Abstract
Krüppel-like factor 4 (KLF4) is a zinc-finger transcription factor with tumor suppressive activity in colorectal cancer. Here, we investigated whether KLF4 is involved in maintaining genetic stability in mouse embryonic fibroblasts (MEFs) isolated from mice wild type (+/+), heterozygous (+/−), or homozygous (−/−) for the Klf4 alleles. Compared to Klf4+/+ and Klf4+/− MEFs, Klf4−/− MEFs had both a higher level of apoptosis and rate of proliferation. Quantification of chromosome numbers showed that Klf4−/− MEFs were aneuploid. A higher number of Klf4−/− MEFs exhibited γ-H2AX foci and had higher amounts of γ-H2AX compared to controls. Cytogenetic analysis demonstrated the presence of numerous chromosome aberrations including dicentric chromosomes, chromatid breaks, and double minute chromosomes in Klf4−/− cells but in few, if any, Klf4+/+ or Klf4+/− MEFs. Approximately 25% of Klf4−/− MEFs exhibited centrosome amplification in contrast to the less than 5% of Klf4+/+ or Klf4+/− MEFs. Finally, only Klf4−/− MEFs were capable of anchorage-independent growth. Taken together, these findings demonstrate that MEFs null for the Klf4 alleles are genetically unstable, as evidenced by the presence of aneuploidy, chromosome aberration and centrosome amplification. The results support a crucial role for KLF4 in maintaining genetic stability and as a tumor suppressor.
Keywords: aneuploidy, centrosome amplification, cell cycle, chromosome aberrations, γ-H2AX, KLF4
Introduction
Krüppel-like factor 4 (KLF4) belongs to the Krüppellike factor family of zinc-finger-containing transcription factors that are involved in diverse biological and pathobiological conditions (Dang et al., 2000b; Bieker, 2001; Black et al., 2001; Kaczynski et al., 2003). Expression of KLF4 is enriched in epithelial tissues including the intestine and epidermis (Garrett-Sinha et al., 1996; Shields et al., 1996). In the intestinal epithelium, KLF4 is highly expressed in the postmitotic, differentiated epithelial cells (Shields et al., 1996; McConnell et al., 2007). In vitro, overexpression of KLF4 leads to growth arrest by activating key checkpoints in the cell cycle (Shields et al., 1996; Chen et al., 2001). Similarly, KLF4 has been shown to exert a checkpoint function following DNA damage (Zhang et al., 2000; Yoon et al., 2003; Yoon and Yang, 2004). Consistent with its role as a checkpoint protein, expression of KLF4 is often reduced in tumors such as colorectal cancer and gastric cancer (Dang et al., 2000a; Zhao et al., 2004; Wei et al., 2005; Kanai et al., 2006; Ghaleb and Yang, 2008). The reason for such reduction has been shown to be due to loss of heterozygosity of the KLF4 locus or hypermethylation of the KLF4 promoter in a subset of colorectal cancer, which leads to the conclusion that KLF4 is a tumor suppressor in colorectal cancer (Zhao et al., 2004).
The in vivo functions of KLF4 have been demonstrated by studies of mice with targeted deletion of the Klf4 gene, which showed that KLF4 is important for the barrier function of the skin (Segre et al., 1999) and terminal differentiation of goblet cells in the colon of newborn mice (Katz et al., 2002). However, the effect of KLF4 on epithelial cell tumorigenesis could not be assessed in these models as mice homozygous for Klf4 deletion die within 1 day after birth (Segre et al., 1999; Katz et al., 2002). Experiments involving conditional Klf4 mutant mice did show that loss of Klf4 from the gastric mucosa resulted in epithelial hyperplasia, confirming the antiproliferative activity of KLF4 in vivo (Katz et al., 2005). Recently, it was shown that the intestinal tumor burden was increased when mice heterozygous for the Klf4 alleles were crossed with the ApcMin/+ mice, indicating for the first time that KLF4 has a tumor suppressive effect in the intestine in vivo (Ghaleb et al., 2007b).
A hallmark of cancer is the presence of genetic instability, frequently manifested as aneuploidy (Rajagopalan and Lengauer, 2004; Ganem et al., 2007; Weaver and Cleveland, 2007). One of the contributing factors of genetic instability is abnormal amplification of centrosomes, which increases the frequency of mitotic defects (D’Assoro et al., 2002; Fukasawa, 2005, 2007). Centrosome amplification has been demonstrated in numerous human cancers (Lingle and Salisbury, 1999; Ghadimi et al., 2000; Lingle et al., 2002; Mayer et al., 2003; Salisbury et al., 2004; Chng et al., 2008). A key mechanism responsible for centrosome amplification is the loss of the tumor suppressor p53. Thus, mouse embryonic fibroblasts (MEFs) null for the p53 alleles contain abnormal centrosome number and genetic instability as demonstrated by the presence of aneuploidy (Fukasawa et al., 1996, 1997; Tarapore et al., 2001; Tarapore and Fukasawa, 2002). As such, p53-null status confers to MEFs a growth advantage and a capacity for transformation (Harvey et al., 1993). These studies therefore underscore the critical role of p53 in the maintenance of centrosome duplication and genetic stability.
Because KLF4 has been shown to be a crucial mediator of p53 in the DNA damage response (Zhang et al., 2000; Yoon et al., 2003; Yoon and Yang, 2004; Ghaleb et al., 2005) and is both necessary and sufficient in preventing centrosome amplification following γ radiation-induced DNA damage (Yoon et al., 2005), we sought to determine the role of KLF4 in maintaining genetic stability using MEFs isolated from Klf4-null embryos. We show that Klf4−/− MEFs exhibit evidence of increased DNA damage, chromosome aberrations, centrosome amplification, aneuploidy, and capacity for transformation. These results implicate a critical role for KLF4 in the maintenance of genetic stability.
Results
Klf4-null MEFs are aneuploid
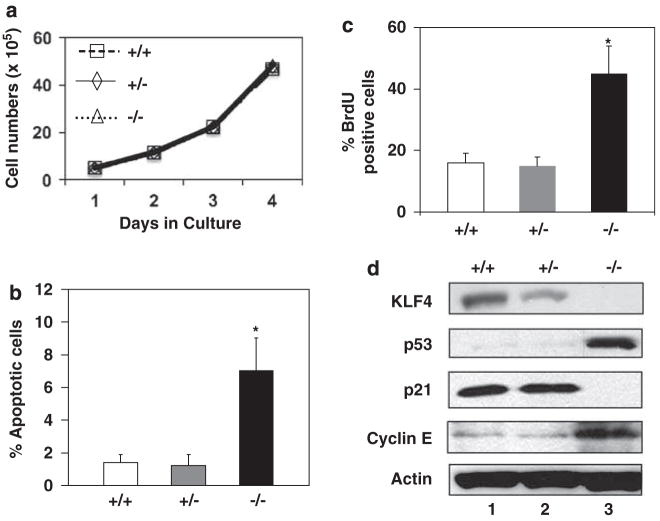
We first examined the growth characteristics in culture of MEFs isolated from days 13.5 Klf4+/+, Klf4+/− and Klf4−/− mouse embryos. As seen in Figure 1a, the growth rates of MEFs of all three genotypes were nearly identical. However, Klf4−/− MEFs had a higher level of apoptosis than Klf4+/+ and Klf4+/− cells as measured by the proportion of cells in the sub-G1 population upon cell-cycle analysis (Figure 1b). Consistent with this finding, Klf4−/− MEFs had a higher amount of cleaved caspase-3 compared to Klf4+/+ MEFs (Supplementary Figure S1), following treatment with tumor necrosis factor-α (TNF-α) at a concentration that induces apoptosis in MEFs (Takada et al., 2007). This result is also similar to the previous finding that Klf4−/− MEFs are more prone to γ-irradiation-induced apoptosis than Klf4+/+ MEFs (Ghaleb et al., 2007a).
Figure 1.
Growth characteristics of Klf4+/+, Klf4+/− and Klf4−/− mouse embryonic fibroblasts (MEFs) in culture. (a) Cells were plated at 105 cells per 60-mm plate. Three plates were counted at each time point and the values represent the mean number of cells per dish. N = 3. (b) The percentages of apoptotic cells 1 day after seeding were measured from the sub-G1 population of cells during flow cytometry. N = 3; *P<0.05 compared to Klf4+/+ MEFs. (c) DNA synthesis was measured by the incorporation of bromodeoxyuridine (BrdU) into replicating cells. Shown are the percentages of cells that stained positive for BrdU. N = 3; *P<0.05 compared to Klf4+/+ MEFs. (d) Western blot analysis of KLF4, p53, p21, cyclin E and β-actin of proteins isolated from cells at 1 day after seeding. Shown are the representative results of four separate experiments.
In addition to having a higher level of apoptosis than Klf4+/+ and Klf4+/− cells, Klf4−/− MEFs had a higher rate of DNA synthesis as measured by the level of incorporation of bromodeoxyuridine (BrdU; Figure 1c and Supplementary Figure S2). This would explain the similar growth rates of MEFs of the three genotypes despite the increased apoptosis in Klf4−/− MEFs. Western blot analysis of Klf4−/− MEFs showed an absence of Klf4 and p21, and a strong induction of p53 and cyclin E when compared to Klf4+/+ and Klf4+/− cells (Figure 1d). These results are consistent with previous reports that KLF4 activates expression of p21 (Zhang et al., 2000; Chen et al., 2001) and represses that of p53 (Rowland et al., 2005) and cyclin E (Yoon et al., 2005).
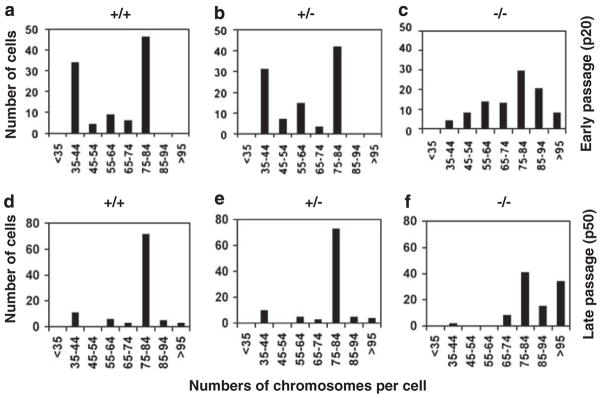
Upon flow cytometric analysis of the cell-cycle profiles of the MEFs, we noticed that Klf4−/− MEFs had a slight shift to a higher DNA content than Klf4+/+ and Klf4+/− MEFs (Supplementary Figure S3). This trend was more apparent in late-passage cells (P50) compared to early-passage cells (P20) (Supplementary Figure S3). We therefore measured the number of chromosomes in cells derived from the three genotypes. As seen in Figure 2, although Klf4+/+ and Klf4+/− MEFs contained a similar distribution of chromosome numbers between the 35–44 and 75–84 ranges at both early and late passages, Klf4−/− MEFs consistently had higher numbers of chromosomes with many cells displaying greater than 85 chromosomes per cell regardless of passage numbers. Even in very early passage cells (P2), a greater proportion of Klf4−/− MEFs had more than 85 chromosomes per cell when compared to Klf4+/+ and Klf4+/− MEFs (Supplementary Figure S4). These results demonstrate that Klf4−/− MEFs are aneuploid.
Figure 2.
Determination of chromosome numbers in mouse embryonic fibroblasts (MEFs). Karyotyping was performed 1 day after seeding in early passages (P20; a–c) and late passages (P50; d–f) MEFs. Spreads from 100 cells were examined per genotype.
Klf4-null MEFs exhibit evidence of DNA damage and chromosome aberrations
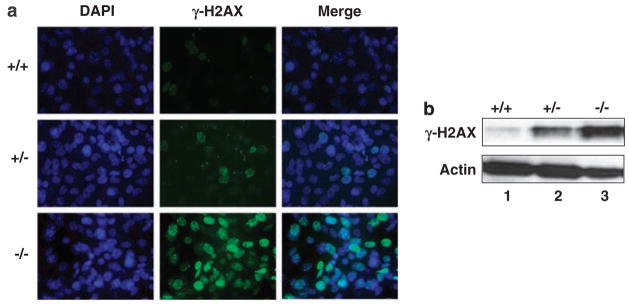
Previous reports indicate that KLF4 is crucial for the cell-cycle checkpoint functions in response to DNA damage (Zhang et al., 2000; Yoon et al., 2003; Yoon and Yang, 2004; Ghaleb et al., 2005). To determine whether cells null for Klf4 exhibit evidence of increasing DNA damage, we performed immunostaining ofMEFs for the presence of γ-H2AX foci, a marker for the DNA damage response (Rogakou et al., 1998). As can be seen in Figure 3a and Table 1, while 16±0.7and 21±1.4% of the Klf4+/+ and Klf4+/− MEFs, respectively, were positive for the presence of γ-H2AX foci, 81±1.4% of the Klf4−/− cells were positive. The increase in γ-H2AX foci formation in Klf4−/− MEFs was confirmed by western blot analysis of γ-H2AX in the three different cell types (Figure 3b).
Figure 3.
Immunostaining and western blotting for γ-H2AX in mouse embryonic fibroblasts (MEFs). (a) Immunostaining was carried out on γ-H2AX in MEFs of the three different genotypes. 4′,6-Diamidino-2-phenylindol (DAPI) stain (blue) was used to visualize nuclei. Shown is a representative result of three independent experiments. (b) Western blot analysis was carried out using an antibody against γ-H2AX or β-actin. Shown is a representative result of three experiments.
Table 1.
Quantification of γ-H2AX stain in MEFsa
| MEF genotype | Total no. of cells counted | No. of cells positive for γ-H2AX | % of cells positive for γ-H2AX |
|---|---|---|---|
| Kl4+/+ | 210 | 34 | 16±0.7 |
| Klf4+/− | 275 | 58 | 21±1.4 |
| Klf4−/− | 264 | 214 | 81±1.4* |
Abbreviation: MEF, mouse embryonic fibroblast.
N = 3 for each genotype.
P<0.05 compared to Klf4+/+ or Klf4+/− MEFs.
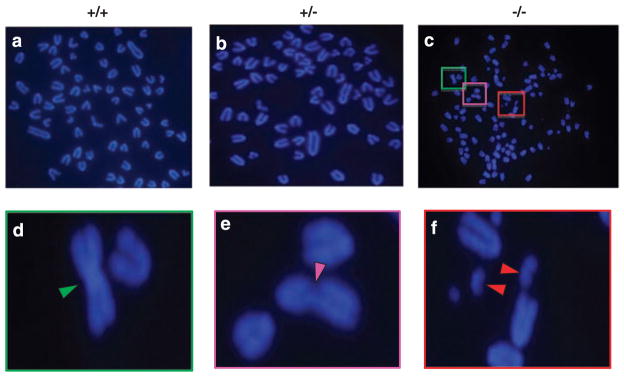
We then performed cytogenetic analysis of the MEFs. As seen in Figure 4, many of the Klf4−/− MEFs exhibited a myriad of chromosome aberrations including dicentric chromosomes, chromatid breaks and double minute chromosomes. In contrast, such aberrations were rare in either Klf4+/+ or Klf4+/− cells (Table 2). These results indicate that deletion of Klf4 in MEFs leads to genetic instability.
Figure 4.
Cytogenetic analysis of mouse embryonic fibroblasts (MEFs). Cytogenetic analysis was carried out in metaphase chromosome spreads prepared from MEFs of the three genotypes. Shown is a typical result of three independent experiments for Klf4+/+ (a), Klf4+/− (b) and Klf4−/− (c) MEFs. The colored boxes illustrate a magnified view of three different chromosome aberrations: (d) dicentric chromosome, (e) chromatid breaks and (f) double minute chromosomes. Arrowheads point to the aberrant chromosomes.
Table 2.
Quantification of chromosome aberrations in MEFsa
| MEF genotype | % of cells with dicentric chromosomes | % of cells with chromatid breaks | % of cells with double minute chromosomes |
|---|---|---|---|
| Kl4+/+ | 2±0.7 | 1±0.7 | 3±0.7 |
| Klf4+/− | 4±0.7 | 2±0.7 | 3±1.4 |
| Klf4−/− | 34±1.4* | 10±0.7* | 40±1.4* |
Abbreviation: MEF, mouse embryonic fibroblast.
One-hundred cells of each cell type were examined for the presence of chromosome aberrations. N=3 for each genotype.
p<0.05 compared to Klf4+/+ or Klf4+/− MEFs.
Loss of Klf4 in MEFs results in centrosome amplification
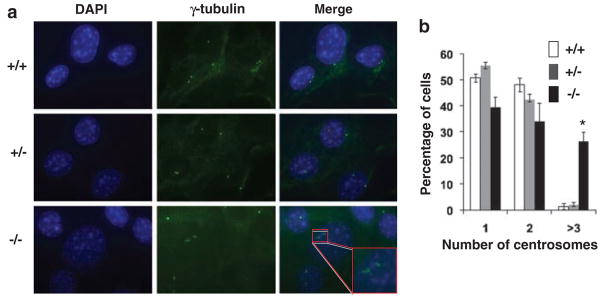
A previous report showed that KLF4 is both necessary and sufficient in preventing centrosome amplification following γ-irradiation-induced DNA damage in the human colon cancer cell line HCT116 (Yoon et al., 2005). To determine whether Klf4 plays a role in regulating centrosome duplication inMEFs, we performed immunostaining for γ-tubulin. As seen from the results in Figure 5, approximately 25% of the Klf4−/− MEFs contained three or more centrosomes per cell, indicating centrosome amplification. In contrast, relatively few Klf4+/+ and Klf4+/− cells exhibited centrosome amplification. A similar centrosome amplification was noted in very early passage (P2) Klf4−/− MEFs (Supplementary Figure S5). These results indicate that Klf4 is involved in the maintenance of centrosome stability in MEFs.
Figure 5.
Centrosome staining of mouse embryonic fibroblasts (MEFs). (a) Centrosome staining was carried out with an antibody against γ-tubulin (Yoon et al., 2005). 4′,6-Diamidino-2-phenylindol (DAPI) stain (blue) was used to visualize the nuclei. Shown is a typical result of four independent experiments. The insert shows a cell with abnormal number (≥3). (b) Histogram showing quantification of centrosome numbers in MEFs with the three genotypes. In total, 100 cells were counted per cell type per experiments. N = 5; *P<0.005 compared to Klf4+/+ cells.
Klf4-null cells are capable of anchorage-independent growth
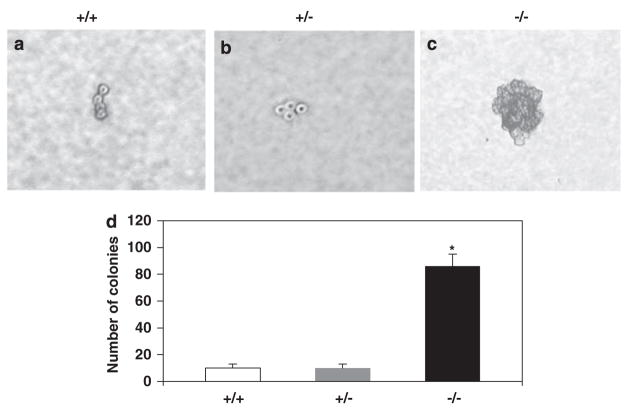
To address whether Klf4−/− cells acquired a transformed property, we examined anchorage-independent growth of MEFs in soft agar. As seen in Figure 6, Klf4−/− but not Klf4+/+ or Klf4+/− MEFs were capable of forming colonies in soft agar. This result suggests that loss of Klf4 renders a transformed phenotype to the MEFs.
Figure 6.
Anchorage-independent growth assays of mouse embryonic fibroblasts (MEFs). MEFs with the three genotypes were seeded in soft agar and formation of colonies determined 3 weeks later. A typical photomicrograph is shown for Klf4+/+ (a), Klf4+/− (b) and Klf4−/− (c) MEFs. Note that Klf4+/+ and Klf4+/− cells typically grew to a four-cell stage as shown in the figure but failed to form any colonies. (d) Quantification of the number of colonies formed in soft agar. Shown are the average numbers of colonies per 10-cm plate. N = 3; *P<0.01 compared to Klf4+/+ MEFs.
Discussion
The control of normal growth process and maintenance of genetic stability requires a complex interacting network of regulatory factors. Genetic instability is commonly present in cancer because of mutation in the genes encoding these regulatory factors (Lengauer et al., 1998). Genetic instability can occur at the level of chromosomes (often manifested as chromosomal instability or CIN) or at the level of nucleotide (often manifested as microsatellite instability or MIN). Aneuploidy, defined as aberrant chromosome numbers, is thought to develop as a result of CIN. The observation that cancer cells harbor aneuploidy was made almost a century ago by Theodor Boveri. Although the exact cause of CIN has not been clearly established, many pathways and processes have been implicated such as chromosomal segregation, checkpoint control and centrosome duplication. Recent studies suggest that aneuploidy acts both to promote tumorigenesis and as a tumor suppressor (Weaver and Cleveland, 2006, 2007).
KLF4 is a member of the Krüppel-like factor family that exhibit important regulatory functions in diverse physiologic processes (Dang et al., 2000b; Bieker, 2001; Black et al., 2001; Kaczynski et al., 2003). Expression of KLF4 is often enriched in tissues that undergo rapid turnover such as the intestine and the epidermis (Garrett-Sinha et al., 1996; Shields et al., 1996). Studies suggest that one of the functions of KLF4 in the intestine is to maintain cells in a quiescent state (Shields et al., 1996; Ghaleb et al., 2005). This is supported by the observation that KLF4 exerts a cell-cycle checkpoint effect in part by acting as a transcriptional activator of the cyclin-dependent kinase inhibitor, p21 (Chen et al., 2001). As such, KLF4 safeguards the G1/S and G2/M checkpoints and mediates the checkpoint functions of p53 following DNA damage (Zhang et al., 2000; Yoon et al., 2003; Yoon and Yang, 2004).
The current study demonstrates that MEFs null for the Klf4 gene exhibit genetic instability as evidenced by the presence of aneuploidy, increasing DNA damage, chromosomal aberrations, centrosome amplification and anchorage-independent growth (Figures 2–6). This does not appear to be a consequence of prolonged propagation in culture as Klf4−/− MEFs at a stage as early as passage 2 exhibit evidence of genetic instability manifested by a trend toward aneuploidy and centrosome amplification (Supplementary Figures S4 and S5). Many of these properties such as aneuploidy and centrosome amplification are similar to those observed in MEFs null for the p53 alleles (Harvey et al., 1993; Fukasawa et al., 1996, 1997). Similarly, p53-null mice are susceptible to radiation-induced carcinogenesis and accumulate chromosome breakage (Lee et al., 1994). The findings of our study are therefore consistent with the fact that KLF4 is a downstream mediator of p53 function (Zhang et al., 2000). Moreover, unlike p53−/− MEFs, which exhibit an increased rate of proliferation (Harvey et al., 1993) when compared to control cells, Klf4−/− MEFs proliferate at a similar rate as Klf4+/+ and Klf4+/− MEFs (Figure 1a). This is because of the combined effect of both an increased rate of apoptosis and proliferation in Klf4−/− MEFs when compared to controls (Figures 1b and c). The susceptibility to apoptosis of Klf4−/− cells is likely because of the absence of p21 (Figure 1d), which has been shown to be an inhibitor of both p53-dependent and -independent apoptosis (Gartel and Tyner, 2002). Consistent with these findings, Klf4−/− MEFs are more susceptible to apoptosis following treatment with TNF-α (Supplementary Figure S1) or γ-irradiation (Ghaleb et al., 2007a) than Klf4+/+ MEFs. These results are also consistent with previous studies that KLF4 exhibit antiapoptotic activity in a context-dependent manner (Rowland et al., 2005; Rowland and Peeper, 2006; Ghaleb et al., 2007a).
It is of interest to note that the level of p53 is elevated in Klf4−/− MEFs in comparison to Klf4+/+ and Klf4+/− cells (Figure 1d). This result is consistent with the previous report that KLF4 acts as a transcriptional repressor of p53 (Rowland et al., 2005). However, despite the relatively high level of p53, Klf4−/− MEFs exhibit genetic instability in a manner similar to p53−/− MEFs. These results are suggestive that KLF4 is downstream from p53 in the ability of p53 to maintain genetic stability.
The centrosome is the major microtubule-organizing center of animal cells and plays a fundamental role in cell division and cell polarity (Kirschner and Mitchison, 1986a, b). Centrosome amplification is often observed in cancers and is thought to contribute to cancer development (Fukasawa, 2005, 2007). This is illustrated by the finding that p53-null cells exhibit centrosome amplification (Fukasawa et al., 1996). We previously showed that KLF4 is necessary and sufficient in preventing centrosome amplification following γ-irradiation-induced DNA damage (Yoon et al., 2005). Here, we show that a significant fraction of Klf4−/− MEFs exhibit evidence of spontaneous centrosome amplification, at both passage 20 (P20) (Figure 5) and passage 2 (Supplementary Figure S5). We attribute this observation to the elevated level of cyclin E in Klf4−/− MEFs (Figure 1d). Cyclin E is a critical factor that controls the duplication of centrosome and its overexpression has been shown to result in centrosome amplification (Tokuyama et al., 2001; Hinchcliffe and Sluder, 2002; Tarapore et al., 2002; Kawamura et al., 2004; Hanashiro et al., 2008). The increased cyclin E level in Klf4−/− MEFs is consistent with our previous report that KLF4 suppresses cyclin E (Yoon et al., 2005). We presume that the resultant centrosome amplification in Klf4−/− MEFs is a contributing factor to the genetic instability in cells lacking Klf4. However, the presence of increased DNA damage and chromosomal aberration in Klf4−/− MEFs would suggest that KLF4 may be involved in the regulation of DNA repair. Alternatively, overexpression of cyclin E has been shown to lead to the formation of double-stranded DNA breaks because of replication fork collapse (Bartkova et al., 2006). Lastly, the mechanism by which Klf4 deletion results in aneuploidy is an open question although, again, deregulated cyclin E has been shown to induce chromosome instability (Spruck et al., 1999). It is also of interest to note that among the target genes suppressed by KLF4, some function to control the spindle assembly checkpoints (Chen et al., 2003). This coupled with the recent finding that overexpression of certain spindle assembly checkpoint genes promotes aneuploidy and tumorigenesis (Sotillo et al., 2007) would suggest that KLF4 may be involved in controlling genetic stability by regulating the spindle assembly checkpoint.
In summary, we provide direct evidence that the absence of KLF4 results in genetic instability and subsequent transformation. This supports a tumor suppressive role for KLF4 in certain tumors as previously observed. Further investigation of the mechanism by which KLF4 controls genetic stability will provide new information on how KLF4 functions as a tumor suppressor.
Materials and methods
Isolation of MEFs and cell culture
Mice heterozygous for the Klf4 alleles (Klf4+/−) on a C57BL/6 background (Katz et al., 2002) were crossbred. MEFs that are wild type (Klf4+/+), heterozygous (Klf4+/−), or null (Klf4−/−) for Klf4 were derived from day 13.5 embryos using the 3T3 protocol as previously described (Todaro and Green, 1963). Briefly, 106 MEFs were plated on 10-cm dishes and maintained in Dulbecco’s modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin at 37 °C in atmosphere containing 5% CO2. Cells were passed every 3 days at a density of 106 cells per 10-cm dish. Unless otherwise specified, experiments were performed on cells at P20.
Cell proliferation and soft-agar assays
For cell proliferation assay, cells were seeded onto six-well plates at a density of 105 cells per well. On a daily basis, cells were trypsinized and counted using a Bright-Line Hemacytometer (Sigma, St Louis, MO, USA). For anchorage-independence assay, MEFs were seeded at a density of 5 × 104 cells per plate in triplicate in 5-cm soft-agar dishes (0.5 and 0.3% bottom and top agar, respectively). The cells were fed fresh media (DMEM with 10% FBS) every 3 days until foci were counted 21 days later.
Bromodoxyuridine uptake studies
Cells were seeded onto coverslips overnight until 70–80% confluence. Cells were pulsed with BrdU for 30 min at a final concentration of 100 μM. Following incubation with BrdU, cells were fixed in cold methanol for 20 min at −20 °C and then rehydrated in phosphate-buffered saline (PBS). Briefly, 800 μl of 2M HCl was added to each wall and incubated at room temperature for 30 min. Cells were washed twice for 5 min in 1ml of 0.1M sodium borate (pH 8.5) and washed for 5 min in PBS before blocking in 2% bovine serum albumin (BSA)/PBS for 1.5 h at room temperature. Anti-BrdU was diluted at 1:50 in 2% BSA/PBS and added to each coverslip and incubated at 4 °C overnight. Coverslips were washed three times in PBS for 5 min and antibody–antigen complexes were detected with Alexa Fluor 488-conjugated goat antimouse antibody diluted 1:500 in 2% BSA/PBS and incubated at room temperature for 1 h. Cells were then washed four times with PBS and counterstained with 4′,6-diamidino-2-phenylindol (DAPI) for 5 min at room temperature in the dark. Finally, cells were washed five times with PBS and mounted in Prolong Antifade kit (Invitrogen), and visualized with a Zeiss 510 confocal microscope. Each experiment was performed in triplicate, and 200 cells were counted per replicate.
TNF-α treatment
MEFs (1 × 105) were plated onto six-well plates 1 day before addition of TNF-α. Cells were then treated or not with 40 ng/ml TNF-α (Sigma) for 18 h. TNF-α-induced cell death was measured by western blot using cleaved caspase-3.
Flow cytometry
Cell-cycle analysis was performed as previously described (Yoon et al., 2003). Cells were rinsed in PBS, trypsinized and resuspended in DMEM containing 10% FBS. Pelleted cells were fixed in 70% with ethanol in PBS and incubated at −20 °C overnight. The fixed cells were pelleted and resuspended in PBS that contain 50 μg/ml propidium iodide, 50 μg/ml RNase A, 0.1% Triton X-100 and 0.1mM ethylene diaminetetraacetic acid at room temperature for 30 min before analysis. Cell-cycle profile analysis was performed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Cytogenetic analysis
Cytogenetic analysis by metaphase spreading of MEFs was performed following standard protocols (Lee et al., 1990) with slight modifications. In brief, cells were initially plated in DMEM containing 10% FBS until they reached 60–70% confluency. Cells then were incubated in the presence of 0.1 μg/ml colcemid (Invitrogen, Carlsbad, CA, USA) for 4 h to induce metaphase arrest, centrifuged and resuspended in 75mM potassium chloride for 10 min. Cells were then fixed with freshly prepared methanol:acetic acid (3:1, v/v) solution drop wise whereas the tubes were vortexed at low speed. The cells were collected by low-speed centrifugation (800 r.p.m.) for 5 min. The cell suspension was then spread onto glass slides then air-dried. Slides were aged at 60 °C overnight before the addition of DAPI. Metaphase spreads images were acquired using an Axioskop 2 plus microscope (Zeiss, Thornwood, NY, USA) equipped with an AxioCam MRc5 CCD camera (Zeiss). The numbers of chromosomes in metaphase (n = 100 cells) from each genotype were counted and analysed.
Centrosome and γ-H2AX immunostaining
Mouse embryonic fibroblasts grown on coverslips were washed with PBS. They were then fixed with cold 100% methanol at −20 °C for 20 min. Cells were then washed three times in PBS before blocking in PBS/0.3% BSA for 1 h at room temperature. FITC-conjugated γ-tubulin antibody was added to final concentration of 10 μg/ml in blocking solution and incubated for 1 h. Cells were then washed three times with PBS and counterstained with DAPI for 5 min at room temperature in the dark. Finally, cells were washed five times with PBS and mounted in Prolong Antifade kit (Molecular probe), and visualized with a Zeiss 510 confocal microscope. Immunostaining for γ-H2AX was carried out as previously described (Dalton et al., 2007).
Western blot analysis
Protein extraction and western blot analysis were as previously described (Yoon et al., 2003). The membranes were immunoblotted with primary antibodies against KLF4, p53, p21, cyclin E and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), γ-H2AX (Upstate Biotechnology, Billerica, MA, USA) and cleaved caspase-3 (Cell Signaling, Danvers, MA, USA). The blots were then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The antibody–antigen complex was visualized by ECL chemiluminescence (Amersham, Pittsburgh, PA, USA).
Supplementary Material
Acknowledgments
We thank Dr Klaus Kaestner and Dr Jonathan Katz for providing the Klf4+/− mice. This work was in part supported by grants from the National Institutes of Health (DK52230, DK64399, and CA84197). EGH was an Emory Fellowships in Research and Science Teaching (FIRST) fellow. AMG was the recipient of a NIH National Research Service Award (CA130308). WBD was supported in part by an Emory Biochemistry, Cell and Developmental Biology (BCDB) training grant.
Abbreviations
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- CIN
chromosomal instability
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- KLF4
Krüppel-like factor 4
- MEFs
mouse embryo fibroblasts
- PBS
phosphatebuffered saline
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and Kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, et al. Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng WJ, Braggio E, Mulligan G, Bryant B, Remstein E, Valdez R, et al. The centrosome index is a powerful prognostic marker in myeloma and identifies a cohort of patients that might benefit from aurora kinase inhibition. Blood. 2008;111:1603–1609. doi: 10.1182/blood-2007-06-097774. [DOI] [PubMed] [Google Scholar]
- D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- Dalton WB, Nandan MO, Moore RT, Yang VW. Human cancer cells commonly acquire DNA damage during mitotic arrest. Cancer Res. 2007;67:11487–11492. doi: 10.1158/0008-5472.CAN-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DT, Bachman KE, Mahatan CS, Dang LH, Giardiello FM, Yang VW. Decreased expression of the gut-enriched Kruppel-like factor gene in intestinal adenomas of multiple intestinal neoplasia mice and in colonic adenomas of familial adenomatous polyposis patients. FEBS Lett. 2000a;476:203–207. doi: 10.1016/s0014-5793(00)01727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000b;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Fukasawa K, Wiener F, Vande Woude GF, Mai S. Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene. 1997;15:1295–1302. doi: 10.1038/sj.onc.1201482. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- Ghadimi BM, Sackett DL, Difilippantonio MJ, Schrock E, Neumann T, Jauho A, et al. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer. 2000;27:183–190. [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Kruppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene. 2007a;26:2365–2373. doi: 10.1038/sj.onc.1210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007b;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb AM, Yang VW. The pathobiology of Kruppel-like factors in colorectal cancer. Curr Colorectal Cancer Rep. 2008;4:59–64. doi: 10.1007/s11888-008-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashiro K, Kanai M, Geng Y, Sicinski P, Fukasawa K. Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells. Oncogene. 2008;27:5288–5302. doi: 10.1038/onc.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P, et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993;8:2457–2467. [PubMed] [Google Scholar]
- Hinchcliffe EH, Sluder G. Two for two: Cdk2 and its role in centrosome doubling. Oncogene. 2002;21:6154–6160. doi: 10.1038/sj.onc.1205826. [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, et al. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395–6402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Izumi H, Ma Z, Ikeda R, Moriyama M, Tanaka T, et al. Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression. Cancer Res. 2004;64:4800–4809. doi: 10.1158/0008-5472.CAN-03-3908. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986a;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, Mitchison T. Microtubule dynamics. Nature. 1986b;324:621. doi: 10.1038/324621a0. [DOI] [PubMed] [Google Scholar]
- Lee JM, Abrahamson JL, Kandel R, Donehower LA, Bernstein A. Susceptibility to radiation-carcinogenesis and accumulation of chromosomal breakage in p53 deficient mice. Oncogene. 1994;9:3731–3736. [PubMed] [Google Scholar]
- Lee JJ, Warburton D, Robertson EJ. Cytogenetic methods for the mouse: Preparation of chromosomes, karyotyping, and in situ hybridization. Anal Biochem. 1990;189:1–17. doi: 10.1016/0003-2697(90)90036-9. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Lingle WL, Barrett SL, Negron VC, D’Assoro AB, Boeneman K, Liu W, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA. 2002;99:1978–1983. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle WL, Salisbury JL. Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol. 1999;155:1941–1951. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F, Stoop H, Sen S, Bokemeyer C, Oosterhuis JW, Looijenga LH. Aneuploidy of human testicular germ cell tumors is associated with amplification of centrosomes. Oncogene. 2003;22:3859–3866. doi: 10.1038/sj.onc.1206469. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- Salisbury JL, D’Assoro AB, Lingle WL. Centrosome amplification and the origin of chromosomal instability in breast cancer. J Mammary Gland Biol Neoplasia. 2004;9:275–283. doi: 10.1023/B:JOMG.0000048774.27697.30. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- !Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ichikawa H, Pataer A, Swisher S, Aggarwal BB. Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 2007;26:1201–1212. doi: 10.1038/sj.onc.1209906. [DOI] [PubMed] [Google Scholar]
- Tarapore P, Fukasawa K. Loss of p53 and centrosome hyperamplification. Oncogene. 2002;21:6234–6240. doi: 10.1038/sj.onc.1205707. [DOI] [PubMed] [Google Scholar]
- Tarapore P, Horn HF, Tokuyama Y, Fukasawa K. Direct regulation of the centrosome duplication cycle by the p53-p21Waf1/Cip1 pathway. Oncogene. 2001;20:3173–3184. doi: 10.1038/sj.onc.1204424. [DOI] [PubMed] [Google Scholar]
- Tarapore P, Okuda M, Fukasawa K. A mammalian in vitro centriole duplication system: evidence for involvement of CDK2/cyclin E and nucleophosmin/B23 in centrosome duplication. Cell Cycle. 2002;1:75–81. [PubMed] [Google Scholar]
- Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Horn HF, Kawamura K, Tarapore P, Fukasawa K. Specific phosphorylation of nucleophosmin on Thr(199) by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J Biol Chem. 2001;276:21529–21537. doi: 10.1074/jbc.M100014200. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Ghaleb AM, Nandan MO, Hisamuddin IM, Dalton WB, Yang VW. Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene. 2005;24:4017–4025. doi: 10.1038/sj.onc.1208576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Yang VW. Requirement of Kruppel-like factor 4 in preventing entry into mitosis following DNA damage. J Biol Chem. 2004;279:5035–5041. doi: 10.1074/jbc.M307631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, et al. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395–402. doi: 10.1038/sj.onc.1207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.