related indexes of heart rate variability: a novel sleep approach. Am J Physiol Heart Circ Physiol 2004;287(6):H2813–H2818 [DOI] [PubMed] [Google Scholar]
- 35.Buchheit M, Gindre C. Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 2006;291(1):H451–H458 [DOI] [PubMed] [Google Scholar]
- 36.Mourot L, Bouhaddi M, Perrey S, Rouillon JD, Regnard J. Quantitative Poincare plot analysis of heart rate variability: effect of endurance training. Eur J Appl Physiol 2004;91(1):79–87 [DOI] [PubMed] [Google Scholar]
- 37.Boutcher SH, Nugent FW, McLaren PF, Weltman AL. Heart period variability of trained and untrained men at rest and during mental challenge. Psychophysiology 1998;35(1):16–22 [PubMed] [Google Scholar]
- 38.Genovesi S, Zaccaria D, Rossi E, Valsecchi MG, Stella A, Stramba-Badiale M. Effects of exercise training on heart rate and QT interval in healthy young individuals: are there gender differences? Europace 2007;9(1):55–60 [DOI] [PubMed] [Google Scholar]
- 39.Melo RC, Santos MD, Silva E, et al. Effects of age and physical activity on the autonomic control of heart rate in healthy men. Braz J Med Biol Res 2005;38(9):1331–1338 [DOI] [PubMed] [Google Scholar]
- 40.Reland S, Ville NS, Wong S, Senhadji L, Carre F. Does the level of chronic physical activity alter heart rate variability in healthy older women? Clin Sci (Lond) 2004;107(1):29–35 [DOI] [PubMed] [Google Scholar]
- 41.Lazoglu AH, Glace B, Gleim GW, Coplan NL. Exercise and heart rate variability. Am Heart J 1996;131(4):825–826 [DOI] [PubMed] [Google Scholar]
- 42.Smith SA, Querry RG, Fadel PJ, et al. Differential baroreflex control of heart rate in sedentary and aerobically fit individuals. Med Sci Sports Exerc 2000;32(8):1419–1430 [DOI] [PubMed] [Google Scholar]
- 43.Davy KP, Desouza CA, Jones PP. Elevated heart rate variability in physically active young and older adult women. Clin Sci 1998;94:579–584 [DOI] [PubMed] [Google Scholar]
- 44.Catai AM, Chacon-Mikahil MP, Martinelli FS, et al. Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. Braz J Med Biol Res 2002;35(6):741–752 [DOI] [PubMed] [Google Scholar]
- 45.Davy KP, Willis WL, Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clin Physiol 1997;17(1):31–40 [DOI] [PubMed] [Google Scholar]
- 46.Forte R, De Vito G, Figura F. Effects of dynamic resistance training on heart rate variability in healthy older women. Eur J Appl Physiol 2003;89(1):85–89 [DOI] [PubMed] [Google Scholar]
- 47.Baumert M, Brechtel L, Lock J, Voss A. Changes in heart rate variability of athletes during a training camp. Biomed Tech (Berl) 2006;51(4):201–204 [DOI] [PubMed] [Google Scholar]
- 48.Schuit AJ, van Amelsvoort LG, Verheij TC, et al. Exercise training and heart rate variability in older people. Med Sci Sports Exerc 1999;31(6):816–821 [DOI] [PubMed] [Google Scholar]
- 49.Jurca R, Church TS, Morss GM, Jordan AN, Earnest CP. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J 2004;147(5):e8–e15 [DOI] [PubMed] [Google Scholar]
- 50.Kiviniemi AM, Hautala AJ, Makikallio TH, Seppanen T, Huikuri HV, Tulppo MP. Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R-R interval. Eur J Appl Physiol 2006;96(6):686–692 [DOI] [PubMed] [Google Scholar]
- 51.Myslivecek PR, Brown CA, Wolfe LA. Effects of physical conditioning on cardiac autonomic function in healthy middle-aged women. Can J Appl Physiol 2002;27(1):1–18 [DOI] [PubMed] [Google Scholar]
- 52.La Rovere MT, Mortara A, Sandrone G, Lombardi F. Autonomic nervous system adaptations to short-term exercise training. Chest 1992;101:299S–303S [DOI] [PubMed] [Google Scholar]
- 53.Uusitalo ALT, Laitinen T, Vaisanen SB, Lansimies E, Rauramaa R. Physical training and heart rate and blood pressure variability: a 5-yr randomized trial. Am J Physiol Heart Circ Physiol 2004;286(5):H1821–H1826 [DOI] [PubMed] [Google Scholar]
- 54.Verheyden B, Eijnde BO, Beckers F, Vanhees L, Aubert AE. Low-dose exercise training does not influence cardiac autonomic control in healthy sedentary men aged 55–75 years. J Sports Sci 2006;24(11):1137–1147 [DOI] [PubMed] [Google Scholar]
- 55.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J 1993;125:726–731 [DOI] [PubMed] [Google Scholar]
- 56.McKinley PS, King A, Shapiro PA, et al. The impact of menstrual cycle phase on cardiac autonomic regulation. Psychophysiology In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao HT, Kuo CD, Su YJ, Chuang SS, Fang YJ, Ho LT. Short-term effect of transdermal estrogen on autonomic nervous modulation in postmenopausal women. Fertil Steril 2005;84(5):1477–1483 [DOI] [PubMed] [Google Scholar]
- 58.Farag NH, Nelesen RA, Parry BL, Loredo JS, Dimsdale JE, Mills PJ. Autonomic and cardiovascular function in postmenopausal women: the effects of estrogen versus combination therapy. Am J Obstet Gynecol 2002;186(5):954–961 [DOI] [PubMed] [Google Scholar]
- 59.Liu CC, Kuo TBJ, Yang CCH. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol 2003;285(5):H2188–H2193 [DOI] [PubMed] [Google Scholar]
- 60.Mercuro G, Podda A, Pitzalis L, et al. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol 2000;85(6):787–789 [DOI] [PubMed] [Google Scholar]
- 61.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17[beta]-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci 2000;84(1-2):78–88 [DOI] [PubMed] [Google Scholar]
- 62.Earnest CP, Lavie CJ, Blair SN, Church TS. Heart rate variability characteristics in sedentary postmenopausal women following six months of exercise training: the DREW study. PLoS ONE 2008;3(6):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minami N, Mori N, Nagasaka M, et al. Exercise training fails to modify arterial baroreflex sensitivity in ovariectomized female rats. Tohoku J Exp Med 2007;211(4):339–345 [DOI] [PubMed] [Google Scholar]
- 64.Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237–267 [Google Scholar]
- 65.Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999;281(8):727–735 [DOI] [PubMed] [Google Scholar]
- 66.Strong JP, McGill HC., Jr The pediatric aspects of atherosclerosis. J Atheroscler Res 1969;9(3):251–265 [DOI] [PubMed] [Google Scholar]
- 67.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994;89(5):2462–2478 [DOI] [PubMed] [Google Scholar]