Abstract
Objectives. We sought to estimate the effect of universal implementation of a clinic-based, psychosocial smoking cessation intervention for pregnant women.
Methods. We used data from US birth certificates (2005) and the Pregnancy Risk Assessment Monitoring System (2004) to estimate the number of women smoking at conception. To calculate the number of women eligible to receive the cessation intervention, we used estimates from the literature of the percentage of women who quit spontaneously (23%), entered prenatal care before the third trimester (96.5%), and disclosed smoking to their provider (75%). We used the pooled relative risk (RR) for continued smoking from the 2004 Cochrane Review as our measure of the intervention's effectiveness (RR = 0.94).
Results. We estimated that 944 240 women smoked at conception. Of these, 23.0% quit spontaneously, 6.3% quit with usual care, and an additional 3.3% quit because of the intervention, leaving 67.4% smoking throughout pregnancy. The calculated smoking prevalence in late pregnancy decreased from 16.4% to 15.6% because of the intervention.
Conclusions. Universal implementation of a best-practice, clinic-based intervention would increase the total number of quitters but would not substantially reduce smoking prevalence among pregnant women.
An extensive body of literature demonstrates that maternal smoking during pregnancy has numerous adverse effects on maternal, fetal, and infant health, including increased risk of abruption, placenta previa, premature rupture of membranes, preterm birth, fetal growth restriction, and sudden infant death syndrome.1,2 Increased awareness of the dangers of smoking and the implementation of tobacco control policies likely have contributed to declines in smoking during pregnancy over recent decades, but the prevalence remains unacceptably high. A goal of Healthy People 2010 is to decrease the percentage of women who smoke during pregnancy to 1.2%,3 but recent estimates indicate that more than 16% of pregnant women still smoke.4
Numerous approaches to prenatal smoking cessation have been studied, including counseling, cognitive and behavioral therapy, hypnosis, acupuncture, and pharmacotherapy. Because of concerns about the safety and efficacy of pharmacologic therapies, approaches such as nicotine replacement therapy or use of bupropion are recommended for consideration only in women who fail nonpharmacologic methods and in whom the potential benefits of the therapy outweigh the unknown risks of the medications.5,6 Many efforts to further decrease smoking among pregnant women focus on clinic-based cessation interventions. Current recommendations for a first-line approach (beyond simply advising pregnant smokers to quit) advocate extended or augmented psychosocial intervention delivered in a clinical setting.5,6 This endorsement is based on numerous studies that found that augmented cessation interventions were more effective than was usual care in achieving smoking cessation during pregnancy.
An augmented psychosocial intervention is commonly implemented in the form of the 5 A's (ask, advise, assess, assist, and arrange); clients receive a provider-administered, 5- to 15-minute counseling intervention and self-help materials. This approach, which can be integrated into routine clinical care, is endorsed by the American College of Obstetricians and Gynecologists as best practice for smoking cessation during pregnancy.6 A study that used findings from a previous study that an intervention would achieve a 30% to 70% improvement over baseline quit rates7 found the 5 A's to be cost effective or cost neutral.8 To date, however, the efficacy of an augmented clinic-based intervention in reducing the prevalence of smoking during pregnancy at the population level has not been formally evaluated. Therefore, we sought to estimate the number of additional women who would stop smoking during pregnancy and the change in US prenatal smoking prevalence if an augmented psychosocial intervention were implemented universally.
METHODS
We used the most recently available data from US birth certificates on women who delivered live infants and from the Pregnancy Risk Assessment Monitoring System (PRAMS). PRAMS is an ongoing, population-based surveillance system that collects self-reported information in participating states on maternal behaviors before, during, and after pregnancy. All participants are state residents who delivered a live infant in the preceding 2 to 4 months. A mother completes a self-administered questionnaire or phone interview, with results linked to her child's birth certificate. Details of PRAMS have been published elsewhere.9
Estimates of Women Eligible for Intervention
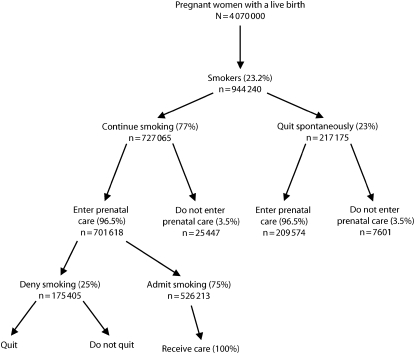
We included pregnancies ending in a live birth in 2005 as our initial hypothetical cohort. After correcting for multifetal pregnancies, we estimated that 4.07 million women delivered a live infant in the United States in 2005 (Figure 1).10 We then estimated the number of women who were smoking when they learned they were pregnant by using data from the 2004 PRAMS question, “In the three months before you got pregnant, how many cigarettes or packs of cigarettes did you smoke on an average day?” In the 26 states participating in PRAMS in 2004 with response rates over 70%, 23.2% of the women stated that they smoked more than 0 cigarettes per day during the 3 months before pregnancy.9 We applied this percentage to the 2005 US birth cohort and estimated that of 4 070 000 women, 944 240 were smokers at the time they became pregnant.
FIGURE 1.
Estimated number of pregnant smokers who go on to have a live birth and are eligible to receive a clinic-based smoking cessation intervention: United States.
Note. For the initial scenario, we used the best available published population-based or summary estimates of women eligible to receive a smoking cessation intervention.9–14
We reviewed the relevant literature and derived an estimate of the percentage of women who quit smoking spontaneously when they found out they were pregnant and before they entered prenatal care. To our knowledge, the most recent available data on spontaneous quit rates in the US population was collected by the 1990 National Health Interview Survey on Health Promotion and Disease Prevention, in which 23% of self-reported female smokers who had a live birth in the preceding 5 years reported that they stopped smoking when they learned of their pregnancy.11 Therefore, of the estimated 944 240 women who were smoking at the time they became pregnant, we estimated that 727 065 (77%) continued to smoke after learning of their pregnancy and before entering prenatal care (Figure 1).
We assumed that women would have to enter prenatal care before the third trimester to receive a clinic-based, augmented cessation intervention. Data on time of entry into prenatal care from the 1989 and 2003 birth certificates were not directly comparable. Therefore, we used 2005 data from the 37 states that used the 1989 birth certificate to estimate the percentage of women entering prenatal care before the third trimester. These 37 states reported that in 2005, 3.5% of women received prenatal care beginning in the third trimester or received no prenatal care at all.10 Thus, of the estimated 727 065 women who continued to smoke after learning of their pregnancy, we estimated that 701 618 (96.5%) of them entered prenatal care before the third trimester (Figure 1).
Next we estimated the percentage of smokers who likely would disclose to their provider on entry into prenatal care that they smoked. To our knowledge, there are no national estimates of the prevalence of nondisclosure of smoking status among pregnant smokers. In the 3 clinic-based studies conducted between 1990 and 2003 that biochemically validated self-reported smoking among pregnant women, nondisclosure at baseline (before receiving the intervention) ranged from 24% to 28%.12–14 For the prevalence of nondisclosure in our study, we used the median of this range (25%). Therefore, of an estimated 701 618 active smokers who entered prenatal care, we estimated that 75% (n = 526 213) would disclose their smoking status to their providers and so would be eligible for the intervention (Figure 1).
Because we wanted to estimate the greatest possible effect of the intervention on prenatal smoking prevalence, we assumed that the intervention would be fully implemented and that 100% of the 526 213 eligible women would receive it.
Analysis
To calculate how many more women would quit smoking if the augmented intervention were implemented than if they received usual care, we used a pooled relative risk (RR) estimate for continued smoking with exposure to the intervention (0.94; 95% confidence interval [CI] = 0.92, 0.95), which we obtained from the 2004 Cochrane Review.15 This RR translated to an absolute difference in the proportion of women continuing to smoke with the augmented intervention of 6% (out of every 100 smokers, an extra 6 would quit if exposed to the intervention). It included data from 36 randomized controlled trials (33 behavioral intervention trials and 3 trials of nicotine replacement therapy) of biochemically validated smoking cessation programs implemented during pregnancy. A pooled RR based on behavioral intervention trials alone with biochemically validated quit status was not available in the Cochrane Review. However, we assumed that an RR based on behavioral interventions would be similar to the overall RR found in the Cochrane Review, because the RR for the 3 nicotine replacement trials (RR = 0.94) did not differ from the pooled RR for the 36 trials.
To better understand the contributions of the intervention to overall smoking prevalence and quit rates at a population level, we first estimated the quit rate among smokers who received usual care. Among US-based, biochemically validated, randomized controlled trials of smoking cessation interventions from 1990 to 2003,15 the quit rates in the control groups (who received only usual care) ranged from 0% to 34%. For our analysis, we used the median of these estimates (11.3%). An increase of 6 percentage points would change the quit rate from 11.3% to 17.3%. We used this usual care quit rate and the increase of 6 percentage points to estimate the overall change in smoking prevalence in late pregnancy resulting from implementation of the intervention.
To determine the effects of the intervention under a best-case scenario (i.e., if we were able to maximize the number of women eligible to receive it and the efficacy of the intervention), we then recalculated the number of new quitters gained and the decrease in overall smoking prevalence attributable to implementation of the intervention, assuming that 100% of women entered prenatal care before the third trimester, 100% disclosed their smoking status to their provider, and the RR for continued smoking was 0.92 (the lower bound of the CI from the Cochrane estimate). Our estimates for this scenario and for our initial scenario are shown in Table 1.
TABLE 1.
Estimates Used to Calculate the Effect of a Clinic-Based Cessation Intervention Among Pregnant Women in the Initial and Best-Case Scenarios: United States
| Initial Scenario | Best-Case Scenario | |
| Women entering prenatal care, % | 96.5 | 100 |
| Women smoking at the time they became pregnant, % | 23.2 | 23.2 |
| Smokers who quit spontaneously,a % | 23 | 23 |
| Smokers who do not disclose their smoking status to their provider, % | 25 | 0 |
| Eligible women receiving the intervention, % | 100 | 100 |
| Risk ratio for continued smoking | 0.94 | 0.92 |
| Quit rate with usual care, % | 9.7 | 9.7 |
Note. For the best-case scenario, our estimates assumed that the maximum number of women were eligible to receive the smoking cessation intervention and that the intervention had maximum possible efficacy.
Women who quit when they found out they were pregnant but before they entered prenatal care.
Finally, we used the usual care quit rate and the Cochrane RR estimate of 6 percentage points to calculate the change in the overall quit rate (the percentage of smokers who quit at any time during pregnancy, including those who quit spontaneously and those who quit with usual care) with implementation of the intervention.
RESULTS
We used the Cochrane estimated RR of 0.94 to estimate that of the 526 213 women who informed their providers that they smoked (Figure 1), 31 573 (6%) new quitters would be gained with universal implementation of a clinic-based, augmented smoking cessation intervention. These new quitters would be in addition to the women who would have quit in the absence of the intervention (i.e., those who quit spontaneously before entering prenatal care and those who would have quit had they received usual care).
Among all pregnant women (4 070 000), the calculated overall smoking prevalence by late pregnancy would decrease from 16.4% to 15.6% with implementation of the intervention, an absolute difference of 0.8 percentage points.
If we were able to maximize the efficacy of the intervention and the number of women eligible to receive it, 58 165 more pregnant women would quit smoking and the calculated overall smoking prevalence by late pregnancy would decrease from 15.8% to 14.4% (an absolute difference of 1.4 percentage points).
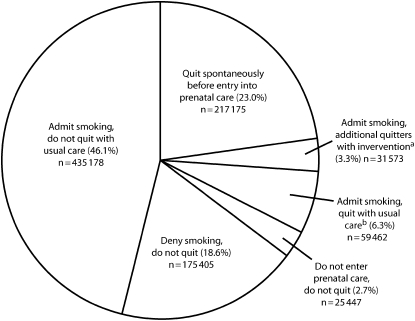
In our initial scenario, of the 944 240 smokers who became pregnant, the overall quit rate would increase from 29.3% ([217 175 + 59 462]/944 240) to 32.6% ([217 175 + 59 462 + 31 573]/944 240) with implementation of the intervention (Figure 2). In other words, 67.4% of the 944 240 women who were smoking when they became pregnant would still be smoking by late pregnancy ([435 178 + 175 405 + 25 447]/944 240; Figure 2).
FIGURE 2.
Proportion of pregnant smokers (n = 944 240) who quit or continue to smoke, by entry into prenatal care, nondisclosure, and exposure to the intervention.
aAdditional quitters with the intervention among women who are eligible for intervention (n = 526 213) is 6% of 526 213, which is 31 573.
bQuit with usual care among women who are eligible for intervention (n = 526 213) is 11.3% of 526 213, which is 59 462.
DISCUSSION
As awareness of the risks associated with smoking during pregnancy has increased, the prevalence of prenatal smoking has decreased, but it remains unacceptably high. We estimated the extent to which universal implementation of a clinic-based, augmented smoking cessation intervention could increase the number of quitters. We calculated that the gain in quitters would be more than 31 000 women because of the intervention, but of the 944 240 women who were smokers at the time they became pregnant, 67.4% would still be smoking by the end of pregnancy and the overall decline in prenatal smoking prevalence would be only 0.8 percentage points.
Data suggest that smoking cessation before the third trimester reduces the risk of adverse pregnancy outcomes.5,6 Therefore, increasing quit rates during pregnancy should result in improvements in infant health. For example, a gain of 58 165 quitters (our best-case scenario) could prevent approximately 2560 cases of low birth weight, assuming that 7.5% of nonsmokers deliver low-birthweight babies and that infants born to smokers have a relative risk of 1.59.10 However, nearly 28 000 infants would still be born with low birthweight because of smoking.
We chose to use the recent Cochrane Review's estimated RR for continued smoking of 0.94 (95% CI = 0.92, 0.95) because that review provided the most recent and most comprehensive meta-analysis of smoking cessation interventions during pregnancy. If we assumed a baseline quit rate of 11.3% among women who enter prenatal care still smoking, and an increase of 6 percentage points in the quit rate to 17.3%, our estimated RR for cessation with the intervention was 1.5. This estimated RR is similar in magnitude to other widely cited risk estimates.7,16
Because we wanted to estimate the greatest possible effect of the intervention on prenatal smoking prevalence, we assumed that all women who disclosed their smoking status to their provider after entering prenatal care would receive a clinic-based, augmented intervention, but in reality, the evidence suggests that universal implementation of the intervention would be extremely difficult to achieve. For example, Hartmann et al. recently reported that only one third of prenatal care providers administered the 5 A's to their pregnant patients.17 If we assumed that only 30% of eligible women would receive the intervention, the gain in quitters would be 9 472 and there would be no change in smoking prevalence.
Several factors may limit the potential effect of a clinic-based, augmented intervention on smoking cessation at the population level. We found that a large percentage of smokers quit before or upon learning they were pregnant, and those who continue to smoke may be a highly addicted subgroup and especially resistant to cessation interventions.18–20 This phenomenon has been observed in the general population of smokers, among whom it has been noted that cessation interventions appear to have become less effective over time because of their “hardening.” This “hardening” may be attributable to an increase in the proportion of residual smokers who are heavily addicted or who have a mental illness or codependency on drugs or alcohol.21 Consistent with this phenomenon, smoking among pregnant women has become increasingly concentrated among lower socioeconomic populations, who are least able to give up tobacco.22–24
Moreover, a substantial percentage of smokers do not reveal their smoking status to their providers and so are never identified as being eligible to receive an intervention. Although nondisclosure rates theoretically could be improved, more research is needed to determine how to minimize this problem.25 Moreover, women who do not disclose their smoking status may be more resistant to cessation interventions than are women who readily admit to smoking. If so, improving disclosure of smoking status may not result in the expected improvement in cessation rates.
The RR estimates we used from the Cochrane Review were based on results from clinical trials, which involve a unique subset of the population: those who are willing to participate in research trials. Smokers in the general population may be less compliant with the requirements of an intervention than are smokers in clinical trials, and thus the magnitude of the effect of the intervention on quit rates might be lower than our estimates. Evidence of this potential obstacle can be found in numerous clinical trials with low participation and high attrition.13,20,26 If we presume that those declining to participate in trials are more resistant to quitting, then clinical trials are likely to produce more quitters than are interventions in typical community settings. Realistic estimates of the effect of a cessation intervention are best assessed through studies with clustered designs in which the clinic is the unit of randomization. Unfortunately, to our knowledge, pooled estimates of the effect size from studies with such designs among pregnant women are not available.
Researchers have long contended that clinic-based interventions alone are unlikely to have more than a modest effect on smoking prevalence in either the pregnant27 or the general28 population. The importance of comprehensive strategies such as increasing excise taxes on cigarettes, banning all forms of tobacco advertisement, improving the enforcement of laws that prohibit sales to children and adolescents, and promoting smoke-free areas in public places and the workplace was recently emphasized by the Institute of Medicine,29 and these strategies were found to be effective after widespread implementation through the World Health Organization Framework Convention for Tobacco Control.30–32
The Centers for Disease Control and Prevention promotes several comprehensive tobacco-use reduction strategies, including community-based programs (such as developing partnerships with local organizations and promoting governmental and voluntary policies to promote clean indoor air), restricting access to tobacco products, and providing insurance coverage for cessation treatment.33 In addition, the agency promotes the enforcement of tobacco control policies, including restricting minors' access to tobacco, outlawing smoking in public places, and an increased availability of cessation programs. These strategies are derived from analysis of evidence-based comprehensive state tobacco control programs and could decrease smoking initiation among youths, which in turn could lead to declines in smoking during pregnancy.33
Limitations
Our analysis had several limitations. Implementation of a smoking cessation intervention could have a greater or lesser effect in specific communities or subpopulations, depending on local demographics and spontaneous quit rates. Our hypothetical cohort population included only women with a live birth and so did not include women whose pregnancies ended in a miscarriage, fetal death, or stillbirth. Although it has been documented that some spontaneous quitters relapse to smoking during pregnancy,34 our estimate of the number of spontaneous quitters was based on data from women who reported after delivery that they did not smoke after learning they were pregnant and so did not include quitters who later relapsed. Excluding women who had quit at the time they entered prenatal care but later relapsed may have caused us to overestimate the number of women eligible for the intervention.
We also made several assumptions. We assumed that women who did not disclose their smoking status to their providers did not quit later in pregnancy. If a substantial proportion of these women quit on their own, then we would have underestimated the percentage of spontaneous quitters. We also assumed that women needed to enter prenatal care before the third trimester to receive a clinic-based, augmented cessation intervention and that cessation later in pregnancy did not improve pregnancy outcomes.5 It is possible that there are some health benefits from cessation in the third trimester.
Some of the estimates used in our analysis were based on data that were not current or may not have been nationally representative. For example, our estimate of the percentage of women who were smoking at the time they became pregnant (23.2%) was derived from population-based surveillance conducted in 27 states, and thus it may not be generalizable to the United States as a whole. However, we believe our estimate is a reasonable approximation for the entire country because it is consistent with the results of the 2004–2005 National Survey on Drug Use and Health, in which 16.6% of pregnant women and 29.6% of nonpregnant women of reproductive age reported that they smoked cigarettes.4 Our estimate of the rate of spontaneous quitting (23%) was based on national data from 1990, and the rate may have increased since then. However, this estimate is consistent with recent studies conducted in health care settings in which rates of spontaneous quitting ranged from 23% to 29%.35–38
Similarly, our estimate of the percentage of women who enter prenatal care was based on data from the 1989 version of the birth certificate. Comparisons of data on prenatal care from the 1989 and 2003 versions of the birth certificate indicated that the percentage of women entering prenatal care before the third trimester was lower for the 2003 version. This discrepancy is believed to be attributable to changes in reporting and not changes in utlization.10 Therefore, data from the 1989 birth certificate may have overestimated the percentage of women entering prenatal care before the third trimester. If the percentage of women receiving early prenatal care is lower than the estimate we used, the effect of the intervention on quit rates and prenatal smoking prevalence might be less than we estimated.
Finally, population-based estimates of nondisclosure rates were not available for pregnant women, and thus we relied on studies based in medical centers, which may not be representative of the US population as a whole. We addressed this limitation by conducting a subanalysis in which we assumed that 100% of smokers disclosed their smoking status to their providers.
Conclusions
Universal use of a clinic-based, augmented intervention for smoking cessation in pregnant women would likely increase the total number of quitters, but it would not result in substantial changes in smoking prevalence among pregnant women in the United States. Even under the most optimistic assumptions, the vast majority of smokers would continue to smoke during pregnancy. Therefore, comprehensive tobacco control approaches at the population level, particularly those targeting youths, will be needed if most pregnant smokers are to be reached. Efforts to promote these approaches should be intensified.
Acknowledgments
The author thank F. Carol Bruce for her insightful comments feedback on previous drafts of this article.
Human Participant Protection
No protocol approval was needed for this analysis because data were from published sources.
References
- 1.Office on Smoking and Health Women and Smoking: A Report of the Surgeon General Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; 2001 [Google Scholar]
- 2.Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol 2000;5:231–241 [DOI] [PubMed] [Google Scholar]
- 3.Healthy People 2010: With Understanding and Improving Health and Objectives for Improving Health 2nd ed Washington, DC: US Dept of Health and Human Services; 2000 [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration Results From the 2005 National Survey on Drug Use and Health: National Findings Rockville, MD: Office of Applied Studies; 2006. NSDUH Series H-30, DHHS publication SMA 06-4194 [Google Scholar]
- 5.Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline Rockville, MD: Public Health Service; 2000 [Google Scholar]
- 6.ACOG committee opinion. Number 316, October 2005 Smoking cessation during pregnancy. Obstet Gynecol 2005;106:883–888 [DOI] [PubMed] [Google Scholar]
- 7.Mullen PD. Maternal smoking during pregnancy and evidence-based intervention to promote cessation. Prim Care 1999;26:577–589 [DOI] [PubMed] [Google Scholar]
- 8.Ayadi MF, Adams EK, Melvin CL, et al. Costs of a smoking cessation counseling intervention for pregnant women: comparison of three settings. Public Health Rep 2006;121:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Angelo D, Williams L, Morrow B, et al. Preconception and interconception health status of women who recently gave birth to a live-born infant—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 reporting areas, 2004. MMWR Surveill Summ 2007; 56(10):1–35 [PubMed] [Google Scholar]
- 10.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep 2007;56(6):1–103 [PubMed] [Google Scholar]
- 11.LeClere FB, Wilson JB. Smoking behavior of recent mothers, 18–44 years of age, before and after pregnancy: United States, 1990. Adv Data 1997; July25(288):1–11 [PubMed] [Google Scholar]
- 12.Petersen L, Handel J, Kotch J, Podedworny T, Rosen A. Smoking reduction during pregnancy by a program of self-help and clinical support. Obstet Gynecol 1992; 79(6):924–930 [PubMed] [Google Scholar]
- 13.Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in medicaid maternity care. Am J Obstet Gynecol 2000;182:68–75 [DOI] [PubMed] [Google Scholar]
- 14.Kendrick JS, Zahniser SC, Miller N, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. Am J Public Health 1995;85:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2004Oct18(4):CD001055. [DOI] [PubMed] [Google Scholar]
- 16.Melvin CL, Dolan-Mullen P, Windsor RA, Whiteside HP, Jr, Goldenberg RL. Recommended cessation counselling for pregnant women who smoke: a review of the evidence. Tob Control 2000;9(suppl 3):III80–III84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstet Gynecol 2007;110:765–770 [DOI] [PubMed] [Google Scholar]
- 18.McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health 1999;89:706–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennrikus DJ, Lando HA, McCarty MC, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Prev Med 2005;40:249–258 [DOI] [PubMed] [Google Scholar]
- 20.Cinciripini PM, McClure JB, Wetter DW, et al. An evaluation of videotaped vignettes for smoking cessation and relapse prevention during pregnancy: the very important pregnant smokers (VIPS) program. Tob Control 2000;9(suppl 3):III61–III63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns DM, Warner KE. Smokers who have not quit: is cessation more difficult and should we change our strategies?. In: Amacher R, Marcus S, eds Smoking and Tobacco Control Monograph No. 15 Bethesda, MD: National Cancer Institute; 2003. NIH publication 03–5370 [Google Scholar]
- 22.Healton C, Nelson K. Reversal of misfortune: viewing tobacco as a social justice issue. Am J Public Health 2004;94:186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Tobacco use among adults–United States, 2005. MMWR Morb Mortal Wkly Rep 2006;55(42):1145–1148 [PubMed] [Google Scholar]
- 24.Ebert LM, Fahy K. Why do women continue to smoke in pregnancy?. Women Birth 2007;20:161–168 [DOI] [PubMed] [Google Scholar]
- 25.Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn't enough. Nicotine Tob Res 2004; Apr(6 suppl 2): S141–S151 [DOI] [PubMed] [Google Scholar]
- 26.O'Connor AM, Davies BL, Dulberg CS, et al. Effectiveness of a pregnancy smoking cessation program. J Obstet Gynecol Neonatal Nurs 1992;21:385–392 [DOI] [PubMed] [Google Scholar]
- 27.Lumley J. Stopping smoking–again. Br J Obstet Gynaecol 1991;98:847–849 [DOI] [PubMed] [Google Scholar]
- 28.Lando HA. Reflections on 30+ years of smoking cessation research: from the individual to the world. Drug Alcohol Rev 2006;25:5–14 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine Ending the Tobacco Problem: A Blueprint for the Nation Washington, DC: National Academies Press; 2007 [Google Scholar]
- 30.Fong GT, Cummings KM, Shopland DR. Building the evidence base for effective tobacco control policies: the International Tobacco Control Policy Evaluation Project (the ITC Project). Tob Control 2006;15(suppl 3):iii1–iii2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong GT, Hyland A, Borland R, et al. Reductions in tobacco smoke pollution and increases in support for smoke-free public places following the implementation of comprehensive smoke-free workplace legislation in the Republic of Ireland: findings from the ITC Ireland/UK Survey. Tob Control 2006;15(suppl 3):iii51–iii58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Framework Convention on Tobacco Control Geneva, Switzerland: World Health Organization; 2005 [Google Scholar]
- 33.Office of Smoking and Health Best Practices for Comprehensive Tobacco Control Programs—2007 Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; 2007 [Google Scholar]
- 34.Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstet Gynecol 2003;101(1):140–147 [DOI] [PubMed] [Google Scholar]
- 35.Morasco BJ, Dornelas EA, Fischer EH, Oncken C, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: a preliminary investigation. Addict Behav 2006;31:203–210 [DOI] [PubMed] [Google Scholar]
- 36.Ockene J, Ma Y, Zapka J, Pbert L, Valentine GK, Stoddard A. Spontaneous cessation of smoking and alcohol use among low-income pregnant women. Am J Prev Med 2002;23:150–159 [DOI] [PubMed] [Google Scholar]
- 37.Pbert L, Ockene JK, Zapka J, et al. A community health center smoking-cessation intervention for pregnant and postpartum women. Am J Prev Med 2004;26:377–385 [DOI] [PubMed] [Google Scholar]
- 38.Avidano Britton GR, Brinthaupt J, Stehle JM, James GD. The effectiveness of a nurse-managed perinatal smoking cessation program implemented in a rural county. Nicotine Tob Res 2006;8:13–28 [DOI] [PubMed] [Google Scholar]