Abstract
The objective of this experiment was to identify circulating growth factors, hormones, and cellular and molecular mechanisms that account for the effects of physical activity on mammary carcinogenesis. A total of 120 female Sprague-Dawley rats were injected with 1-methyl-1-nitrosourea (50 mg/kg) and 7 days thereafter were randomized to either a physically active or a sedentary control group. Individually housed rats were given free access to a nonmotorized, computer-controlled activity wheel and running behavior was reinforced by food reward. Rats self-determined their daily intensity and duration of running. Sedentary control rats received the same amount of food as the physically active rats to which they were paired. Physical activity reduced mammary cancer incidence (P = 0.015) and cancer multiplicity (P = 0.01). Physical activity induced changes in plasma insulin, insulin-like growth factor-I, and corticosterone, suggesting that mechanisms regulating glucose homeostasis were affected. Western blot analyses of mammary carcinomas revealed that proteins involved in cell proliferation were reduced (P < 0.001) and those involved in apoptosis via the mitochondrial pathway were elevated (P < 0.001) by physical activity. The hypothesis that these effects were mediated by activation of AMP-activated protein kinase, and down-regulation of protein kinase B, which collectively down-regulate the activity of the mammalian target of rapamycin, was evaluated. Evidence in support of this hypothesis was found in the Western blot analyses of mammary carcinomas, mammary gland, liver, and skeletal muscle. Collectively, these findings provide a rationale for additional studies of energy-sensing pathways in the elucidation of mechanisms that account for the inhibition of carcinogenesis by physical activity.
Introduction
Physical activity is simply defined as skeletal muscle contraction that results in a quantifiable expenditure of energy (1). Accordingly, investigations into the effects of physical activity on carcinogenesis in human populations involve an effort to quantify a very heterogeneous set of physical activity exposures. Those efforts have frequently categorized physical activity as either occupational (the daily activities of life) or recreational (with exercise being considered as a type of recreational activity; ref. 2). Despite the recognized limitations of the various approaches used to quantify physical activity behaviors, there is substantial evidence that an inverse relationship exists between physical activity and cancer incidence at multiple organ sites (2). However, it is important to recognize that key questions that affect the translation of these observations to public health recommendations remain unanswered. Those issues include the identification of the types, frequencies, durations, and intensities of physical activity that afford protection against cancer and the elucidation of the mechanisms that account for protective activity (3).
Because of the complexity and heterogeneity of physical activity in human populations and the long latency period involved in the development of human cancers, the combined use of animal models for carcinogenesis and physical activity offers the opportunity to study the effects of physical activity in a systematic manner. However, the models available by which to study the effects of physical activity suffer from several limitations that have reduced their usefulness in carcinogenesis experiments; they include the general decline in physical activity observed in experiments of several months duration and the behavior of some animals in which they turn their wheels without actually running in them (reviewed in refs. 4, 5). In this study, a newly developed wheel running instrument was used to investigate how physical activity affects the carcinogenic process in the mammary gland. Using this instrument, physically active animals were given free access to an activity wheel and their running behavior was reinforced via the periodic distribution of food of a predetermined amount for a prescribed distance run. This served to maintain running behavior throughout the experiment. We report inhibition in physically active rats relative to their sedentary pair-fed controls during the postinitiation stage of experimentally induced breast cancer. The postinitiation design of this experiment simulates the promotional/progressional events of the disease process, which is highly relevant to women at increased risk for breast cancer and to breast cancer survivors. The relationship between plasma biomarkers affected by physical activity and the carcinogenic response also was investigated since growth factors such as insulin-like growth factor-I (IGF-I) and insulin, hormones such as estrogen and glucocorticoids, and cytokines such as leptin have been reported to be affected by physical activity and to be involved in the development of cancer. In addition, evidence that intracellular energy-sensing pathways were involved in accounting for protective activity is presented.
Materials and Methods
Chemicals
Primary antibodies used in this study were anti-cyclin D1, anti-E2F-1, and anti-p27Kip1 from Thermo Fisher Scientific; anti-retinoblastoma (Rb), anti-Bcl-2, anti-hILP/X-linked inhibitor of apoptosis protein, and anti-Bax from BD Biosciences; anti-apoptosis protease-activating factor-1 from Millipore; anti-phospho-AMP-activated protein kinase (AMPK; Thr172), anti-AMPK, anti-phospho-mammalian target of rapamycin (mTOR; Ser2448), anti-mTOR, anti-phospho-70-kDa ribosomal protein S6 kinase (p70S6K; Thr389), anti-p70S6K, anti-phospho-eukaryote initiation factor 4E-binding protein 1 (4E-BP1; Thr37/Thr46), anti-4E-BP1, anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-acetyl-CoA carboxylase (ACC; Ser79), anti-ACC, anti-glyceraldehyde-3-phosphate dehydrogenase, and anti-rabbit immunoglobulin-horseradish peroxidase–conjugated secondary antibody, as well as LumiGLO reagent with peroxide, all from Cell Signaling Technology. Anti-p21Cip1 and anti-mouse immunoglobulin-horseradish peroxidase–conjugated secondary antibody were from Santa Cruz. Mouse anti-β-actin primary antibody was obtained from Sigma.
Physical Activity Instrument
The running wheel used for physical activity was developed based in part on earlier work indicating the feasibility of training rats to run for a food reward (6, 7), with numerous modifications including computer-controlled distribution of food to both animals that ran and their paired, sedentary controls. Physically active animals were given free access to an activity wheel and their running behavior was reinforced via the periodic distribution of food of a predetermined amount for a prescribed distance run. This process was automated via the use of a pellet dispenser whose function was integrated with the running wheel under computer control with a proximity sensor being used to verify that the animal was actually running in the wheel. By coupling this running system with the use of a rapid emergence model for breast cancer (8), it was possible to study the effects of physical activity over an 8-week timeframe with the induction of mammary cancer in the majority of animals in the sedentary control group within this experimental duration. Rats voluntarily decided when, how much, and how fast to run each day. Because each physically active rat was paired to a sedentary control animal that received the same amount of food at the same time of day throughout the study, effects on carcinogenesis specifically attributed to physical activity and its associated energy expenditure were investigated.
Experimental Design
Female Sprague-Dawley rats were obtained from Taconic Farms at age 20 days. At age 21 days, rats were injected with 50 mg 1-methyl-1-nitrosourea/kg body weight (i.p.) as described previously (8). Rats were housed individually in solid-bottomed polycarbonate cages. Seven days following carcinogen injection, all rats were randomized into one of two groups, a physically active group and a pair-fed sedentary control group (60 rats per group), and were fed AIN-93G pellet diet (Research Diet). The animals in the physically active group were housed in the cages equipped with a running wheel for physical activity and earned pellets according to the distance they ran. The animals in the pair-fed group ate the same amount as the physically active group. Animal rooms were maintained at 22 ± 1°C with 50% relative humidity and a 12-h light/12-h dark cycle. Rats were weighed daily and palpated for detection of mammary tumors twice per week starting from 29 days post-carcinogen. The work reported was reviewed and approved by the Institutional Animal Care and Use Committee and conducted according to the committee guidelines.
Necropsy
Following an overnight fast, rats at rest were killed over a 3-h time interval via inhalation of gaseous carbon dioxide. The sequence in which rats were euthanized was stratified across groups to minimize the likelihood that order effects would masquerade as treatment-associated effects. After the rats lost consciousness, blood was directly obtained from the retro-orbital sinus and gravity fed through heparinized capillary tubes (Fisher Scientific) into EDTA-coated tubes (Becton Dickinson) for plasma. The bleeding procedure took ∼1 min/rat. Plasma was isolated by centrifugation at 1,000 × g for 10 min at room temperature. Following blood collection and cervical dislocation, liver and gastrocnemius muscle were immediately excised and frozen between clamps that were precooled in liquid nitrogen. Rats were then skinned and the skin to which the mammary gland chains were attached was examined under translucent light for detectable mammary pathologies. All grossly detectable mammary gland lesions were excised for histologic classification. When possible, the pathology-free (by gross examination) contralateral abdominal inguinal mammary gland chain was excised, prepared as a whole mount on transparency film, and snap frozen in liquid nitrogen.
Assessment of Plasma Molecules
Insulin and leptin were determined by commercial ELISA kit from Millipore. IGF-I was determined using a commercial rat enzyme immunoassay kit from Diagnostic Systems Laboratories. Estradiol and corticosterone were determined by commercial enzyme immunoassay kits from Cayman Chemical. C-reactive protein was determined using ELISA kit from BD Biosciences.
Western Blotting
Mammary carcinomas, mammary gland, liver, or muscle were homogenized in lysis buffer [40 mmol/L Tris-HCl (pH 7.5), 1% Triton X-100, 0.25 mol/L sucrose, 3 mmol/L EGTA, 3 mmol/L EDTA, 50 Amol/L β-mercaptoethanol, 1mmol/L phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail (Calbiochem)]. The lysates were centrifuged at 7,500 × g for 10 min in a tabletop centrifuge at 4°C and clear supernatant fractions were collected and stored at −80°C. The protein concentration in the supernatants was determined by the Bio-Rad protein assay.
Western blotting was done as described previously (9). Briefly, protein lysate per sample (40−60 Ag) was subjected to 8% to 16% SDS-PAGE after being denatured by boiling with SDS sample buffer [63 mmol/L Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mmol/L DTT, and 0.01% bromophenol blue] for 5 min and the proteins were transferred to a nitrocellulose membrane. The levels of cyclin D1, E2F-1, Rb, p21Cip1, p27Kip1, Bcl-2, hILP/X-linked inhibitor of apoptosis protein, Bax, apoptosis protease-activating factor-1, phospho-AMPK (Thr172), AMPK, phospho-ACC (Ser79), ACC, phospho-Akt (Ser473), Akt, phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, phospho-4E-BP1 (Thr37/Thr46), 4E-BP1, β-actin, and glyceraldehyde-3-phosphate dehydrogenase were determined using specific primary antibodies followed by treatment with the appropriate peroxidase-conjugated secondary antibodies and visualized by LumiGLO reagent Western blotting detection system. The chemiluminescence signal was captured using a ChemiDoc densitometer (Bio-Rad) that is equipped with a CCD camera having a resolution of 1,300 × 1,030 and run under the control of Quantity One software (Bio-Rad) and analyzed by the software. The actin-normalized or glyceraldehyde-3-phosphate dehydrogenase–normalized scanning density data were reported.
Statistical Analyses
Cancer incidence and multiplicity served as the primary endpoints for this experiment. Cancer burden and plasma, cellular, and molecular determinants were considered secondary endpoints. Differences between groups in the incidence and multiplicity of mammary carcinomas were evaluated, respectively, by χ2 analysis (10) or ANOVA after square-root transformation of cancer counts (11). Differences among groups in the cancer burden (tumor mass), body weight, and plasma molecules were analyzed by ANOVA (12). For Western blots, representative Western bands were shown in the figures. The data displayed in the bar graphs of the figures were either the actin-normalized scanning data for proteins involved in cell cycle or apoptosis or the ratio of the actual scanning units derived from the densitometric analysis of each Western blot for the phospho-proteins involved in energy-sensing pathways. For statistical analyses, the actin-normalized or glyceraldehyde-3-phosphate dehydrogenase–normalized (used for muscle) scanning density data obtained from the ChemiDoc scanner using Quantity One (Bio-Rad) were first rank transformed. This approach is particularly suitable for semiquantitative measurements that are collected as continuously distributed data as is the case with Western blots. The ranked data were then subjected to multivariate ANOVA (13). Ratio data were computed from the scanning units derived from the densitometric analysis (the arbitrary units of absorbance for variables stated), and the ratios were rank transformed and evaluated via multivariate ANOVA. All analyses were done using Systat statistical analysis software version 12.
Results
Physical Activity and Growth
A total of 60 pairs of rats were assigned to the study. Of the 60 rats that were randomized to wheel running, 52 (87%) were judged to be compliant to running in response to food reward and completed the experiment. The 8 rats eliminated either refused to run or ran to a limited extent and failed to gain weight during the first week of the study. The average distance run per day by the 52 rats that completed the study was 7,650 ± 498 m/d. As expected, sedentary rats had a slightly greater rate of body weight gain than their wheel running counterparts, and their final body weights were 7.5% higher (200 ± 11 versus 185 ± 10 g; P < 0.01) than the physically active group (Table 1).
Table 1.
Effect of physical activity on final body weight and the carcinogenic response in the mammary gland
| Treatment | Carcinogenic response |
Final body weight (g) | ||
|---|---|---|---|---|
| Incidence | Average no. per rat | Average mass per rat (g) | ||
| Sedentary control | 98.1 | 3.72 ± 0.32 | 1.16 ± 0.21 | 200.4 ± 1.6 |
| Physically active | 84.6* | 2.67 ± 0.28* | 0.62 ± 0.14 | 185.2 ± 1.4* |
| P | <0.01 | <0.01 | 0.17 | <0.01 |
NOTE: Percent or mean ± SE (n = 52). Differences between groups in the incidence and average number of cancers per rat were evaluated, respectively, by χ2 analysis or ANOVA after square-root transformation. Differences between groups in the body weight and average cancer mass per rat were analyzed by ANOVA.
P < 0.01, compared with sedentary control.
Carcinogenic Response
Greater than 98% of the mammary tumors detected at necropsy were adenocarcinomas and the distribution of benign versus malignant tumors did not differ by treatment group. The effect of physical activity on the carcinogenic response is summarized in Table 1. Cancer incidence (98.1 versus 84.6%; P < 0.01) and average number of cancers per rat (3.72 versus 2.67, respectively; P < 0.01) were reduced by physical activity. The average cancer mass per rat was 0.62 g in the physically active group and 1.16 g in the sedentary control group (P = 0.17).
Plasma Growth Factors and Hormones
The effects of physical activity on growth factors and hormones that have been reported to be affected by physical activity and that may play a role in carcinogenesis are shown in Table 2. Of the plasma analytes reported, only the concentration of corticosterone, IGF-I, insulin, and leptin were shown to be affected by physical activity. Plasma corticosterone was elevated and IGF-I, insulin, and leptin were reduced by physical activity (P < 0.01).
Table 2.
Effect of physical activity on the concentration of growth factors and hormones in plasma
| Treatment | Insulin (ng/mL) | IGF-I (ng/mL) | Corticosterone (ng/mL) | C-reactive protein (μg/mL) | Leptin (ng/mL) | Estradiol (pg/mL) |
|---|---|---|---|---|---|---|
| Sedentary | 1.42 ± 0.12 | 1341 ± 52 | 256 ± 9 | 443 ± 72 | 2.19 ± 0.17 | 16.6 ± 1.5 |
| Physical activity | 0.99 ± 0.09* | 1041 ± 71* | 546 ± 22* | 418 ± 80 | 0.70 ± 0.09* | 14.3 ± 1.3 |
| P | 0.005 | 0.001 | <0.0001 | 0.189 | <0.001 | 0.259 |
NOTE: Mean ± SE (n = 52). Differences between groups were analyzed by ANOVA.
P < 0.01, compared with sedentary control.
Cell Proliferation, Apoptosis, and Angiogenesis
Despite the marked variation among animals in cancer burden, which is typically observed in this cancer model, the 50% reduction in cancer mass in the physical activity treatment was consistent with effects of physical activity on cell proliferation, apoptosis, and/or blood vessel formation within the carcinomas induced. Therefore, mammary carcinomas were analyzed by Western blotting for proteins involved in these processes. As shown in Fig. 1A, levels of cyclin D1, E2F-1, and hyperphosphorylated Rb were reduced and p21Cip1 and p27Kip1 were elevated in physically active versus sedentary controls. Multivariate ANOVA indicated that the pattern of change observed was significant (Hotelling-Lawley statistic, P = 0.001) with the univariate statistics indicating that the greatest effects were on cyclin D1 (P = 0.025) and p21 (P < 0.0001). Figure 1B shows representative Western blots for proteins involved in apoptosis. Mammary carcinomas from physically active rats had lower levels of Bcl-2 and X-linked inhibitor of apoptosis protein and higher levels of Bax and apoptosis protease-activating factor-1. This pattern of protein expression is consistent with physical activity inducing a proapoptotic environment (Hotelling-Lawley multivariate statistic, P = 0.013). Univariate statistics from the multivariate analysis indicated that the effects of physical activity on Bax (P = 0.034), Bcl-2 (P = 0.011), and X-linked inhibitor of apoptosis protein (P = 0.001) were significant. Only one marker of angiogenesis was measured. Vascular endothelial growth factor levels were lower in mammary carcinomas from physically active rats, but the 23% reduction that was observed was not statistically significant (P = 0.07; data not shown).
Figure 1.
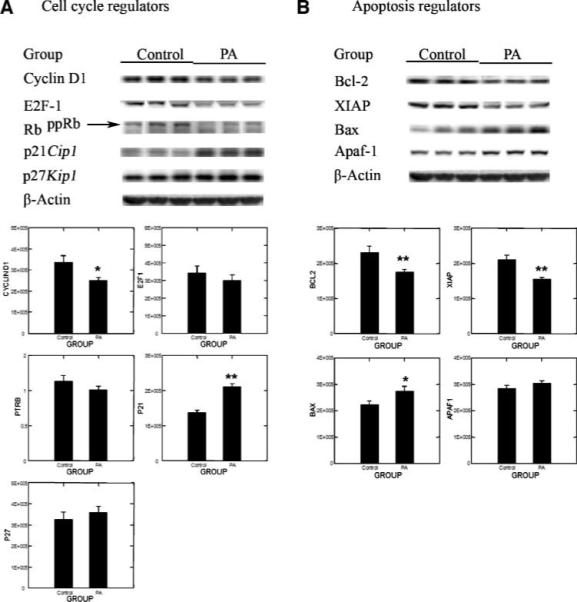
Effects of physical activity (PA) on cell cycle regulators: (A) cyclin D1, E2F-1, RbppRb (hyperphosphorylated Rb), p21Cip1, and p27Kip1 and on apoptosis regulators and (B) Bcl-2, X-linked inhibitor of apoptosis protein (XIAP), Bax, and apoptosis protease-activating factor-1 (Apaf-1) in mammary carcinoma of rats. Representative Western blot images and column graphs for the levels of proteins or the ratio of hyperphosphorylated Rb to Rb (PTRB; n = 8 for each column). The images shown are those directly acquired from the ChemiDoc work station that is equipped with a CCD camera having a resolution of 1,300 × 1,030. The normalized intensity data from the ChemiDoc were evaluated; statistical analyses were done on the ranks of the absorbance data via multivariate ANOVA as described in Materials and Methods. *, P < 0.05; **, P < 0.01, compared with sedentary control (Control).
Signaling Pathways
Based on the effects of physical activity on plasma hormones and growth factors (Table 2), we hypothesized that the energy-sensing pathway of which AMPK and Akt are components would be affected by physical activity and that this in turn would modulate the activity of the mTOR. Specifically, we hypothesized that activated AMPK would be increased and activated Akt would be decreased with decreases in phosphorylated levels of mTOR substrates p70S6K and 4E-BP1. Representative Western blots for the each protein in the pathway and its phosphorylated counterpart are shown in Fig. 2A, and the actual absorbance data normalized to β-actin were used to compute the ratios, which were then rank transformed and analyzed by multivariate ANOVA. The results of the multivariate analysis supported the working hypothesis (Hotelling-Lawley statistic, P = 0.0007). The univariate statistics from that analysis indicated that activated AMPK [ratio of phospho-AMPK (Thr172)/AMPK] was increased by physical activity (P < 0.0001), that AMPK activity was increased as measured by ratio of phospho-ACC (Ser79)/ACC (P = 0.0037), and that activated Akt [ratio of phospho-Akt (Ser473)/Akt (P = 0.013)], mTOR activity [ratio of phospho-mTOR (Ser2448)/mTOR (P = 0.027)], the ratio of phospho-p70S6K (Thr421/Ser424)/p70S6K (P = 0.0007), and the ratio of phospho-4E-BP1 (Thr37/Thr46)/4E-BP1 (P = 0.0005) were reduced by physical activity in comparison with the sedentary control.
Figure 2.
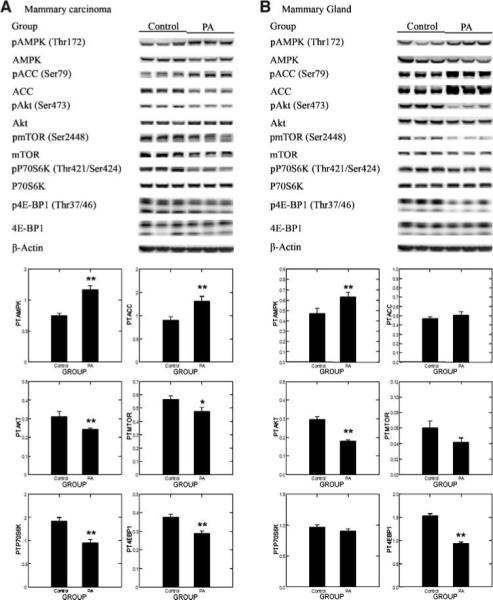
Effects of physical activity on the phosphorylation of AMPK (pAMPK; Thr172), ACC (pACC; Ser79), Akt (pAkt; Ser473), mTOR (pmTOR; Ser2448), p70S6K (pp70S6K; Thr389), and 4E-BP1 (p4E-BP1; Thr37/Thr46) in mammary carcinoma (A) or mammary gland (B) of rats. Representative Western blot images for levels of protein and column graphs for the ratio of phosphorylated to unphosphorylated form (n = 8 for each column). The images shown are those directly acquired from the ChemiDoc work station that is equipped with a CCD camera having a resolution of 1,300 × 1,030. The normalized intensity data from the ChemiDoc were evaluated; statistical analyses were done on the ranks of the ratio data via multivariate ANOVA. *, P < 0.05; **, P <0.01, compared with sedentary control.
To determine if this effect would also be observed in either pathology-free mammary gland or in other tissues involved in energy metabolism, we extended our observations to mammary gland, liver, and muscle. As shown in Fig. 2B, the same pattern of change was observed in pathology-free mammary gland lysates as in the mammary carcinomas, although the effects of physical activity did not reach the level of statistical significance for all the proteins assessed. The multivariate statistic (Hotelling-Lawley statistic, P < 0.0001) supported the hypothesis that this energy-sensing pathway was modulated by physical activity in mammary gland. The effect reached the level of significance for activated AMPK (P = 0.016), 4E-BP1 (P < 0.0001), and Akt (P < 0.0001). Data from the Western blot analysis of liver are shown in Fig. 3A. The multivariate statistic (Hotelling-Lawley statistic, P < 0.00001) supported the hypothesis that this energy-sensing pathway was modulated by physical activity in liver. The effect reached the level of significance for activated AMPK (P = 0.044), AMPK activity measured as phospho-ACC (P = 0.027), 4E-BP1 (P < 0.0001), and Akt (P < 0.007). Data from the Western blot analysis of muscle are shown in Fig. 3B. The multivariate statistic (Hotelling-Lawley statistic, P < 0.000001) supported the hypothesis that this energy-sensing pathway was modulated by physical activity in muscle. The effect reached the level of significance for activated AMPK (P < 0.0001), AMPK activity measured as phospho-ACC (P < 0.00001), and 4E-BP1 (P < 0.0001).
Figure 3.
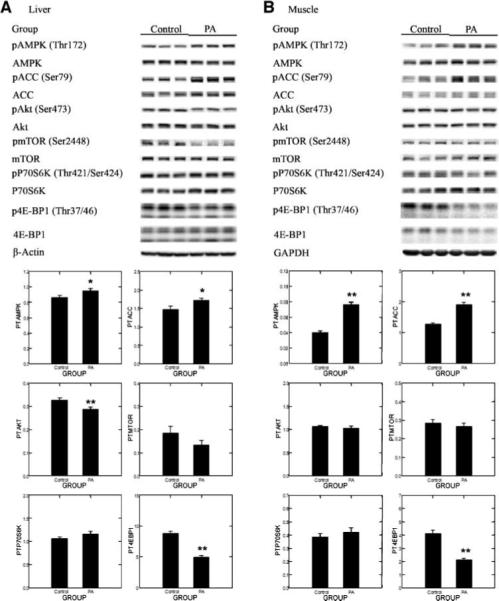
Effects of physical activity on the phosphorylation of AMPK (Thr172), ACC (Ser79), Akt (Ser473), mTOR (Ser2448), p70S6K (Thr389), and 4E-BP1 (Thr37/Thr46) in liver (A) or muscle (B) of rats. Representative Western blot images for the levels of proteins and column graphs for the ratio of phosphorylated to unphosphorylated form (n = 8 for each column). The images shown are those directly acquired from the ChemiDoc work station that is equipped with a CCD camera having a resolution of 1,300 × 1,030. The normalized intensity data from the ChemiDoc were evaluated; statistical analyses were done on the ranks of the absorbance data via ANOVA and/or regression analysis. *, P < 0.05; **, P < 0.01, compared with sedentary control.
Discussion
The objective underlying the experiments reported in this study was to investigate effects on chemically induced carcinogenesis specifically attributed to physical activity and its associated energy expenditure. As shown in Table 1, mammary cancer incidence, cancer multiplicity, and cancer mass/rat were reduced in physically active rats in comparison with sedentary rats fed the same amount as their active counterparts. To our knowledge, this is the first experiment of its type to use a food reward system to reinforce running behavior in the investigation of the effects of physical activity on experimentally induced carcinogenesis. In the following paragraphs, these observations are discussed in greater detail and candidate mechanisms are presented.
Physical Activity Model
In this study, we report for the first time the use of a newly developed running wheel that reinforced physical activity by linking running behavior to a food reward. Because rats voluntarily decided when, how much, and how fast to run, this experiment modeled the effects of occupational activity or what is also called the daily activity of living. The advantages of this approach included (a) the use of Sprague-Dawley rats, (b) the use of food reward instead of adverse stimuli to reinforce running behavior, and (c) the pairing of physically active rats to a sedentary control. Because of the reinforcement system, it was possible to use Sprague-Dawley rats, which are highly susceptible to chemically induced mammary carcinogenesis; in our previous exercise work, it was necessary to use female Fisher 344 rats (14, 15). Fisher rats have inherently higher running activity than Sprague-Dawley rats but are genetically less sensitive to chemically induced mammary carcinogenesis (16). Because of the ability to use Sprague-Dawley rats, our carcinogenesis bioassay in the present study had greater sensitivity to detect changes mediated by physical activity. Moreover, an added advantage of using a food reward system to reinforce running behavior was that it permitted us to adjust caloric intake to the amount run. The final advantage of the system we developed was that each physically active rat was paired to another rat that was designated its sedentary control pair-mate. Although the sedentary control rat did not have access to an activity wheel, it was individually housed and the cage in which it was maintained was equipped with a pellet dispenser that was wired to the pellet dispenser of its physically active partner. Consequently, the sedentary control animal was distributed the same amount of food at the same time of the day as its physically active pair-mate. This approach minimized the likelihood that differences among groups in the pattern of eating would masquerade as physical activity–associated effects on the carcinogenic process.
Physical Activity and the Carcinogenic Response
Physical activity was associated with a modest but statistically significant reduction in several indicators of the carcinogenic response. Cancer incidence was reduced by 16% (P = 0.015) and cancer multiplicity by 30% (P < 0.01). The large differences observed in cancer mass per rat (average cancer mass per rat was 50% lower in physically active rats compared with the sedentary control rats) suggested the value of further investigations of the effects of physical activity on processes that affect tissue size homeostasis, including cell proliferation, apoptosis, and angiogenesis (17-19) as discussed in a subsequent section.
The physically active rats in this study were all very physically active. Rats were categorized into tertiles based on the average distance they ran on a daily basis. Although there were small differences among tertiles in both cancer incidence and cancer multiplicity, these differences were not statistically significant (data not shown). Although it could be concluded from these data that there is no effect of physical activity dose on the carcinogenic response, we judge that such a conclusion would be premature because rats differed markedly in the intensity, duration, timing, and frequency of wheel running bouts that comprised their daily activity profile. Refinements in the design of the activity wheel and the acquisition of data from the wheel and of its computerized control are being made to facilitate investigation of how specific physical activity components affect the carcinogenic process.
Energy Expenditure versus Muscle Contraction
The physical activity model and experimental protocol used in this study focused on evaluating the effects of physical activity on carcinogenesis that were associated with a chronic positive energy balance that resulted in a final group mean body weight that was 7.5% lower in the physically active group than in the sedentary control group. Because the final body weights reflected a relatively small difference in net energy balance that is generally considered to be below the threshold (10%) that is known to confer protection against mammary carcinogenesis (20), our results prompted the question of whether the observed inhibition of carcinogenesis was attributable to effects of physical activity on energy expenditure or to the state of being physically active. In the epidemiologic literature, physical activity has been reported to have effects associated with energy balance as well as effects that are independent of body size and therefore presumably energy balance (21). That physical activity could exert effects on the carcinogenic process that are related to bodily movement per se (to muscle contraction) is consistent with an experiment reported over four decades ago indicating that contracting skeletal muscle produces a factor(s) that inhibits tumor cell growth (22). Moreover, emerging evidence indicates that contracting muscle releases cytokines, called myokines, which have endocrine activity (23); this finding has important implications in that it suggests that skeletal muscle represents the largest endocrine organ in the body. Additional experiments will be required to distinguish between the contributions to the inhibition of mammary carcinogenesis of skeletal muscle contraction per se and those effects due to increased energy expenditure. The new physical activity system introduced in this study has the flexibility to permit the design of experiments to address this important issue.
Cell Proliferation, Apoptosis, and Angiogenesis
As noted above, average cancer mass was reduced by physical activity. To investigate this observation further, mammary carcinomas from the sedentary control and physically active groups were analyzed for levels of proteins that are involved in cell proliferation, apoptosis, and angiogenesis. As shown in Fig. 1, physical activity was associated with changes in cell cycle regulatory proteins that are involved in the G1-S transition and the induction of apoptosis via the mitochondrial (intrinsic) pathway without marked effects on a key regulator of angiogenesis (vascular endothelial growth factor). These observations provide a foundation for in-depth analyses of the effects of physical activity on components of the cellular machinery that are invariably misregulated during the development of cancer (24).
Plasma Hormones and Growth Factors
As reviewed in ref. 3, there is a paucity of data from experimental models about the mechanisms that account for the effects of physical activity on the development of cancer, although a long list of candidate mechanisms has been proposed based on epidemiologic reports (21). Relative to breast cancer, the most frequently cited mechanism is that physical activity decreases circulating levels of estrogen (25). Although the role of estrogen in breast cancer is clear and the contributions of reduced estrogen to protection against breast cancer are best exemplified by the effects of aromatase inhibitors, which inhibit estrogen biosynthesis (26), no evidence was found that indicated that the effects of physical activity on carcinogenesis reported in Table 1 were associated with changes in estrogen metabolism (Table 2), although detailed studies on estrous cycle periodicity would be required to study this mechanism in detail. Nonetheless, although the effects of physical activity on estrogen metabolism are important to understand and may play a role in protecting against breast cancer in some individuals based on the characteristics of their lifestyle including their physical activity behaviors, other factors are likely to be involved in explaining protection. Hence, the model system reported here appears to provide an opportunity to investigate effects of physical activity unrelated to changes in plasma estrogen levels.
As shown in Table 2, evidence was obtained indicating that the physical activity modulated circulating factors that regulate glucose homeostasis. Whereas fasting glucose levels were unaffected by physical activity (data not shown), levels of plasma insulin and IGF-I were reduced and corticosterone was increased. Collectively, these data imply that physical activity induced changes in growth factors and hormones that exert effects on glucose production and utilization. Based on this finding, we formulated the hypothesis that the changes in insulin, IGF-I, and corticosterone induced by physical activity were part of a coordinated response to maintain glucose homeostasis in the face of increased energy expenditure induced by physical activity. We reasoned that if this were the case that intracellular energy networks would be perturbed within tissues in a manner that would constrain biosynthetic activity and increase catabolic processes to maintain intracellular energy homeostasis.
Energy-Sensing Networks
We tested the hypothesis that physical activity would induced AMPK while decreasing the activation of Akt in mammary carcinomas as well as in pathology-free mammary gland and in other tissues (liver and muscle) involved in energy metabolism. As shown in Figs. 2 and 3, evidence consistent with this hypothesis was obtained.
AMPK is an exquisitely sensitive detector of small changes in the intracellular ratio of AMP to ATP, and some investigators have even proposed that AMPK plays a central role in homeostatic regulation of whole-body energy metabolism (27). AMPK is activated via phosphorylation of Thr172, located on the α-catalytic subunit of this heterotrimeric protein. AMPK is phosphorylated on Thr172, by LKB-1, a reaction that occurs due to conformational changes induced in the γ-regulatory subunit of AMPK by its binding of AMP, an event that occurs with greater prevalence when the ratio of AMP/ATP increases within the cell (28). We studied the effects of physical activity on the levels of AMPK and phospho-AMPK (Thr172). It was observed via Western blot analyses that the amount of phospho-AMPK and the ratio of phospho-AMPK/AMPK in mammary carcinomas and mammary gland increased with physical activity. To confirm that physical activity actually increased AMPK activity, the site-specific phosphorylation of ACC, a known substrate for AMPK, was measured. The phosphorylation of ACC (Ser79) increased in response to physical activity. Given that ACC is not directly related to the energy-sensing pathway that we were investigating, such independent confirmation of increased AMPK activity strengthens the observation that physical activity activated AMPK. Moreover, given that inhibition of fatty acid synthesis also has been associated with anticancer activity, and that phosphorylation of Ser79-ACC by AMPK inhibits the activity of ACC, this observation suggests yet another mechanism by which physical activity may inhibit carcinogenesis (29). We extended our investigation of physical activity activation of AMPK to liver and muscle with the benefit of the relative homogeneity of cell types within these tissues. A highly significant positive relationship was observed between activated AMPK and physical activity (data not shown); collectively, these observations were consistent with emerging evidence that AMPK functions as a cellular energy sensor (30) and that physical activity activates AMPK both in the target tissue for the inhibition of cancer and in tissues involved in energy metabolism.
Akt
Physical activity has been reported to decrease circulating levels of IGF-I (31) and reduced levels of circulating IGF-I were observed in this study (Table 2). Lower levels of IGF-I would be expected to down-regulate signaling via the pathway of which IGF-I receptor, phosphoinositide kinase-3, and Akt are components. Of these proteins, activated Akt, a serine/threonine kinase, is the critical affecter molecule. Akt is activated by its phosphorylation on Ser473. Phospho-Akt serves important roles in cell proliferation, cell survival, and new blood vessel formation that are associated with tumor development (32). As shown in Figs. 2 and 3, physical activity was observed to be associated with reduce levels of phospho-Akt (total amount or the ratio of phospho-Akt/Akt) in all tissues assessed, but the effect reached the level of statistically significance only in mammary carcinomas, mammary gland, and liver.
mTOR
Activated AMPK has several targets that are likely to be directly relevant to carcinogenesis, but one of particular interest is mTOR, an evolutionarily conserved serine/threonine kinase that is a key regulator of protein translation and synthesis. mTOR is centrally involved in cell growth (increase in cell size and cell mass) and these processes are tightly coupled to cell division (reviewed in ref. 33). Although it is clear that reduced levels of activated Akt are likely to affect proliferation, apoptosis, and angiogenesis by mechanisms independent of mTOR (reviewed in ref. 34), the finding that physical activity induced both AMPK activation and down-regulation of growth factor signaling via Akt points to mTOR as a downstream target mediating the effects of physical activity because the activity of AMPK and Akt is integrated via tuberous sclerosis 2, which regulates mTOR. That mTOR may represent a molecular target that is regulated by physical activity to inhibit carcinogenesis is a currently unappreciated possibility. The observed changes in p70S6K and 4E-BP1 reported in Figs. 2 and 3 provide additional evidence consistent with the down-regulation of mTOR activity by physical activity. The linkage of this effect with the effects of physical activity on cell proliferation and apoptosis is the topic of ongoing investigations in our laboratory.
Other Considerations
As noted above, there are a host of candidate mechanisms that have been cited to explain the effects of physical activity on the carcinogenic process. One of these is that physical activity suppresses chronic inflammation. As a preliminary test of this idea, plasma levels of C-reactive protein were assessed. Physical activity was found to be without effect on circulating levels of C-reactive protein. Another molecule frequently cited is leptin, a cytokine produced by adipocytes. Plasma leptin was observed to be reduced by physical activity (Table 2), a finding that is likely to reflect changes in body fat due to physical activity.
Summary
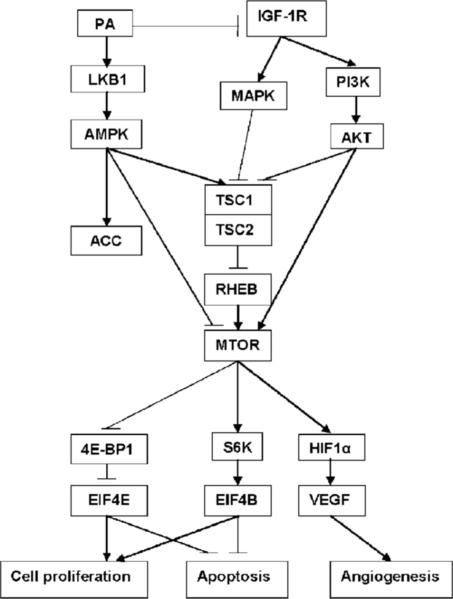
Although evidence continues to grow indicating that a physically active lifestyle has beneficial effects against several chronic diseases and also prolongs survival (retards aging), limited progress has been made in the identification of the molecular events that account for health benefits. A combination of models for physical activity and experimentally induced breast cancer in rats has been delineated in this article that permitted investigation of mechanisms that may be involved in mediating the effects of a physically active lifestyle on the development of cancer. Our finding that physical activity induced the activation of AMPK while reducing levels of activated Akt provides a new lens through which to search for common causal mechanisms that account for the health benefits of physical activity. As diagrammed in Fig. 4, our findings point to key pathways involved in energy metabolism and in biosynthetic activity that have been reported to be misregulated during carcinogenesis as targets by which physical activity protects against cancer. These new insights about candidate mechanisms of action are likely to have significant translational implications that not only permit more specific recommendations about the type, intensity, duration, and frequency of physical activity that protect against cancer but also identify how physical activity can be used in combination with therapeutic modalities to improve cancer treatment efficacy.
Figure 4.
Energy-sensing pathways affected by physical activity.
Acknowledgments
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Nicholas Fernandez, Vanessa Fitzgerald, Andre Powell, Jennifer Price, Denise Rush, and Jay Waterman for excellent technical assistance.
Grant support: National Cancer Institute, USPHS grant CA100693.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 2.IARC . Weight control and physical activity. IARC Press; Lyon: 2002. pp. 1–355. IARC Handbook of Cancer Prevention. [Google Scholar]
- 3.Thompson HJ. Pre-clinical investigations of physical activity and cancer: a brief review and analysis. Carcinogenesis. 2006;27:1946–9. doi: 10.1093/carcin/bgl117. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman-Goetz L. Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc. 2003;35:1828–33. doi: 10.1249/01.MSS.0000093621.09328.70. [DOI] [PubMed] [Google Scholar]
- 5.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46:135–41. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 6.Bronson FH. Puberty in female rats: relative effect of exercise and food restriction. Am J Physiol. 1987;252:R140–4. doi: 10.1152/ajpregu.1987.252.1.R140. [DOI] [PubMed] [Google Scholar]
- 7.Manning JM, Bronson FH. Effects of prolonged exercise on puberty and luteinizing hormone secretion in female rats. Am J Physiol. 1989;257:R1359–64. doi: 10.1152/ajpregu.1989.257.6.R1359. [DOI] [PubMed] [Google Scholar]
- 8.Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16:2407–11. doi: 10.1093/carcin/16.10.2407. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Zhu Z, Thompson HJ. Effect of energy restriction on cell cycle machinery in 1-methyl-1-nitrosourea-induced mammary carcinomas in rats. Cancer Res. 2003;63:1228–34. [PubMed] [Google Scholar]
- 10.Snedecor GW, Cochran WG. Statistical methods. 8th ed. Iowa State University Press; Ames (IA): 1989. pp. 1–503. [Google Scholar]
- 11.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd ed. W.H. Freeman; New York: 1995. [Google Scholar]
- 12.Hochberg Y, Tamhane AC. Multiple comparison procedures. John Wiley & Sons; New York (NY): 1987. pp. 1–450. [Google Scholar]
- 13.Morrison DF. Multivariate statistical methods. 3rd ed. McGraw-Hill Publishing Co.; New York: 1990. [Google Scholar]
- 14.Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 1994;54:1960–3s. [PubMed] [Google Scholar]
- 15.Thompson HJ, Westerlind KC, Snedden JR, Briggs S, Singh M. Inhibition of mammary carcinogenesis by treadmill exercise. J Natl Cancer Inst. 1995;87:453–5. doi: 10.1093/jnci/87.6.453. [DOI] [PubMed] [Google Scholar]
- 16.Gould MN, Lubet RA, Kelloff GJ, Haag JD. Inherited susceptibility and acquired allelic imbalance in rat mammary carcinogenesis. J Cell Biochem Suppl. 1996;25:37–40. [PubMed] [Google Scholar]
- 17.Thompson HJ, Strange R, Schedin PJ. Apoptosis in the genesis and prevention of cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:597–602. [PubMed] [Google Scholar]
- 18.Thompson HJ, Jiang W, Zhu Z. Mechanisms by which energy restriction inhibits carcinogenesis. Adv Exp Med Biol. 1999;470:77–84. doi: 10.1007/978-1-4615-4149-3_8. [DOI] [PubMed] [Google Scholar]
- 19.Thompson HJ, McGinley JN, Wolfe P, Spoelstra NS, Knott KK. Targeting angiogenesis for mammary cancer prevention: factors to consider in experimental design and analysis. Cancer Epidemiol Biomarkers Prev. 2004;13:1173–84. [PubMed] [Google Scholar]
- 20.Zhu Z, Haegele AD, Thompson HJ. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis. 1997;18:1007–12. doi: 10.1093/carcin/18.5.1007. [DOI] [PubMed] [Google Scholar]
- 21.McTiernan A, Stanford JL, Weiss NS, Daling JR, Voigt LF. Occurrence of breast cancer in relation to recreational exercise in women age 50−64 years. Epidemiology. 1996;7:598–604. doi: 10.1097/00001648-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman SA, Paschkis KE, Debias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res. 1962;22:597–9. [PubMed] [Google Scholar]
- 23.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Friedenreich CM. Physical activity and breast cancer risk: the effect of menopausal status. Exerc Sport Sci Rev. 2004;32:180–4. doi: 10.1097/00003677-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Briest S, Davidson NE. Aromatase inhibitors for breast cancer. Rev Endocr Metab Disord. 2007;8:215–28. doi: 10.1007/s11154-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 28.Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–73. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 29.Brunet J, Vazquez-Martin A, Colomer R, et al. BRCA1 and acetyl-CoA carboxylase: the metabolic syndrome of breast cancer. Mol Carcinog. 2008;47:157–63. doi: 10.1002/mc.20364. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Z, Jiang W, McGinley J, Wolfe P, Thompson HJ. Effects of dietary energy repletion and IGF-1 infusion on the inhibition of mammary carcinogenesis by dietary energy restriction. Mol Carcinog. 2005;42:170–6. doi: 10.1002/mc.20071. [DOI] [PubMed] [Google Scholar]
- 32.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 33.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younes H, Leleu X, Hatjiharissi E, et al. Targeting the phosphatidylinositol 3-kinase pathway in multiple myeloma. Clin Cancer Res. 2007;13:3771–5. doi: 10.1158/1078-0432.CCR-06-2921. [DOI] [PubMed] [Google Scholar]