Abstract
Objective
The inflammatory cytokine interleukin-1 (IL-1) decreases mineralization by immortalized mouse-derived cementoblastic cells (OC-CM cells), while various prostanoids, including fluprostenol (flup) increase it. Subtraction hybridization conducted on flup- minus IL-1-treated OC-CM cells revealed that one of the primary response genes preferentially induced by flup is the transcription factor Nur77. The objective of this study was to examine the signal transduction cascades regulating prostanoid induction of Nur77 gene expression in OC-CM cells.
Methods
Confluent OC-CM cells were treated with prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), specific activators of the various EP prostanoid receptors and of the FP prostanoid receptor, and direct activators/inhibitors of the cyclic AMP-protein kinase A (PKA), protein kinase C (PKC) and intracellular calcium pathways. Nur77 gene expression was examined by mRNA extraction and northern blot analysis.
Results
PGE2 and PGF2α treatment of OC-CM cells significantly increased Nur77 mRNA expression in a time- and dose-dependent fashion. Both the EP1 prostanoid receptor-specific activator 16, 16 dimethyl PGE2 and the FP prostanoid receptor-specific activator flup significantly increased Nur77 gene expression by OC-CM cells as compared to vehicle-treated controls. Increase in Nur77 gene expression was also observed when direct activators of the PKA, PKC and intracellular calcium pathways were used to treat OC-CM cells. Direct inhibition of the PKA, PKC and intracellular calcium pathways abrogated Nur77 gene expression induced by OC-CM cell treatment with PGE2 and PGF2α.
Conclusion
Nur77 is a primary gene expressed by OC-CM cells and its induction appears to be mediated by the PKA, PKC and intracellular calcium pathways. Nur77 may affect expression of downstream target genes in OC-CM cells and partially regulate cementoblast cell function.
Keywords: Cementum, cementogenesis, periodontal regeneration, prostaglandin
INTRODUCTION
Periodontitis is an inflammatory condition of the supporting structures of the teeth and is characterized by clinical attachment loss and alveolar bone destruction (1). As periodontitis progresses periodontal pocket depth increases and that contributes to the perpetuation of the disease process (2). Deep pockets favor plaque accumulation, which results in more inflammation and tissue destruction (3). An important goal in the treatment of periodontitis is the reduction of periodontal pockets to a physiologic depth, which will lead to the creation of an environment that facilitates plaque control (4). Ideal pocket reduction involves the regeneration of bone, periodontal ligament (PDL) fibers and cementum (5). However, de novo formation of cementum in the periodontal regenerative process is difficult and often incomplete (6). Therefore, understanding cementoblast biology and the cellular and molecular events associated with cementogenesis may lead to therapeutic strategies aimed at predictable periodontal regeneration.
Interleukin-1 (IL-1) and prostaglandins (PGs) are inflammatory mediators that have been associated with the initiation and progression of periodontitis (7). Paradoxically, the effects of IL-1 and PGs on osteoblastic function are distinctly different. While IL-1 has a catabolic effect on bone (8-10), prostaglandin E2 (PGE2) has a biphasic effect on osteoblast function (11-14). Depending on the dosage and mode of administration, PGE2 can either promote or inhibit the formation of bone (15-17).
The effects of IL-1 and PGs on cementum are less well understood than those on bone. Locally delivered PGE1 increases the formation of alveolar bone, PDL and cementum in adult dogs (18). IL-1 inhibits mineralization by an immortalized cementoblastic cell line (OC-CM cells) while PGE2 and PGF2α have an anabolic effect on mineral nodule formation by OC-CM cells (19). Fluprostenol (flup), a synthetic prostanoid agent with a molecular structure similar to PGF2α, exerts a well-defined, dose-dependent anabolic effect on mineralization by OC-CM cells (19). While this anabolic effect appears to be, in part, mediated by activation of the protein kinase C (PKC) signaling pathway (19), the exact molecular mechanism of the prostanoid-induced mineralization by OC-CM cells is not well understood.
The effects of prostanoids on cells are mediated through G protein-coupled prostaglandin receptors (20). PGE2 activates four EP receptor subtypes (EP1-4), while PGF2α, and the synthetic PGF2α analog flup, activate FP receptors. The EP1 and FP receptors preferentially activate the PKC signaling pathway and calcium signaling. EP2 and EP4 receptors activate, while EP3 receptors inhibit, the cyclic AMP-protein kinase A (PKA) signaling pathway (19, 21).
In an attempt to elucidate the mechanism by which prostanoids increase mineralization by cementoblastic cells, cDNA subtraction hybridization of OC-CM cells treated with flup minus those treated with IL-1 was conducted in presence of the protein synthesis inhibitor cycloheximide (22). The subtraction hybridization revealed several primary response genes that were preferentially induced by flup, which may therefore be involved in the regulation of cementoblast function (22). One of the preferentially flup-induced genes in OC-CM cells is Nur77, an NGFI-B nuclear orphan receptor whose role as a transcription factor is well documented (23, 24). From these findings, it could be hypothesized that induction of the Nur77 gene affects expression of downstream target genes in OC-CM cells and partially regulates cementoblast function. The purpose of this study was to examine the signaling cascades that mediate prostanoid regulation of Nur77 gene expression in OC-CM cells.
MATERIALS AND METHODS
Materials
PGE2, PGF2α, phorbol 12-myristate 13-acetate (PMA), forskolin (FSK), ionomycin, H89 and 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA) were purchased from Sigma (Saint Louis, MO); 16,16-Dimethyl-prostaglandin E2 (16,16-DM-PGE2) from Biomol (Plymouth Meeting, PA); and butaprost, sulprostone, misoprostol, and flup from Cayman (Ann Arbor, MI).
Cell culture
OC-CM cells (a gift from Dr. Martha J. Somerman, University of Washington, Seattle) were grown on 10 cm tissue culture dishes (Corning, Corning, NY) in DMEM (Gibco, Grand Rapids, MI) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco), 100 mU/ml penicillin and 100μg/ml streptomycin (Pen/Strep, Gibco) at 37°C in 5% CO2 and were passaged at 30,000 cells/ml. Cells between passages 14 and 19 were used for experiments. Upon confluence, the culture medium was changed and cell treatment with various agents was initiated. Cell treatment was performed for 30 minutes up to 8 hours in various experiments.
RNA isolation and northern blot analysis
Total RNA was isolated by a single-step method using Trizol (Invitrogen, Carlsbad, CA). Twelve micrograms of RNA were fractionated by electrophoresis on a 1% agarose gel containing 9% formaldehyde for 2.5 hours. RNA was transferred overnight to a Gene Screen Plus hybridization membrane (NEN Life Science Products, Boston, MA) and exposed to UV using a SpectroLinker XL-100 (Spectronics Corporation, Westbury, NY). Radioactive cDNA probes were made using Nur77 and GAPDH cDNA, random primers, DNA polymerase, α32P-radiolabelled cytosine, and a mixture of thymine, guanine, and adenine. Membranes were hybridized using QuikHyb hybridization solution (Stratagene, La Jolla, CA) for 1 hour at 68°C in the presence of 1×107 to 2×107 counts of 32P-labeled probe.
Membranes were washed twice in 2 × SSC and 0.1% SDS for 15 minutes at room temperature and once in 0.1 × SSC and 0.1% SDS for 15 minutes at 55°C. Membranes were used to expose radiographic films at -80°C using an intensifying screen.
Phosphoimaging
Membranes were exposed to a phospho-imager screen (Fisher Scientific, Pittsburgh, PA) at room temperature overnight. Band intensity was assessed with the use of a Storm 840 PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and ImageQuant software (General Electric Healthcare Bio-Sciences Corporation, Piscataway, NJ).
Data analysis and statistics
All experiments were performed at least in triplicate. Sample means and standard errors were calculated utilizing Microsoft Excel (Redmond, WA). The independent variables for all experiments were treatments administered to OC-CM cell cultures. The dependent variable was the mRNA gene expression of Nur77. The outcome variable was the ratio of Nur77 to GAPDH mRNA as determined by ImageQuant. Parametric statistics were used, as the outcome was quantitative and continuous. T-tests and two-way ANOVA were used to compare means of the three experiments across treatment groups. T-tests were performed using Excel. ANOVA was performed using the Prism software (GraphPad, San Diego, CA), with post-test repeated measures analyzed by the Bonferroni procedure. P-values ≤ 0.05 were considered significant.
RESULTS
PGE2 and PGF2α increased Nur77 mRNA gene expression by OC-CM cells
The expression of Nur77 in the mRNA of OC-CM cells treated with PGE2 and PGF2α occurred in a time- and dose-dependent fashion. Nur77 mRNA gene expression by OC-CM cells treated with PGE2 and PGF2α was significantly increased at 30 minutes after treatment, peaked at 1 hour, and returned to baseline levels by 4 hours (figure 1). Maximum expression of Nur77 in mRNA was observed when OC-CM cells were treated for 1 hour with PGE2 at a concentration of 10-5M, with a significant effect occurring at a concentration as low as 10-8M (figure 2). Similarly, PGF2α treatment of OC-CM cells resulted in maximum expression of Nur77 in mRNA of OC-CM cells at a concentration of 10-5M, with a significant effect observed at a concentration as low as 10-9M (figure 2).
Figure 1.
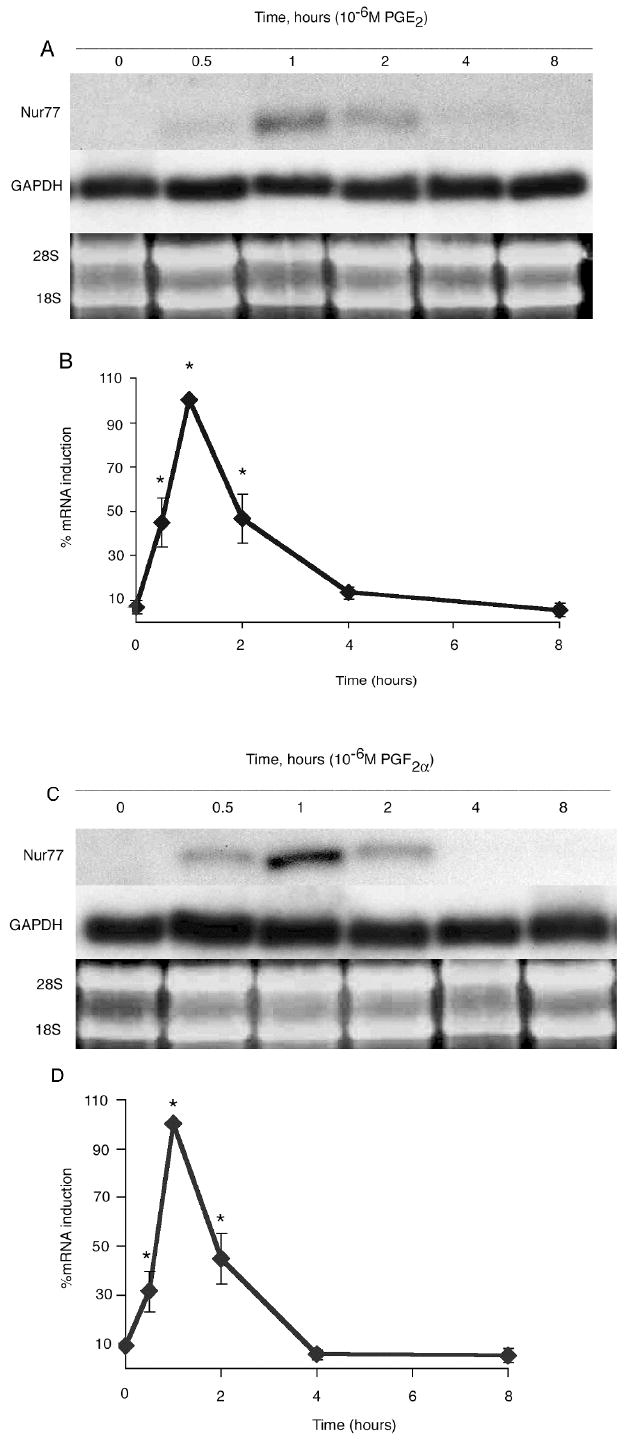
Nur77 mRNA gene expression by OC-CM cells treated 10-6M PGE2 and 10-6M PGF2α: time courses. Confluent OC-CM cells were treated with 10-6M PGE2 or 10-6M PGF2α for 0 to 8 hours. A) PGE2 treatment: Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) PGE2 treatment: Densitometric values of Nur77 mRNA relative to GAPDH mRNA. C) PGF2α treatment: Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. D) PGF2α treatment: Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Significantly different from baseline, p<0.05.
Figure 2.
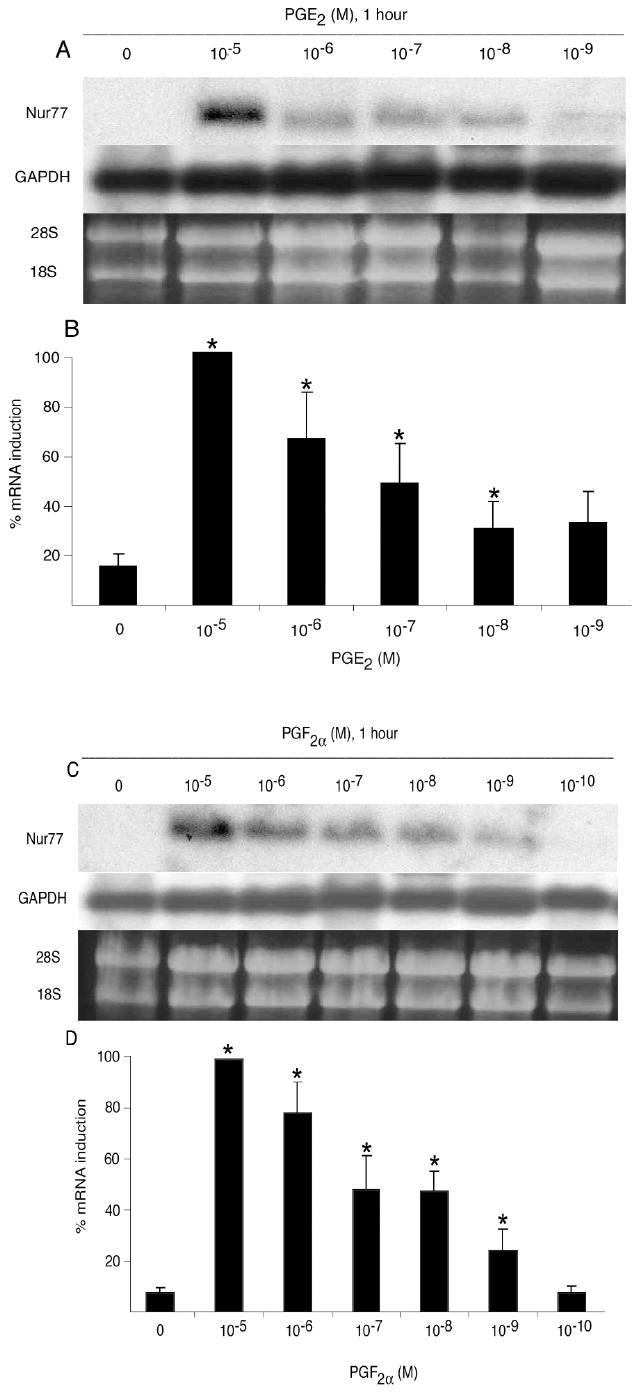
Nur77 mRNA gene expression by OC-CM cells treated with PGE2 and PGF2α: dose responses. Confluent cells were treated with various concentrations of PGE2 and PGF2α for 1 hour. A) PGE2 treatment: Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) PGE2 treatment: Densitometric values of Nur77 mRNA relative to GAPDH mRNA. C) PGF2α treatment: Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. D) PGF2α treatment: Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Significantly different from vehicle-treated control, p<0.05.
Effect of selective EP and FP prostanoid receptor agonists on Nur77 mRNA gene expression by OC-CM cells
PGE2 and PGF2α signal through activation of EP and FP prostanoid receptors. In order to examine the role played by the four EP prostanoid receptors as well as by the FP prostanoid receptor on the increased expression of Nur77 in mRNA of cementoblastic cells treated with PGE2 and PGF2α, OC-CM cells were treated with various EP and FP prostanoid receptor activators (figure 3). Selective activators of the EP2 (butaprost), EP3 (sulprostone) and of the EP2, EP3 and EP4 (misoprostol) prostanoid receptors did not affect Nur77 mRNA gene expression by OC-CM cells. However, the selective EP1 prostanoid receptor agonist 16,16-DM-PGE2 and the selective FP prostanoid receptor agonist flup significantly increased Nur77 mRNA gene expression by OC-CM cells as compared to vehicle-treated controls.
Figure 3.
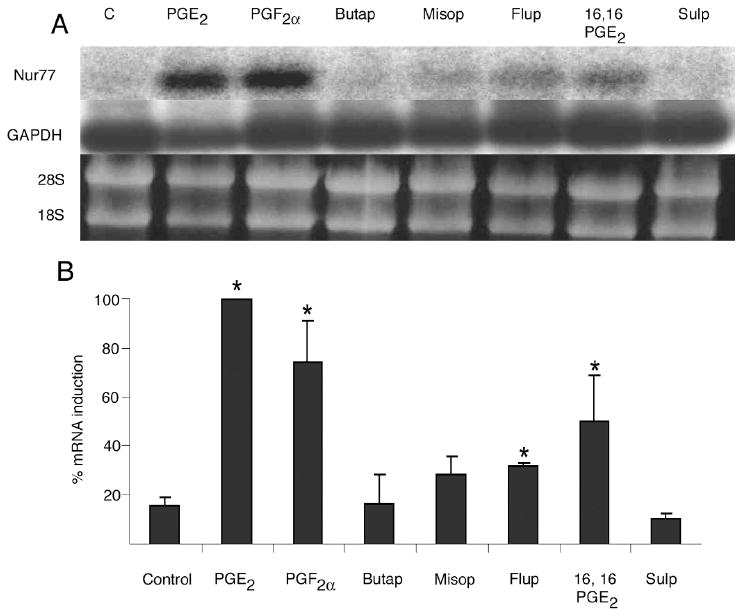
Effect of specific prostanoid receptor activators on Nur77 mRNA gene expression by OC-CM cells. Confluent cells were treated for 1 hour with vehicle (EtOH), 10-6M PGE2, 10-6M PGF2α, 10-7M butaprost (butap), 10-5M misoprostol (misop), 10-6M fluprostenol, 10-6M 16,16 dimethyl PGE2 (16,16 PGE2), 10-4M sulprostone (sulp). A) Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Significantly different from vehicle-treated control, p<0.05.
The effect of direct intracellular signaling pathway activators and inhibitors on Nur77 mRNA gene expression by OC-CM cells
PGE2 and PGF2α, by virtue of binding to EP and FP prostanoid receptors, can activate both the PKA and the PKC signaling pathways and can also regulate intracellular calcium (20). As an attempt to dissect the intracellular pathway(s) involved in the modulation of Nur77 mRNA gene expression by OC-CM cells treated with PGE2 and PGF2α, cells were treated with direct activators/inhibitors of the PKA and PKC signaling pathways and with a direct regulator of intracellular calcium. FSK (a direct activator of the PKA pathway) at a concentration of 10-5M, PMA (a direct activator of the PKC pathway) and ionomycin (a direct regulator of intracellular calcium levels) both at a concentration of 10-6M, significantly increased Nur77 mRNA gene expression as compared to vehicle-treated controls (figure 4).
Figure 4.
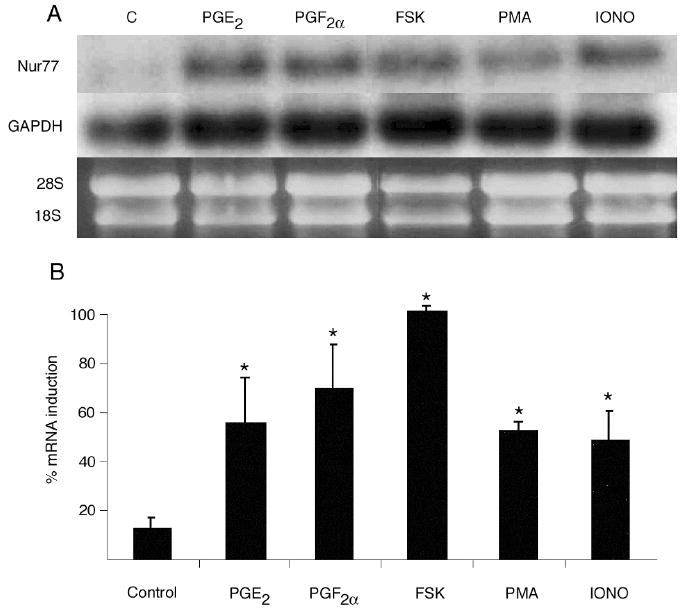
Effect of direct signaling pathway activators on Nur77 mRNA gene expression by OC-CM cells. Confluent cells were treated for 1 hour with vehicle (EtOH), 10-6M PGE2, 10-6M PGF2α, 10-5M forskolin (FSK – a direct PKA pathway activator), 10-6M phorbol 12-myristate 13-acetate (PMA – a direct PKC pathway activator), 10-6M ionomycin (Iono – a regulator or intracellular calcium level). A) Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Significantly different from vehicle-treated control, p<0.05.
Inhibition of the PKA pathway by incubating OC-CM cells with H89 at a concentration of 3 × 10-5M for 1 hour completely abrogated the increased Nur77 mRNA gene expression observed when OC-CM cell were treated with PGE2, PGF2α or FSK (figure 5). When OC-CM cells were incubated overnight with 10-6M PMA in order to exhaust and therefore inhibit the PKC pathway, Nur77 mRNA gene expression was significantly decreased as compared to OC-CM cells that were incubated overnight with the vehicle only prior to treatment with PGE2, PGF2α or PMA (figure 6). Similarly, inhibition of the intracellular calcium pathway by incubation of OC-CM cells with 2 × 10-5M BAPTA for 1 hour resulted in a significant decrease in Nur77 mRNA gene expression relative to controls as compared to cell cultures pre-incubated with BAPTA vehicle only (figure 7).
Figure 5.
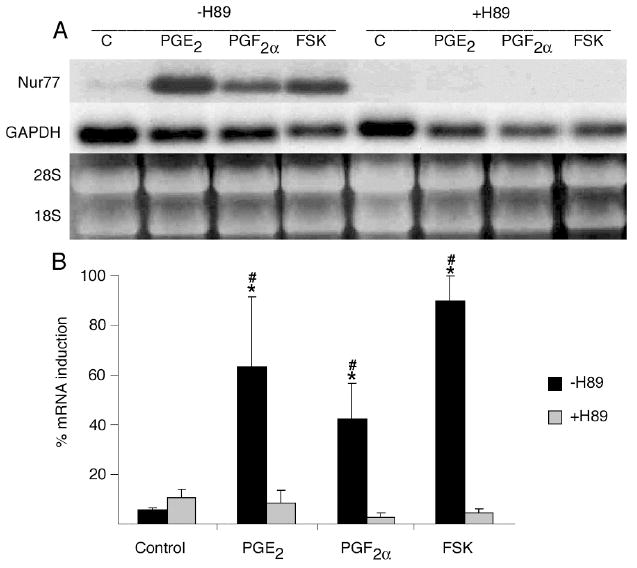
Effect of PKA pathway inhibition on Nur77 mRNA gene expression by OC-CM cells. Confluent OC-CM cells were incubated for 1 hour with vehicle or 3 × 10-5M H89 (a PKA signaling pathway inhibitor) and then treated with 10-6M PGE2, 10-6M PGF2α or 10-5M forskolin (FSK – a direct PKA signaling pathway activator) for 1 hour. A) Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Non-H89 treated group: significantly different from vehicle-treated control, p<0.05. #H89 vs. non-H89 treated groups: significantly different, p< 0.05.
Figure 6.
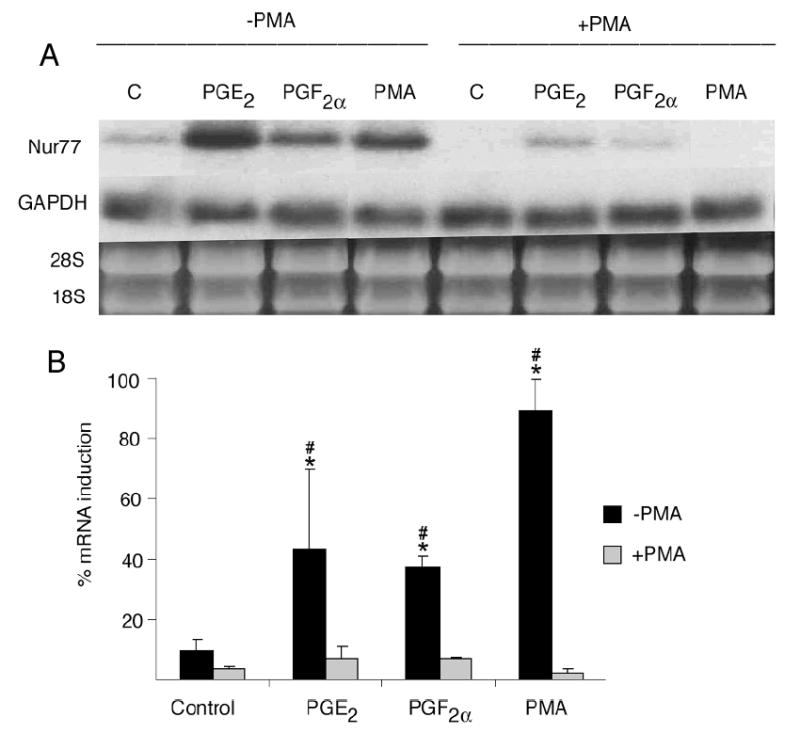
Effect of PKC pathway inhibition on Nur77 mRNA gene expression by OC-CM cells. Confluent OC-CM cells were incubated overnight with vehicle or 10-6M phorbol 12-myristate 13-acetate (PMA – a direct PKC pathway activator) as means to exhaust the PKC pathway and then treated with 10-6M PGE2, 10-6M PGF2α or 10-6M PMA for 1 hour. (A) Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Non-PMA treated group: significantly different from vehicle-treated control, p<0.05. #PMA vs. non-PMA treated groups; significantly different p< 0.05.
Figure 7.
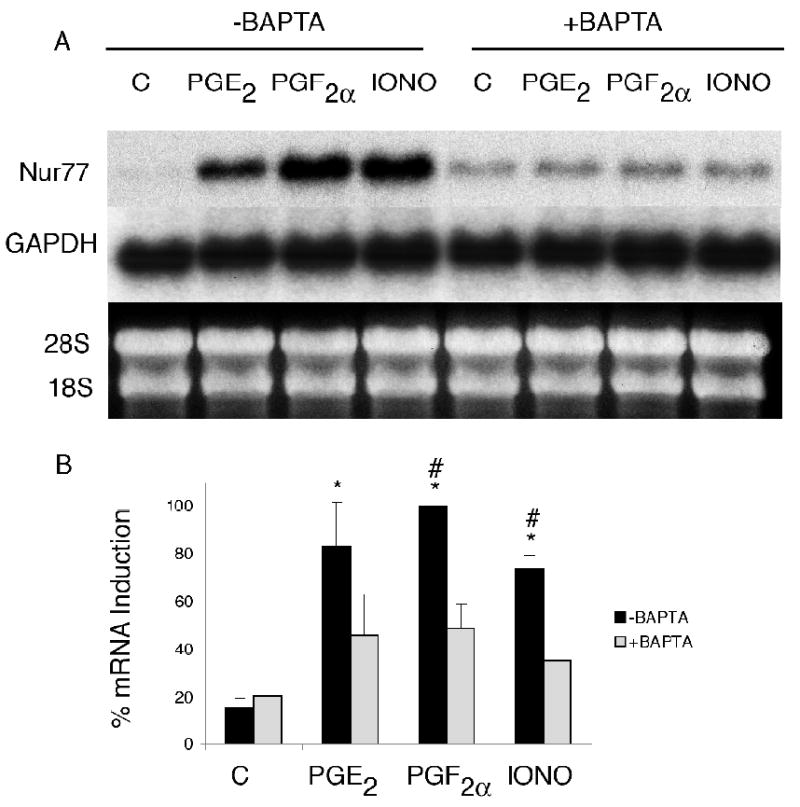
Effect of intracellular calcium pathway inhibition on Nur77 mRNA gene expression by OC-CM cells. Confluent OC-CM cells were incubated for 1 hour with vehicle or 2 × 10-5M 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA – a direct intracellular calcium pathway inhibitor) and then treated with 10-6M PGE2, 10-6M PGF2α or 10-6M PMA for 1 hour. (A) Northern blot analysis of Nur77 and GAPDH mRNA levels and ethidium bromide staining of RNA. B) Densitometric values of Nur77 mRNA relative to GAPDH mRNA. Each value is the mean +/- standard error of three experiments. *Non-BAPTA treated group: significantly different from vehicle-treated control, p<0.05. #BAPTA vs. non-BAPTA treated groups; significantly different p< 0.05.
DISCUSSION
Exogenous signals alter cell function by inducing changes in gene expression. The first genes to be induced in response to a stimulus are called primary response genes. Primary response genes are expressed rapidly and transiently, and do not require de novo protein synthesis (25). We were interested in identifying early changes in the cementoblastic cell gene expression cascade. In order to examine this cascade, OC-CM cells were treated with cycloheximide in order to indentify primary response genes. Expression of primary response genes subsequently regulates downstream late gene expression, which may result in phenotypic and functional changes in cells. Therefore, the variations in Nur77 expression when OC-CM cells are treated with prostanoids reported in this study are not due changes in the in the expression of other genes upstream of Nur77 but due to changes induced by the direct activation of existing cellular proteins in both PKC and PKA signal transduction pathways.
Among several other genes, Nur77 was identified as a primary response gene preferentially induced by flup in cementoblastic cells (22). Nur77 belongs to the family of NGF1-B nuclear orphan receptors, is constitutively expressed in various mammalian tissues(23, 26-29) and its expression is induced by treatment of luteal cells with PGF2α (30). The role of Nur77 as transcription factor has been documented on a variety of cell models (23, 24, 31-34). Hence, based on data obtained from other cell models, Nur77 may potentially play a significant role in cementoblast function. However, no data examining the expression of Nur77 on cementoblasts was available and merited further investigation.
The prostanoids PGE2, PGF2α, and flup increase, while IL-1 decreases, mineralization by cementoblastic cells (19). The anabolic effect exerted by prostanoids on cementoblastic cells is mediated, as least in part, by induction of the PKC pathway, which occurs through activation of the EP1 and FP prostanoid receptors (19). Induction of the PKA pathway, as observed when cementoblastic cells are treated with parathyroid hormone, decreases mineralization by cementoblastic cells (19). With the Nur77 gene being preferentially induced by flup treatment of cementoblastic cells and not by IL-1, it was hypothesized that its induction played an important role in mineralization by OC-CM cells and that such an induction occurred through activation of the PKC pathway (22).
Herein, a series of experiments was designed to examine the signaling cascades mediating prostanoid-induction of Nur77 in cementoblastic cells. Nur77 mRNA gene expression peaked between 0 and 2 hs following treatment of OC-CM cells with PGE2 and PGF2α and that expression returned to baseline levels by 4 hs after treatment (figure 1), confirming the primary response characteristics of the gene. Nur77 mRNA gene expression by PGE2- and PGF2α- treated OC-CM cells was dose-dependent, with maximum effects observed at 10-5 and 10-6 M (figure 2), which were concentrations at which these PGs also increased mineralization by OC-CM cells (19). These findings may indirectly provide support to the hypothesis that Nur77 participates in cementoblast function when those cells are exposed to prostanoid agents, as shown previously (19).
Direct induction of the PKC pathway and direct increase in intracellular calcium with PMA and ionomycin, respectively, increased Nur77 mRNA gene expression by OC-CM cells (figures 4 and 6). These agents bypass the prostanoid receptors present on the cell surface and work intracellularly to activate the PKC and calcium signaling pathways. The increase in Nur77 mRNA gene expression by OC-CM cells following treatment with PMA and ionomycin was expected because direct activation of the PKC pathway with PMA treatment of OC-CM cells results in an increase in mineral nodule formation (19) and also because the Nur77 gene is preferentially induced by flup (22), whose effect occurs through activation of the PKC pathway. It is also known that the PKC pathway interacts with the intracellular calcium channel (35). Release of intracellular calcium stores promotes influx of extracellular calcium via store-operated channels. A rise in cytosolic calcium recruits PKC from the cytosol to the membrane, where diacylglycerol (DAG) activates it. Activation of the PKC depends on both calcium and DAG, suggesting an interaction between the two branches of the inositol-lipid signaling pathway. Therefore, it was not surprising that direct activation of the PKC pathway and an increase in intracellular calcium had similar effects on Nur77 mRNA gene expression by OC-CM cells.
The direct activator or the PKA pathway FSK also significantly increased Nur77 mRNA gene expression by OC-CM cells. There is evidence to support that Nur77 gene induction is regulated through the PKA, PKC and calcium pathways in other organ systems (36-40). Findings from the current study suggest that the mechanism involved in the expression of Nur77 by cementoblastic cells involves similar mechanisms as compared to other types of cells. Direct inhibition of the PKA, PKC and intracellular calcium pathways resulted in abrogation of the induction of Nur77 mRNA gene expression observed when OC-CM cells were treated with FSK, PMA or BAPTA (figures 5, 6 and 7). This further confirms that both intracellular pathways are involved in Nur77 mRNA gene expression by OC-CM cells.
Nur77 mRNA gene expression increased when OC-CM cells were treated with the selective EP1 prostanoid receptor agonist 16,16-DM-PGE2 and the selective FP prostanoid receptor agonist flup. Both the EP1 prostanoid receptor and the FP prostanoid receptor activate the PKC pathway, which is anabolic in the process of OC-CM mineralization (19). These findings support the theory that the Nur77 gene is induced via activation of the PKC pathway, which occurs through binding of PGs to specific prostanoid receptors on the cell surface. Induction of the Nur77 gene may result in the expression of late response genes, which regulate cell function. Of interest was the fact that sulprostone, a selective agonist of the EP3 prostanoid receptor which signals by inhibiting the PKA pathway, while having an anabolic effect on mineralization by OC-CM cells (19), had no significant effect on the expression of the Nur77 gene in mRNA as compared to non-treated controls. Also, sulprostone-treated OC-CM cells showed significantly less mRNA expression of the Nur77 gene than PGE2-, PGF2α-, 16, 16-DM-PGE2 - and flup-treated OC-CM cells. A possible explanation for the fact that activation of the EP3 prostanoid receptor increases mineralization by OC-CM cells but does not increase Nur77 mRNA gene expression is that there may be other primary response genes involved in the cascade of events leading to mineral nodule formation by cementoblastic cells. This proposed mechanism deserves further investigation.
It is also noteworthy that misoprostol, which is an agonist of the EP2, EP3 and EP4 prostanoid receptors did not significantly increase Nur77 gene expression by OC-CM cells. By virtue of binding to EP prostanoid receptors 2 through 4, misoprostol signals in two distinct, yet opposed ways: inducing the PKA pathway (EP2 and EP4 receptors) and inhibiting the PKA pathway (EP3 receptor). Therefore, it is likely that the concomitant activation and inhibition of the PKA pathway neutralized each other, and the net effect on Nur77 gene expression did present with a significant increase.
Butaprost is a selective agonist of the EP2 prostanoid receptor and as such, signals through activation of the PKA pathway. Because direct activation of the PKA pathway with FSK resulted in an increase in Nur77 gene expression by OC-CM cells, treatment of cementoblastic cells with butaprost was also expected to increase expression of the Nur77 gene. The fact that butaprost treatment had no significant effect on Nur77 gene expression by OC-CM cells may be explained by the fact that PKA activation occurs through the EP4 - and not through the EP2 - prostanoid receptor when cementoblastic cells are treated with butaprost. In order to confirm this proposed explanation, Nur77 gene expression by OC-CM cells needed to be examined under treatment of a selective agonist of the EP4 prostanoid receptor, and such agonist is not currently available.
Taken all together, findings herein support the initial hypothesis that Nur77 mRNA gene expression by cementoblastic cells is modulated by treatment with prostanoid agents and delineate the signaling cascades that mediate such regulation. Combined with published results demonstrating regulation of osteoblastic and cementoblastic differentiation markers by members of the NGIF-B nuclear orphan receptor family (41, 42), results from this study suggest that Nur77 gene activation may be involved, at least partially, in OC-CM cell function and differentiation. This involvement possibly occurs by the Nur77 gene regulating late response gene expression, which subsequently causes phenotypic and functional changes on cementoblastic cells. Clarifying the exact mechanism involved in the relationship between PKA activation, Nur77 mRNA gene expression and changes in cementoblastic cell function, warrants further investigation.
Acknowledgments
This study was supported by NIH/NIDCR grant R01DE015111. JMN was supported by NIH/NIDCR training grant K08DE014881.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glossary of Periodontal Terms. Chicago, IL: The American Academy of Periodontology; 2001. [Google Scholar]
- 2.Carranza FAJ, Camargo PM. The periodontal pocket. In: Newman M, Takei H, Carranza F, editors. Carranza’s Clinical Periodontology. Philadelphia: WB Saunders; 2002. pp. 336–353. [Google Scholar]
- 3.Haffajee AD, Socransky SS, Lindhe J, Kent RL, Okamoto H, Yoneyama T. Clinical risk indicators for periodontal attachment loss. J Clin Periodontol. 1991;18:117–125. doi: 10.1111/j.1600-051x.1991.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 4.Waerhaug J. Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth. J Periodontol. 1978;49:119–134. doi: 10.1902/jop.1978.49.3.119. [DOI] [PubMed] [Google Scholar]
- 5.Carranza FAJ, McClain P, Schallborn R. Regenerative osseous surgery. In: Newman M, Takei H, Carranza F, editors. Carranza’s Clinical Periodontology. Philadelphia: WB Saunders; 2002. pp. 804–824. [Google Scholar]
- 6.Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol. 1996;1:621–666. doi: 10.1902/annals.1996.1.1.621. [DOI] [PubMed] [Google Scholar]
- 7.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 8.Stashenko P, Dewhirst FE, Rooney ML, Desjardins LA, Heeley JD. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987;2:559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- 9.Ellies LG, Heersche JN, Pruzanski W, Vadas P, Aubin JE. The role of phospholipase A2 in interleukin-1 alpha-mediated inhibition of mineralization of the osteoid formed by fetal rat calvaria cells in vitro. J Dent Res. 1993;72:18–24. doi: 10.1177/00220345930720010101. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen L, Dewhirst FE, Hauschka PV, Stashenko P. Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1991;10:15–21. [PubMed] [Google Scholar]
- 11.Raisz LG, Alander CB, Fall PM, Simmons HA. Effects of prostaglandin F2α on bone formation and resorption in cultured neonatal mouse calvariae: role of prostaglandin E2 production. Endocrinology. 1990;126:1076–1079. doi: 10.1210/endo-126-2-1076. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy TL, Centrella M, Raisz LG, Canalis E. Prostaglandin E2 stimulates insulin-like growth factor I synthesis in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1991;128:2895–2900. doi: 10.1210/endo-128-6-2895. [DOI] [PubMed] [Google Scholar]
- 13.Raisz LG, Fall PM, Gabbitas BY, McCarthy TL, Kream BE, Canalis E. Effects of prostaglandin E2 on bone formation in cultured fetal rat calvariae: role of insulin-like growth factor I. Endocrinology. 1993;133:1504–1510. doi: 10.1210/endo.133.4.7691577. [DOI] [PubMed] [Google Scholar]
- 14.Fall PM, Breault DT, Raisz LG. Inhibition of collagen synthesis by prostaglandins in the immortalized rat osteoblastic cell line Py1a: structure-activity relations and signal transduction mechanisms. J Bone Miner Res. 1994;9:1935–1943. doi: 10.1002/jbmr.5650091213. [DOI] [PubMed] [Google Scholar]
- 15.Jee WSS, Ueno K, Deng YP, Woodbury DM. The effects of prostaglandin E2 in growing rats: Increased metaphyseal hard tissue and corticoendosteal bone formation. Calcif Tissue Int. 1985;37:148–156. doi: 10.1007/BF02554834. [DOI] [PubMed] [Google Scholar]
- 16.Akamine T, Jee WSS, Ke HZ, Li XJ, Lin BY. Prostaglandin E2 prevents bone loss and adds extra bone to immobilized distal femoral metaphysis in female rats. Bone. 1992;13:11–22. doi: 10.1016/8756-3282(92)90356-2. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen HRI, Svanholm H, Host A. Bone formation induced in an infant by systemic prostaglandin E2 administration. Acta Orthop Scand. 1988;59:464–466. doi: 10.3109/17453678809149406. [DOI] [PubMed] [Google Scholar]
- 18.Marks SC, Jr, Miller SC. Local delivery of prostaglandin E1 induces periodontal regeneration in adult dogs. J Periodontal Res. 1994;29:103–108. doi: 10.1111/j.1600-0765.1994.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 19.Camargo PM, Lagos R, Pirih FQ, Benitez A, Nervina JM, Tetradis S. Prostaglandins E(2) and F(2alpha) enhance differentiation of cementoblastic cells. J Periodontol. 2005;76:303–309. doi: 10.1902/jop.2005.76.2.303. [DOI] [PubMed] [Google Scholar]
- 20.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 22.Nervina JM, Camargo PM, Bezouglaia O, Tetradis S. Prostanoid- and interleukin-1 (IL-1)-induced primary genes in cementoblastic cells. J Periodontol. 2006;77:1362–1370. doi: 10.1902/jop.2006.050354. [DOI] [PubMed] [Google Scholar]
- 23.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama K, Tsukada T, Ohkura N, Bandoh S, Hosono T, Yamaguchi K. The NGFI-B subfamily of the nuclear receptor superfamily (review) Int J Oncol. 1998;12:1237–1243. doi: 10.3892/ijo.12.6.1237. [DOI] [PubMed] [Google Scholar]
- 25.Herschman HR. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Q, Castillo SO, Nikodem VM. Distribution of messenger RNAs for the orphan nuclear receptors Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ hybridization. Neuroscience. 1996;75:221–230. doi: 10.1016/0306-4522(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 27.Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 28.Saucedo-Cardenas O, Conneely OM. Comparative distribution of NURR1 and NUR77 nuclear receptors in the mouse central nervous system. J Mol Neurosci. 1996;7:51–63. doi: 10.1007/BF02736848. [DOI] [PubMed] [Google Scholar]
- 29.Bandoh S, Tsukada T, Maruyama K, Ohkura N, Yamaguchi K. Differential expression of NGFI-B and RNR-1 genes in various tissues and developing brain of the rat: comparative study by quantitative reverse transcription-polymerase chain reaction. J Neuroendocrinol. 1997;9:3–8. doi: 10.1046/j.1365-2826.1997.00571.x. [DOI] [PubMed] [Google Scholar]
- 30.Stocco CO, Lau LF, Gibori G. A calcium/calmodulin-dependent activation of ERK1/2 mediates JunD phosphorylation and induction of nur77 and 20alpha-hsd genes by prostaglandin F2alpha in ovarian cells. J Biol Chem. 2002;277:3293–3302. doi: 10.1074/jbc.M110936200. [DOI] [PubMed] [Google Scholar]
- 31.Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11:39–47. doi: 10.1210/mend.11.1.9874. [DOI] [PubMed] [Google Scholar]
- 32.Philips A, Lesage S, Gingras R, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–5951. doi: 10.1128/mcb.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol Cell Biol. 1995;15:4316–4331. doi: 10.1128/mcb.15.8.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocco CO, Zhong L, Sugimoto Y, Ichikawa A, Lau LF, Gibori G. Prostaglandin F2alpha-induced expression of 20alpha-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem. 2000;275:37202–37211. doi: 10.1074/jbc.M006016200. [DOI] [PubMed] [Google Scholar]
- 35.Zipursky HLS. Molecular Biology of the Cell. 4. New York: WH Freeman & Co; 2000. [Google Scholar]
- 36.Woronicz JD, Lina A, Calnan BJ, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia I, Pipaon C, Alemany S, Perez-Castillo A. Induction of NGFI-B gene expression during T cell activation. Role of protein phosphatases. J Immunol. 1994;153:3417–3425. [PubMed] [Google Scholar]
- 38.Satoh J, Kuroda Y. The constitutive and inducible expression of Nurr1, a key regulator of dopaminergic neuronal differentiation, in human neural and non-neural cell lines. Neuropathology. 2002;22:219–232. doi: 10.1046/j.1440-1789.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 39.Pichon B, Jimenez-Cervantes C, Pirson I, Maenhaut C, Christophe D. Induction of nerve growth factor-induced gene-B (NGFI-B) as an early event in the cyclic adenosine monophosphate response of dog thyrocytes in primary culture. Endocrinology. 1996;137:4691–4698. doi: 10.1210/endo.137.11.8895335. [DOI] [PubMed] [Google Scholar]
- 40.Park JI, Park HJ, Lee YI, Seo YM, Chun SY. Regulation of NGFI-B expression during the ovulatory process. Mol Cell Endocrinol. 2003;202:25–29. doi: 10.1016/s0303-7207(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 41.Pirih FQ, Tang A, Ozkurt IC, Nervina JM, Tetradis S. Nuclear orphan receptor Nurr1 directly transactivates the osteocalcin gene in osteoblasts. J Biol Chem. 2004;279:53167–53174. doi: 10.1074/jbc.M405677200. [DOI] [PubMed] [Google Scholar]
- 42.Lammi J, Huppunen J, Aarnisalo P. Regulation of the osteopontin gene by the orphan nuclear receptor Nurr1 in osteoblasts. Mol Endocrinol. 2004;18:1546–1557. doi: 10.1210/me.2003-0247. [DOI] [PubMed] [Google Scholar]


