Abstract
NG2-glia are a substantial population of cells in the central nervous system (CNS) that can be identified by their specific expression of the NG2 chondroitin sulphate (CSPG). NG2-glia can generate oligodendrocytes, but it is unlikely this is their only function; indeed, they may be multipotent neural stem cells. Moreover, NG2-glia are a highly reactive cell type and a major function is to help form the axon growth inhibitory glial scar in response to CNS injury. The factors that regulate these diverse behaviours of NG2-glia are not fully resolved, but NG2-glia express receptors to the neurotransmitter glutamate, which has known potent effects on other glia. Here, we have examined the actions of glutamate receptor activation on NG2-glia in the rat optic nerve, a typical CNS white matter tract that does not contain neuronal cell bodies. Glutamate induces an increase in [Ca2+]i in immuno-identified NG2-glia in situ and in vitro. In addition, we examined the effects of glutamate receptor activation in vivo by focal injection of the glutamate receptor agonist kainate into the optic nerve; saline was injected in controls. Changes in glial and axonal function were determined at 7 days post injection (dpi), by immunohistochemistry and electrophysiological measurement of the compound action potential (CAP). Injection of kainate resulted in a highly localized ‘injury response’ in NG2-glia, marked by dense labelling for NG2 at the lesion site, as compared to astrocytes, which displayed a more extensive reactive astrogliosis. Furthermore, injection of kainate resulted in an axonal conduction block. These glial and axonal changes were not observed following injection of saline vehicle. In addition, we provide evidence that endogenous glutamate induces calcium-dependent phosphorylation of extracellular signal-regulated kinases (ERK1/2), which may provide a potential mechanism by which glutamate-mediated changes in raised intracellular calcium could regulate the observed gliosis. The results provide evidence that activation of AMPA-kainate type ionotropic glutamate receptors evoke raised calcium in NG2-glia and induces an injury response in NG2-glia.
Keywords: axon, glia, glutamate, NG2-glia, OPC
Introduction
The NG2 chondroitin sulphate proteoglycan (CSPG) identifies a major population of glial cells in the adult central nervous system (Levine & Card, 1987; Levine & Stallcup, 1987; Stallcup & Beasley, 1987; Nishiyama et al. 1996a,b). These ‘NG2-glia’ are distinct from the other glial cell types, namely astrocytes, oligodendrocytes, microglia and ependymal cells, but NG2-glia do express markers of oligodendrocyte progenitor cells (OPCs), such as platelet-derived growth factor alpha receptors (PDGFαR) and Nkx2.2 (Nishiyama et al. 1996a,b; Watanabe et al. 2004). NG2-glia can generate both oligodendrocytes and astrocytes (Zhu et al. 2008), and so may be the in vivo equivalent of oligodendrocyte-type-2 astrocyte (O-2A) cells isolated from neonatal and adult CNS by Raff and colleagues (Raff et al. 1983; ffrench-Constant et al. 1986). NG2-glia may also be programmed to act as multipotent neural stem cells that can also generate neurons (Kondo & Raff, 2000; Belachew et al. 2003; Aguirre et al. 2004). Many studies refer to NG2-glia as OPCs, but we have also termed them synantocytes to reflect their non-OPC nature in the adult CNS (Butt et al. 2002). The distinguishing feature of these cells is their expression of the NG2 CSPG and so in this paper we will refer to them simply as NG2-glia. The majority of NG2-glia retain an OPC phenotype in the adult CNS, and can regenerate oligodendrocytes following demyelination in multiple sclerosis (MS) in humans and in animal models of MS (Polito & Reynolds, 2005). In addition, NG2-glia are a highly reactive cell type that respond to most CNS insults by a rapid gliosis, comprising cell proliferation and upregulation of the NG2 CSPG (Levine, 1994). Indeed, NG2-glia are an important element of the neuroprotective glial scar and the NG2 CSPG is a major inhibitor of axon regeneration in the CNS (Sandvig et al. 2004; Tan et al. 2005, 2006).
The factors that regulate the diverse responses of NG2-glia to demyelination and injury are unresolved, but several lines of evidence indicate a potential role for the neurotransmitter glutamate (Butt, 2006). Firstly, NG2-glia express AMPA-type glutamate receptors that are activated by glutamate released at synapses in grey matter (Bergles et al. 2000; Lin et al. 2005) and by axons in white matter (Kukley et al. 2007; Ziskin et al. 2007). In addition, there is evidence that AMPA receptors on NG2-glia are permeable to Ca2+ (Fulton et al. 1992; Butt et al. 2005; Ziskin et al. 2007), and this would provide a mechanism by which glutamate could regulate intracellular pathways that control cell proliferation, growth, differentiation and death, such as the calcium-dependent mitogen-associated phosphate kinases (MAPK), extracellular signal-regulated kinases (ERK1/2). Finally, glutamate has a well-defined role in the pathology of oligodendrocyte lineage cells (Alberdi et al. 2005). For example, OPCs and oligodendrocytes are extremely susceptible to glutamate-mediated calcium-dependent excitotoxicity (Liu et al. 2002; Matute et al. 2006), and injection of the potent glutamate receptor agonist kainate into the white matter of the optic nerve has been shown to cause demyelination and the loss of oligodendrocytes (Matute et al. 1997, Matute, 1998). Moreover, injection of kainate into the cerebral cortex induced an extensive gliosis in NG2-glia (Ong & Levine, 1999). We therefore examined the potential role for glutamate in driving calcium-dependent processes that may regulate the responses of NG2-glia to CNS insults. Some preliminary data have been presented in review articles (Butt et al. 2005; Butt, 2006; Wigley et al. 2007). In this paper, we provide evidence that glutamate evokes raised [Ca2+]i in NG2-glia and that injection of kainate into the optic nerve induces an injury response in NG2-glia, in apparent contradiction to the excitotoxic actions of glutamate in OPCs. In addition, we provide evidence that endogenous glutamate induces raised pERK1/2 (calcium-dependent MAPK) in isolated optic nerves. The results support a role for glutamate signalling in the injury response of NG2-glia.
Materials and methods
Animals
Wistar rats (Charles River, UK) of either sex were used. Rats were killed humanely by lethal injection of sodium pentobarbitone (i.p.) and optic nerves removed for calcium imaging, cell culture, Western blot, electrophysiology or immunohistochemistry. All procedures were performed in accordance with regulations issued by the Home Office of the United Kingdom under the Animals (Scientific Procedures) Act, 1986.
Optic nerve explant cultures
The methods have been described in detail previously (Greenwood & Butt, 2003). In brief, optic nerves from rat pups aged postnatal day 6 (P6) were dissected free and cut into small pieces (∼0.5 mm3). Individual pieces were explanted onto a numbered micro-grid coverslip glued to the bottom of Petri dishes and incubated in DMEM (Dulbecco's modified Eagle's medium: 1% fetal calf serum, 0.1% gentamycin, 5.6 mg mL−1 glucose, 0.5 µg mL−1 insulin, 100 µg mL−1 transferrin, 90 µg mL−1 bovine serum albumin, 1.6 µg mL−1 putrescine, 60 ng mL−1 progesterone, 5.2 ng mL−1 selenium, 200 µm glutamine, 50 nm T3 and glutamine) at 37 °C for 15–22 days in vitro (DIV).
Calcium imaging
Fura-2 AM calcium imaging was performed in isolated intact optic nerves and in cultures of optic nerve explants (see above). Rats aged P15–20 were used for calcium imaging of immuno-identified NG2-glia in situ, but calcium imaging of unidentified glia was performed in nerves aged from P10 to P30, and adenosine 5′-triphosphate (ATP) and glutamate evoked raised glial [Ca2+]i at all ages. Isolated intact optic nerves or optic nerve explant cultures were incubated in 4 µm Fura-2 AM (Molecular Probes) for 1 h at room temperature (RT), in oxygenated artificial cerebrospinal fluid (aCSF, mm: NaCl,133; KCl, 3; CaCl2, 1.5; NaH2PO4, 1.2; MgCl2, 1.0; d-glucose. 10; HEPES, 10; pH 7.3). Dye-loaded optic nerves were placed on a numbered micro-grid, like those on which optic nerve explants were cultured, and in both cases this allowed recordings from NG2-glia to be identified post hoc at the end of the experiments using immunohistochemistry (see below). In addition, we performed live cell immunolabelling, in which nerves (n = 5) were incubated in a cocktail of primary NG2 antibody and secondary rabbit IgG antibody (see below), together with Fura-2 for 1 h prior to washes in CSF and calcium imaging. In these cases, the cell somata of NG2-positive cells were identified and cell bodies within a single plane of focus were selected as regions of interest (ROI); several ROI were simultaneously recorded in each experiment, and both NG2-positive and NG2-negative cells in the same field and focal plane were analysed. Calcium imaging was performed on an Olympus upright microscope (BX50W1) using an Achroplan ×20 water immersion lens and an optional ×2 multiplier, and tissues were perfused continuously with oxygenated aCSF. Fura-2 was excited alternately at 340 and 380 nm, using a Cairn monochromator (Cairn Research Ltd, UK). Emissions were detected at 510 nm using an intensified CCD camera or a Photometrics S-Coolsnap CCD camera (both supplied by Cairn Research Ltd., UK). The monochromator and the CCD camera were controlled and synchronized by AxonImagingWorkbench 5.1 (Axon Instruments), and quantitative measurements were made using the same program. Signals were sampled at 2 Hz and the change in the F340:F380 ratio relative to the baseline fluorescence was recorded within a single plane of focus. Multiple ROI in a single plane were simultaneously recorded in each experiment, and at the end of the experiment, NG2 immunolabelling was performed (see below) and the co-ordinates of NG2-glia on the micro-grids were revealed and related to the recordings of the F340:F380 ratio, enabling the calcium responses of identified NG2-glia to be analysed.
Application of test agents and data analysis
Unless otherwise stated, for calcium imaging experiments, agonists were applied by bath application over the nerve for 30 s, and a recovery period of 10 min in aCSF was allowed after each agent. Chemicals were dissolved directly in aCSF, and used at 100 µm unless otherwise stated. The following were used (from Tocris Bioscience, Bristol, UK; or Sigma-Aldrich, Dorset, UK): ATP, l-glutamic acid (glutamate/Glu), kainic acid (kainate), cyclothiazide (CTZ), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzol[f]quinoxaline-7-sulfonamide disodium salt (NBQX), tetrodotoxin citrate (TTX). ZERO-Ca2+ was prepared by the removal of Ca2+ and addition of EGTA (1 mm).
To examine the response to ATP and glutamate, each agonist was applied sequentially, and the responses in multiple ROI (cell somata) to both agents were recorded. Measurements within a single nerve were paired responses in each ROI, and results were expressed as the maximum change in ratio, after background subtraction (ΔF340:F380). The mean (± SEM, where n represents the number of cells, from at least three replicates) maximum ΔF340:F380 was calculated for each agent in NG2-positive and NG2-negative cells, and compared for significance using unpaired t-tests.
In the case of the CTZ and NBQX, the response to glutamate was measured in multiple ROI, from unidentified cells. The nerve was then incubated in CTZ-CSF for 3 min, and the response to glutamate + CTZ measured in the same cells, before return to CTZ-CSF. After a 10-min recovery period, the response to glutamate + CTZ + NBQX was measured in the same cells. In each case, the maximum change in ratio, after background subtraction (ΔF340:F380), was measured and then expressed as a per cent of the glutamate response in the same cell. The experiment was replicated in three nerves, and the data was then expressed as mean per cent glutamate response (± SEM), where n represents the number of cells (n = 148), and tested for significance using Mann–Whitney tests.
Post hoc immunohistochemical identification of NG2-glia for calcium imaging
The same antibodies were used for live cell immunolabelling, and post hoc immunolabelling of cells cultures and isolated nerves: rabbit anti-NG2 (Chemicon), diluted 1:500, and Alexa 568-conjugated secondary anti-rabbit IgG (Molecular Probes), diluted at 1:100. For post hoc immunolabelling, optic nerve explant cultures and intact nerves were immersion fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at RT for 20–60 min. Following washes in PBS, a blocking stage was performed by incubation at RT for 30–60 min with 1.5% normal goat serum (NGS) plus 1% BSA in PBS, prior to incubation in primary antibody (2 h at RT or overnight at 4 °C), and then secondary antibody (2 h at RT). Finally, tissues were washed, mounted in citifluor (Sigma-Aldrich Co.) and imaged using the same Olympus upright microscope described above. NG2-glia were identified and their co-ordinates on the micro-grids were related to the recordings of the changes in glial calcium.
In vivo injections into the optic nerve
Rats (200–250 g) were placed under deep anaesthesia by intraperitoneal injection of combined hypnorm/hypnovel (0.2133 mg kg−1 fentanyl citrate, 6.75 mg kg−1 fluanisone, 3.375 mg kg−1 midazolam). To expose the intra-orbital portion of the optic nerve, an incision was made in the skin above the eye and the musculature and lachrymal glands in the superior aspect of the orbit were deflected. A small window was cut in the dural sheath of the optic nerve and the optic nerve superficially impaled with a micropipette, via which 1 µL of solution was pressure injected into the nerve parenchyma. The injectate was sterile saline vehicle, kainate (1 mg mL−1) or tetrodotoxin (TTX, 100 µm), and contained monastrol blue for subsequent localization of the injection site. Following injection, lachrymal glands and musculature were carefully replaced and the wound was repaired. Rats were maintained at 37 °C until they had recovered from anaesthesia and then returned to their cages. After 7 days post injection (dpi), rats were killed humanely by lethal sodium pentobarbitone overdose (Lethobarb, Fort Dodge Animal Health, UK), and optic nerves removed for immunohistochemistry or electrophysiology.
Immunohistochemistry following in vivo injections
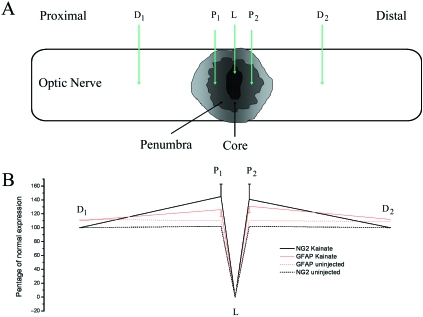
Immediately following dissection, optic nerves were immersion fixed in 4% PFA in PBS for 1 h at RT. Following washes in PBS, nerves were cryoprotected in 30% sucrose overnight at 4 °C, and then embedded in blocks of OCT (BDH Laboratory Supplies, UK) before being frozen. Sections were cut at 25 µm using a cryostat and then washed in PBS prior to double immunofluorescence labelling for NG2 and glial fibrillary acidic protein (GFAP). Sections were incubated at RT for 30–60 min with 1.5% NGS plus 1% BSA in PBS to reduce non-specific binding of antibodies. Sections were incubated in rabbit anti-NG2 (diluted 1:500) overnight at 4 °C, and then incubated for 2 h at RT in Alexa 568-conjugated secondary anti-rabbit IgG (Molecular Probes), diluted at 1:100. For double immunolabelling, intermediary fixation and blocking stages were performed between NG2 and GFAP immunolabelling. Following immunolabelling for NG2, sections were postfixed in 4% PFA for 20 min at RT, and the blocking stage in NGS was repeated prior to incubation in rabbit anti-GFAP (diluted 1:250) overnight at 4 °C, and then incubation in fluorescein-conjugated anti-rabbit IgG (diluted at 1:100). Following washes in PBS, sections were mounted in citifluor and examined on an Olympus Provis microscope. Negative controls in which the primary antibodies were omitted showed no signal, and there was no evidence of cross-reactivity when one or other of the primary antibodies was removed. In addition, single labelling for either antibody revealed an equivalent distribution of NG2 and GFAP as observed in double labelling, further indication that cross-reactivity between NG2 and GFAP immunolabelling was effectively blocked by the intermediary fixation and blocking stages. Double immunofluorescence images of the lesions (n = 3) were captured using a Zeiss axiocam digital camera, with settings maintained constant for each slide. Fluorescence intensity (FI) was measured using KS300 image analysis software (Imaging Associates, UK), in five fields of view (FOV) in each section, at the injection site (A, identified by monastral blue), the penumbra proximal (P1) and distal (P2) to the lesion, and at 500 µm distance from the lesion, both proximally (D1) and distally (D2). For each FOV, the FI was expressed as a per cent of the FI in untreated optic nerve, and the mean (± SEM) per cent normal expression for NG2 and GFAP was calculated.
Measurement of the compound action potential (CAP)
Optic nerves were removed intact and maintained immediately in chilled oxygenated aCSF (Bolton & Butt, 2005). Nerves were then removed to a brain slice chamber and continuously perfused with oxygenated aCSF at room temperature. One end of the nerve was placed in a suction electrode connected to a stimulator (S44 stimulator, Grass Instruments, USA). The other end of the nerve placed in a suction electrode connected to a differential amplifier (Duo 773 Electrometer, World Precision Instruments Inc., USA), which enabled subtraction of the stimulus artefact from the signal. CAPs were acquired on a PC via CED1401 analogue-to-digital interface (Cambridge Electronic Design, UK), and analysed using Signal2 software (Cambridge Electronic Design, UK). The peak CAP amplitude was measured for each nerve, and the mean + SE calculated for each treatment group and tested for significance using Mann–Whitney tests.
Incubation of optic nerves and Western blot analysis
Optic nerves from P10 rats were isolated intact and placed immediately into ice-cold aCSF for 5–10 min prior to the start of the experiment. Nerves were then incubated at 37 °C in either (1) aCSF (untreated controls), (2) ZERO-Ca2+ CSF, (3) NBQX (dissolved in CSF), or (4) kainate (dissolved in CSF). Five nerves were used in each case to provide sufficient protein for Western blot analysis, and each treatment was replicated at least three times (aCSF, n = 7; ZERO-Ca2+ CSF, n = 3; NBQX CSF, n = 3; kainate CSF, n = 7). At the end of 15 min incubation, nerves were washed in ice cold Ca2+-free PBS containing 200 µm EGTA and 200 µm EDTA pH 7.4 and placed immediately on ice to stop any further phosphorylation of ERK. For Western blot analysis, nerves were then homogenized in extraction buffer on ice for 45 min (50 mm Tris, 150 mm NaCl, 1% Triton-X100, 2 mm EDTA, 2 mm EGTA, 0.5 mm phenylmethylsulfonylfluoride, 10 µg mL−1 leupeptin, 10 µg mL−1 antipain, 1 µg mL−1 chymostatin, 1 µg mL−1 pepstatin A, 1 mm sodium orthovanadate and 50 mm Naf, corrected to pH7.4 with HCl). Samples were centrifuged at 5 °C for 5 min at 1000 g and the supernatant sampled for Bradford protein assay determination. Remaining samples were immediately frozen at −20 °C until required. For protein analysis, samples were then solubilized and denatured in SDS boiling buffer (62.5 mm Tris, 2% SDS, 10% glycerol, 5% mercaptoethanol, 0.0025% bromophenol blue, H2O, 0.8) in a 1:5 dilution (i.e. 80 µL of sample to 20 µL of SDS mixture) and heated at 90–100 °C for 2 min. The solubilized, denatured proteins were then separated through a SDS-PAGE gel and then nitrocellulose membranes were placed in blocking buffer for 30 min (Tris-buffered saline, pH 7.5, with 5% w/v skimmed milk powder). Following washes in TTBS (Tris-buffered saline with 0.05% Tween-20), the membrane was incubated with the primary antibodies (Erk1,2, Anti-Active MAPK pAB, pTEpY, Rabbit, Promega, 1:5000) diluted in TTBS containing 1% w/v skimmed milk powder to prevent non-specific binding and left overnight. The membrane was washed in TTBS and incubated for 1 h in goat anti-rabbit IgG conjugated with horseradish peroxidase (Sigma), diluted at 1:1000. Western blot analysis of the levels of total ERK and pERK1/2 were measured for each experiment, and the levels of pERK1/2 were expressed as a per cent of Total ERK in the same nerves (five nerves in each experiment). Each treatment was replicated three to seven times, and the mean (± SE) value of pERK1/2 as per cent of Total ERK was calculated for each treatment group, and tested for significance using Mann–Whitney tests.
Results
Calcium imaging and immuno-identification of NG2-glia in vitro
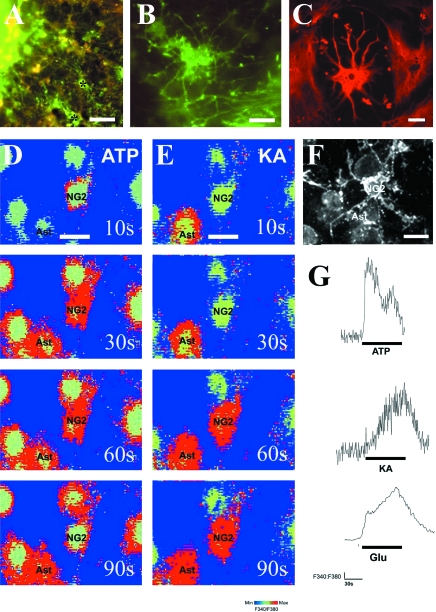
Optic nerves explants from P6 rats were seeded on microgrid coverslips and maintained in culture for 15DIV. During this period, NG2-glia and astrocytes migrate from the explant (Fig. 1A). At DIV15 in serum-containing medium, the vast majority of cells in the cultures were immunopositive for NG2 (Fig. 1B) or GFAP (Fig. 1C). Double labelling for NG2 and GFAP indicated that the majority of NG2-positive cells were GFAP-negative (Fig. 1A). NG2-glia had small somata and extended multiple thin processes that branched three or more times along their length and bore small varicosities along their length (Fig. 1B). Astrocytes were either flat astrocytes (type-1) or process-bearing astrocytes (type-2), which had large somata and numerous thick processes that branched repeatedly (Fig. 1C). Both astrocytes and NG2-glia loaded with Fura-2, but the two cell types could be distinguished readily by their distinctive morphologies at the time of calcium imaging, and NG2-glial cell phenotype was confirmed at the end of the experiment by immunolabelling. Thus, explant cultures were loaded with Fura-2 and a field that contained cells morphologically identified as NG2-glia was selected for calcium imaging (Fig. 1D,E). The grid co-ordinates of cells were noted and immunolabelling for NG2 was performed at the end of the experiment (Fig. 1F) so that calcium recordings could be related to positively identified NG2-glia (Fig. 1G). Fig. 1D–G illustrates a typical experiment, in which cultures of NG2-glia and neighbouring NG2-immunonegative astrocytes respond to bath administration of ATP, the glutamate receptor agonist kainate, and glutamate, all at 100 µm (Fig. 1G). In NG2-glia (n = 19 cells from 6 experiments), ATP evoked a rapid and large increase in [Ca2+]i, which decayed during application of the agonist (Fig. 1G), whereas glutamate and kainate evoked a sustained increase in [Ca2+]i, which was generally slower to peak and to recover to baseline (Fig. 1G).
Fig. 1.
Glutamate evoked calcium signals in immuno-identified NG2-glia in vitro. Optic nerve explants were maintained in culture for 15 days and analysed by immunocytochemistry and calcium imaging. (A–C) Double immunofluorescence labelling for NG2 (green) and GFAP (red) showing astrocytes and NG2-glia (some indicated by asterisks) that have migrated from the optic nerve explant (A), and illustrating the distinct morphologies of NG2-glia (B) and astrocytes (C). (D–F) Cells cultured on microgrids were loaded with Fura-2 and identified as NG2-glia or astrocytes on the basis of their morphology (D). The response to ATP (D) and glutamate or kainate (E) was recorded, and at the end of the experiment, the cellular identify of NG2-glia was confirmed by immunocytochemistry (F). Fura-2 ratiometric images of changes in the F340:F380 ratio illustrate that application of 100 µm ATP (D) or kainate (E) evokes raised [Ca2+]i in identified NG2-glia and neighbouring astrocytes. (G) Traces of the change in [Ca2+]i in individual NG2-glia show that ATP evoked a rapid and transient rise in [Ca2+]i, whereas glutamate and kainate evoked sustained rises in [Ca2+]i. Scale bar 25 µm in (A), 10 µm in (B–F).
Glutamate receptor activation evokes raised [Ca2+]i in NG2-glia in situ
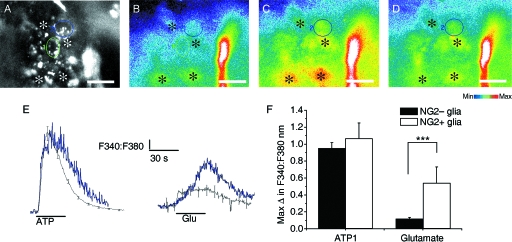
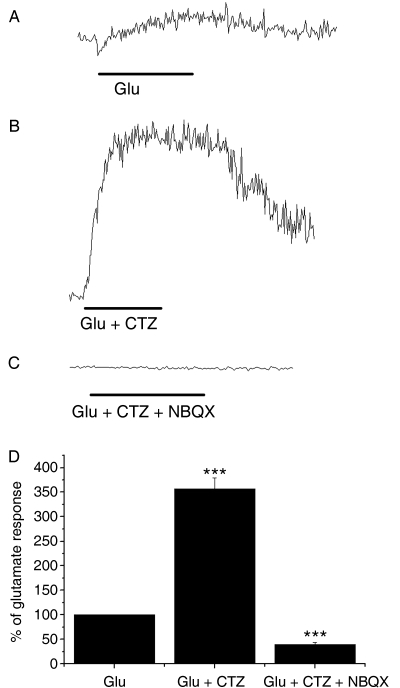
Calcium imaging was performed on isolated intact rat optic nerves aged P15-P20 in which NG2-glia were identified by live cell immunolabelling (n = 5 nerves), and by post hoc immunolabelling (n = 6 nerves) (Fig. 2). Cell somata of NG2-positive (n = 17 cells) and NG2-negative cells (n = 196 cells) were selected as ROI (Fig. 2A), and the response to 100 µm ATP (Fig. 2C) and 100 µm glutamate (Fig. 2D) was measured as the change in the F340:F380 ratio relative to the baseline fluorescence (Fig. 2E) within a single plane of focus. NG2-glia identified either by live cell immunolabelling or by post hoc immunolabelling were observed to respond to both ATP and glutamate, as did NG2-immunonegative glia. The responses were similar to those recorded directly in NG2-glia in vitro (see above): ATP evoked a rapid and transient increase in [Ca2+]i that began to fall in the presence of the agonist and recovered rapidly to baseline on removal of the agonist, whereas glutamate evoked a slower rise in [Ca2+]i that was sustained in the presence of the agonist and recovered slowly to baseline after 50–60 s (Fig. 2E). Comparison of NG2-glia and NG2-negative glia indicated the amplitude of the response to glutamate was significantly greater in NG2-glia (P < 0.05, unrelated t-test), whereas there was no significant difference in the response to ATP (P > 0.05, unrelated t-test). Although the NG2-negative glial cells were not positively identified in these experiments, the published evidence indicates that the vast majority of these loaded cells will be astrocytes (Fern, 1998). Furthermore, we have demonstrated that ATP and glutamate evoke raised [Ca2+]i in astrocytes, using optic nerves from mice in which astrocytes express green fluorescent protein (Hamilton et al. 2008). Moreover, we have not observed expression of NG2 by astrocytes in the optic nerve (Butt et al. 1999, 2005), and so the response describe here for NG2-positive and NG2-negative cells is respectively that of NG2-glia and astrocytes. We took advantage of this fact, and that comparison of NG2-positive and NG2-negative glia indicate they respond similarly to glutamate, to examine the effects of CTZ (10 µm) and NBQX (100 µm) on unidentified glia, which would comprise astrocytes and NG2-glia (Fig. 3). CTZ specifically acts on AMPA receptors to maintain them in an open state, whereas NBQX is a specific antagonist for AMPA receptors. In paired recordings from 148 cells in three nerves, the response to glutamate was measured first (Fig. 3A), then to glutamate in the presence of CTZ (Fig. 3B), and then to glutamate plus CTZ in the presence of NBQX (Fig. 3C). In all cells that responded to glutamate (n = 148) we found that the response to glutamate was markedly increased in CTZ by over 350%, and this was significantly decreased by NBQX to 45 ± 4% of the glutamate response in the same cells (Fig. 3D; Mann–Whitney test, P < 0.001). These results provide evidence that AMPA-type iGluR mediate raised [Ca2+]i in NG2-glia and astrocytes.
Fig. 2.
Glutamate evoked calcium signals in immuno-identified NG2-glia in situ. (A) NG2-glia were identified by live cell immunolabelling, and cell somata were selected as regions of interest (ROI); two NG2-positive cells are identified (encircled), in addition to neighbouring NG2-negative cells (asterisks), which are likely to be astrocytes. (B–D) Images of Fura-2 fluorescence, illustrating baseline levels (B), and the peak response to ATP (C) and glutamate (D), in the cells identified in A. (E) Traces of the change in [Ca2+]i in individual NG2-immunopositive (blue lines) and NG2-immunonegative (black lines). (F) Bar graph illustrating the mean peak responses (± SE) in NG2-immunopositive (n = 17 cells from six nerves) and NG2-immunonegative glia (n = 196 cells from six nerves). ***P < 0.001, unpaired t-test. Scale bars in A–D = 20 µm.
Fig. 3.
Glutamate acts on AMPA receptors. The glutamate response was examined by Fura-2 ratiometry in unidentified glia. In paired experiments, the response to glutamate (A) was markedly increased in 10 µm cyclothiazide (B), which specifically acts on AMPA receptors to maintain them in an open state, and was blocked by 100 µm NBQX (C), an AMPA receptor antagonist. (D) Bar graph of mean data (± SE; n = 148 cells in three nerves) demonstrates the results were statistically significant. ***P < 0.001, Mann–Whitney test.
Glutamate receptor activation induces an injury response in NG2-glia in vivo
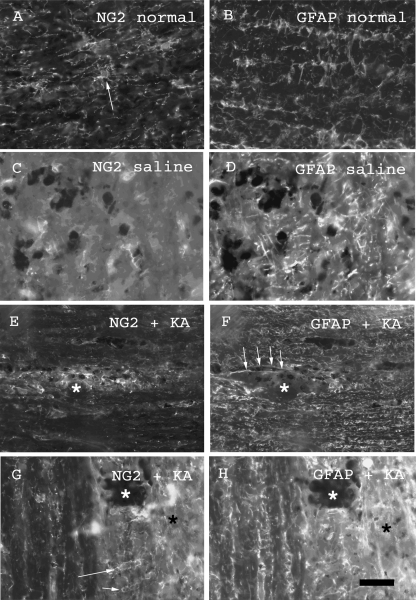
Over-activation of iGluR results in calcium-dependent excitotoxicity in OPCs (Liu et al. 2002), and demyelinating lesions in the optic nerve in vivo which persist for over 2 weeks (Matute et al. 1997; Matute, 1998). We therefore examined the effects of iGluR activation on NG2-glia in vivo 7 dpi following a subpial focal injection of kainate (n = 3; Fig. 4), compared with injection of saline in controls (n = 3). The injection site was identified by monastrol blue and nerve sections were immunolabelled for NG2 and GFAP, and quantitative analysis of immunofluorescence intensity (FI) was performed (Fig. 5). In untreated nerves, NG2-glia are uniformly distributed and have small cell bodies, from which fine branching processes extend radially (Fig. 4A), whereas GFAP-immunopositive astrocyte processes are thicker and extend transversely and longitudinally through the nerve (Fig. 4B). Following injection with saline, there was some disruption of the cytoarchitecture of NG2-glia and astrocytes at the injection site (Fig. 4C,D), but no evidence of increased cellular density or immunolabelling (Fig. 5). In contrast, following injection of kainate, there was marked disruption of the cytoarchitecture of both NG2-glia and astrocytes (Fig. 4E–H). We did not observe cross-reactivity between NG2 and GFAP immunolabelling, but there was close apposition between the processes of astrocytes and NG2-glia, and this is more evident at the site of gliosis, where their processes intertwine to form scar tissue. These may appear as colocalization, but the immunolabelling was quite separate and we did not observe co-expression of NG2 and GFAP. It was not possible to count individual NG2-glia, because of the intensity of NG2 immunolabelling at the injection site, and so we quantified the relative changes in FI compared to normal tissue, keeping all settings constant for each slide (Fig. 5A). The monastral blue used to identify the injection site obscured immunolabelling of both NG2 and GFAP at the injection site, and FI here was zero. Analysis of the penumbra both proximal and distal to the lesion site indicated a significant increase in immunofluorescence, which was more pronounced for NG2 than GFAP (Fig. 5B). In comparison, GFAP immunolabelling was raised above control levels over a wider area, 500 µm away from the injection site, whereas the increase in NG2 immunolabelling was highly localized to the injection site (Fig. 5B).
Fig. 4.
NG2 and GFAP immunolabelling in uninjected optic nerves (A,B) and at 7 dpi following in vivo injection of saline (C,D) or kainate (E–H). Typical distribution of NG2-glia (A) and GFAP-positive astrocyte processes (B) was observed in untreated nerves. NG2 labelling was decreased following saline injection (C), whereas GFAP immunolabelling appeared increased (D). Injection of kainate resulted in increased expression of NG2 (E) and GFAP (F) at the injection site (asterisk). Higher magnification illustrates the increased density of NG2+ (G, arrows) and GFAP+ (H) cells and processes at the injection penumbra (black asterisk). Scale bar = 80 µm in (A,B,E) and 40 µm in (C,D,G,H).
Fig. 5.
Quantification of immunofluorescence intensity for NG2 and GFAP. (A) Illustration of the sites of fluorescence intensity measurements in the injected optic nerve. D1and D2 are the proximal and distal sites up to 500 µm away from the lesion site, L; P1 and P2 measurement sites were in the penumbra. The percentage change in fluorescence of NG2 and GFAP was calculated in randomly selected sections (n = 3). (B) An increase in NG2 and GFAP expression was seen in the penumbra following injection of kainate. A lack of fluorescence is seen at (A) due to the monastrol blue. Data are expressed as a percentage of normal fluorescence intensity and plotted as mean ± SE.
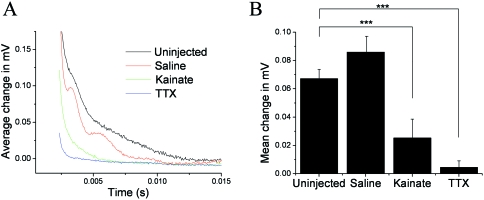
Glutamate receptor activation decreases axonal conduction
Axonal conduction properties were analysed by measuring the CAP at 7 dpi following in vivo injection of saline, kainate or TTX (Fig. 6). The CAP is an average of the action potentials in all the axons of the nerve, related to axon number and diameter. Injection of saline was used as a negative control, and TTX was used as a positive control because it blocks Na+ channels responsible for action potential propagation and blocks myelination (Demerens et al. 1996). Following saline injection, there was no significant change in the CAP amplitude, whereas TTX caused a complete loss of the CAP (Fig. 6A). Administration of kainate resulted in a significant decrease in the CAP (Fig. 6A), with an amplitude 19 ± 14% compared to saline-treated nerves (Fig. 6B).
Fig. 6.
Compound action potentials (CAPs) 7 days after in vivo injection of saline, kainate (1 mg mL−1) or TTX (100 µm). (A) Traces illustrate the typical CAPs measured in each experimental group. (B) Bar graph of mean (± SE) change in peak CAP amplitude (n = 3–6). ***P < 0.001, Mann–Whitney test.
Glutamate receptor activation results in glial ERK1/2 phosphorylation
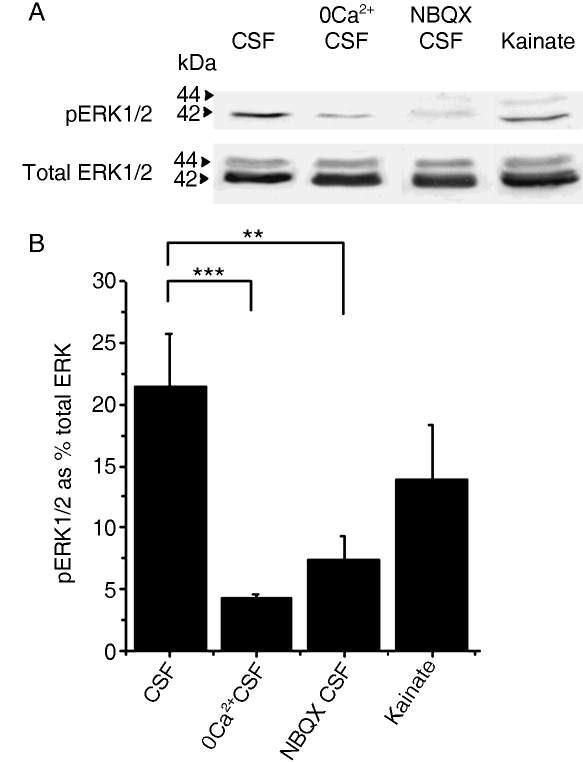
The results presented above show that glutamate evokes raised [Ca2+]i in NG2-glia and astrocytes, and that injection of the glutamate receptor agonist kainate into the optic nerve evoked reactive gliosis in NG2-glia and astrocytes. An important calcium-dependent intracellular signalling pathway that is regulated by glutamate is phosphorylation of ERK1/2, which are mitogen-activated kinases (MAPK) that regulate multiple cellular processes, including cell proliferation. We therefore examined whether there is evidence that glutamate affects levels of phosphorylated ERK1/2 (pERK1/2), using Western blot (Fig. 7). Nerves were isolated intact and incubated in either aCSF (untreated controls), ZERO-Calcium CSF, or aCSF containing NBQX (to block AMPA receptors), or aCSF containing kainate (to activate AMPA-kainate glutamate receptors); five nerves were used each time to provide sufficient protein for Western blot, and each treatment was replicated at least three times. Representative blots of the levels of Total ERK and pERK1/2 are illustrated in Fig. 7A, and the mean levels of pERK1/2 expressed as a per cent of Total ERK in the same nerves for each treatment group are illustrated in Fig. 7B. The results demonstrated significant statistical differences between the mean data for the experimental groups (anovafollowed by Mann–Whitney tests), although a wide range of pERK1/2 levels were observed in control (aCSF) and kainate-treated groups. The results indicated that levels of pERK1/2 were high in isolated nerves incubated in normal aCSF (22 ± 4% of total ERK, n = 7), and significantly decreased by removal of extracellular Ca2+ (4 ± 0.4% of total ERK, n = 3; P < 0.001, Mann–Whitney test), or in CSF containing NBQX (7 ± 2% of Total ERK, n = 3; P < 0.01, Mann–Whitney test); there was no significant difference between nerves incubated in Ca2+-free CSF or in CSF containing NBQX (Fig. 7B). There was no significant difference between nerves incubated in normal aCSF and in CSF containing kainate (17 + 4% of Total ERK, n = 7). The results indicate that endogeneous glutamate is released in the isolated nerves to act on NBQX-sensitive AMPA receptors and mediate calcium-dependent ERK1/2 phosphorylation, and so activation of AMPA-kainate receptors with kainate had no additional effect.
Fig. 7.
Glutamate increases ERK1/2 phosphorylation. Levels of pERK1/2 were measured using Western blot and compared with total ERK1/2, following incubation of nerves in aCSF (untreated controls), ZERO-Calcium aCSF, aCSF containing 10 µm NBQX, or aCSF containing 100 µm kainate. (A) Representative Western blots of pERK1/2 and Total ERK for each treatment. (B) Mean (+ SE, n = 4–7) levels of pERK1/2 expressed as a per cent of total ERK in each treatment group. Removal of extracellular calcium or addition of the AMPA-receptor agonist significantly reduced levels of pERK1/2, compared with controls. Kainate had no significant effect compared with controls. *P < 0.05, **P < 0.01, ***P < 0.001, Mann–Whitney test.
Discussion
We have examined the response of NG2-glia and astrocytes to glutamate receptor activation in the rat optic nerve. Glutamate is shown to evoke raised intracellular [Ca2+] in optic nerve NG2-glia identified by immunolabelling in vitro and in situ. Equivalent results have been show for astrocytes previously (Hamilton et al. 2008). In addition, we show that injection of kainate into the optic nerve to activate AMPA-kainate glutamate receptors results in reactive gliosis in NG2-glia and astrocytes, and in reduced axonal conduction. Finally, we show that levels of pERK1/2 in the isolated nerve are reduced by removal of extracellular calcium and blockade of AMPA receptors with NBQX, indicating that the release of endogenous glutamate activates calcium-dependent MAPK pathways. These results provide a possible mechanism by which glutamate-mediated calcium entry could regulate an injury response of NG2-glia in white matter pathology.
Glutamate and ATP evoked an increase in [Ca2+]i in optic nerve NG2-glia, which is equivalent to that described previously in rat optic nerve glia in situ (Kreigler & Chiu, 1993; James & Butt, 2002; Butt et al. 2004; Hamilton et al. 2008). There have been no previous studies on the calcium responses of NG2-glia, but NG2-glia have an OPC antigenic phenotype in vivo and in vitro (Nishiyama et al. 1996a,b), and it is possible to compare our findings with previous studies on OPCs. The ATP response we observed in NG2-glia is consistent with studies showing ATP-evoked raises [Ca2+]i in perinatal OPCs in vitro (Kastritsis & McCarthy, 1993; Kirischik et al. 1995; Agresti et al. 2005), and a study showing the presence of several ionotropic P2X (1,2,3,4,7) and metabotropic P2Y (1,2,4) receptor subtypes on OPCs (Agresti et al. 2005). Our finding that glutamate evokes calcium signals in NG2-glia corroborates patch-clamp studies showing that NG2-glia express AMPA-type glutamate receptors in situ in the hippocampus, cerebellum, corpus callosum and optic nerve (Bergles et al. 2000; Lin & Bergles, 2004; Lin et al. 2005; Kukley et al. 2007; Ziskin et al. 2007). In addition, a number of studies have shown that AMPA receptors mediate calcium signals in OPCs (Borges et al. 1994; Pende et al. 1994; Holtzclaw et al. 1995). Glutamate-mediated calcium signals in NG2-glia are consistent with calcium entering through calcium-permeable AMPA receptors, as indicated in patch-clamp studies (Ziskin et al. 2007). However, activation of AMPA receptors may also cause depolarization due to sodium influx, and calcium entry through voltage-operated calcium channels (VOCC), which are expressed by OPCs (Blankenfeld et al. 1992). It is not known whether NG2-glia express VOCC in vivo, and further studies on calcium signalling in NG2-glia are required to help resolve this issue.
Our results on the effects of kainate injected into the optic nerve indicate that activation of glutamate receptors can mediate an injury response in NG2-glia, as well as astrocytes. Following injection of kainate into the optic nerve, there was an increase in the density of NG2-glia, similar to that described following intracerebral injection of kainate (Ong & Levine, 1999). A ‘reactive gliosis’ is also seen in NG2-glia and astrocytes following axon degeneration in the optic nerve (Butt et al. 2002, 2004; Greenwood & Butt, 2003). However, axon degeneration resulted in an extensive gliosis along the entire tract (Greenwood & Butt, 2003), whereas the response of NG2-glia and astrocytes to kainate was localized to the injection site, consistent with previous studies showing that kainate does not result in axonal damage and degeneration (Matute et al. 1997; Matute, 1998). Notably, the optic nerve does not contain neuronal somata and so injected kainate acts directly on glial cells, not indirectly via its excitotoxic effect on neurons. It is important to recognize that oligodendrocytes also express AMPA-kainate receptors and injection of kainate in the optic nerve results in demyelination (Matute et al. 1997; Matute, 1998). NG2-glia would be expected to respond to this demyelination by proliferation and migration into the lesion site (Chari & Blakemore, 2002; Watanabe et al. 2002, 2004; Polito & Reynolds, 2005). Nonetheless, NG2-glia express functional AMPA receptors and we show kainate evokes raised calcium in NG2-glia, so it is reasonable to conclude that kainate injected into the nerve could have acted directly on NG2-glia to induce the observed injury response. The electrophysiological recordings show decreased axonal conduction across the lesion, which correlated with glial scar formation and with the demyelination that has been reported previously (Matute et al. 1997; Matute, 1998). Notably, kainate did not induce the loss of NG2-glia in vivo. This is in comparison with findings on OPCs in vitro, where calcium-permeable AMPA-kainate receptors mediate toxicity in OPCs (Kavanaugh et al. 2000; Itoh et al. 2002; Liu et al. 2002; Deng et al. 2003). This may be further evidence that NG2-glia in vivo do not behave the same as OPCs in vitro (Greenwood & Butt, 2002).
Analysis of ERK1/2 phosphorylation indicated a role for glutamate-mediated activation of MAPK signalling pathways in the optic nerve. Phosphorylated ERK1/2 was detected in nerves incubated in normal aCSF, and this was blocked by NBQX and by removal of extracellular Ca2+. This indicates that ERK1/2 phosphorylation was induced by endogenous glutamate, presumably released from axons and astrocytes (Kukley et al. 2007; Ziskin et al. 2007; Hamilton et al. 2008). It is possible that glutamate is released in response to the surgical procedure of nerve isolation, and that the phosphorylation of ERK1/2 represents an early event in the pathological response of glial cells. Notably, kainate had no significant effect on pERK1/2 levels compared with untreated nerves, suggesting that further activation of AMPA-kainate receptors had no additional effect over endogenous glutamate. The Western blot analysis of homogenates of intact tissue did not identify the cells responding to glutamate, and these are likely to include astrocytes, oligodendrocytes and NG2-glia. However, several lines of evidence are consistent with this pathway regulating NG2-glia: (1) glutamate triggers raised calcium in NG2-glia (this study); (2) glutamate regulates OPC proliferation (Yuan et al. 1998); (3) MAPK regulate OPC proliferation (Soliven, 2001); (4) glutamate receptor activation produces calcium-dependent MAPK phosphorylation (Pende et al. 1997; Liu et al. 1999). These results provide a possible intracellular link between glutamate-mediated calcium influx and glutamate-receptor-mediated reactive gliosis in NG2-glia.
In conclusion, we provide evidence that glutamate and ATP evoke calcium signals in NG2-glia, which may be important in regulating the pathological functions of NG2-glia (Matute et al. 2007). It is generally undisputed that NG2-glia have an OPC antigenic phenotype (Nishiyama et al. 2005), and glutamate is known to inhibit OPC differentiation (Gallo et al. 1996; Yuan et al. 1998). Hence, glutamate released by axons (Kukley et al. 2007; Ziskin et al. 2007) may serve to maintain NG2-glia in a quiescent form under physiological conditions. However, over-activation of glutamate receptors mediates an injury response in white matter NG2-glia similar to that observed in grey matter (Ong & Levine, 1999). One of the most prominent features of NG2-glial cell biology is their response to most forms of CNS insults (Levine, 1994; Ong & Levine, 1999; Tan et al. 2005, 2006), and the results support a role for glutamate in this injury response (Butt et al. 2002). Moreover, NG2-glia are a source of remyelinating oligodendrocytes following demyelination, and the gliotic response of NG2-glia to glutamate may be important in promoting the regeneration oligodendrocytes by NG2-glia.
Acknowledgments
Niki Hamilton was in receipt of a PhD studentship from the Anatomical Society of Great Britain and Ireland. Supported by the BBSRC. We thank Dr Rob Williams for advice with the ERK1/2 Western blot experiments.
References
- Agresti C, Meomartini ME, Amadio S, et al. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells that contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Matute C. Calcium and glial cell death. Cell Calcium. 2005;38:417–425. doi: 10.1016/j.ceca.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JDB, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Blankenfeld G, Verhkratsky A, Kettenmann H. Ca2+ channel expression in the oligodendroglial lineage. Eur J Neurosci. 1992;4:1035–1048. doi: 10.1111/j.1460-9568.1992.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Bolton S, Butt AM. The optic nerve: a model for axon-glial interactions. J Pharmacol Toxicol Methods. 2005;51:221–233. doi: 10.1016/j.vascn.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Borges K, Ohlemeyer C, Trotter J, Kettenmann H. AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience. 1994;63:135–149. doi: 10.1016/0306-4522(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Butt AM. Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia. 2006;54:666–675. doi: 10.1002/glia.20424. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Butt AM, Pugh M, Hubbard P, James G. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye. 2004;18:1110–1121. doi: 10.1038/sj.eye.6701595. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J Neurosci. 1998;18:7232–7243. doi: 10.1523/JNEUROSCI.18-18-07232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood K, Butt AM. Evidence that perinatal and adult NG2-glia are not conventional oligodendrocyte progenitors and do not depend on axons for their survival. Mol Cell Neurosci. 2003;23:544–558. doi: 10.1016/s1044-7431(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Kirchhoff F, et al. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia. 2008;56:734–749. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- Holtzclaw LA, Gallo V, Russell JT. AMPA receptors shape Ca2+ responses in cortical oligodendrocyte progenitors and CG-4 cells. J Neurosci Res. 1995;42:124–130. doi: 10.1002/jnr.490420114. [DOI] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, et al. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Kastritsis CH, McCarthy KD. Oligodendroglial lineage cells express neuroligand receptors. Glia. 1993;8:106–113. doi: 10.1002/glia.440080206. [DOI] [PubMed] [Google Scholar]
- Kavanaugh B, Beesley J, Itoh T, Itoh A, Grinspan J, Pleasure D. Neurotrophin-3 (NT-3) diminishes susceptibility of the oligodendroglial lineage to AMPA glutamate receptor-mediated excitotoxicity. J Neurosci Res. 2000;60:725–732. doi: 10.1002/1097-4547(20000615)60:6<725::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-C, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin S-C, Huck JHJ, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Liu HN, Larocca JN, Almazan G. Molecular pathways mediating activation by kainate of mitogen-activated protein kinase in oligodendrocyte progenitors. Brain Res Mol Brain Res. 1999;66:50–61. doi: 10.1016/s0169-328x(99)00009-1. [DOI] [PubMed] [Google Scholar]
- Liu HN, Giasson BI, Mushynski WE, Almazan G. AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves free radical generation and activation of JNK, calpain and caspase 3. J Neurochem. 2002;82:398–409. doi: 10.1046/j.1471-4159.2002.00981.x. [DOI] [PubMed] [Google Scholar]
- Matute C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc Natl Acad Sci U S A. 1998;95:10229–10234. doi: 10.1073/pnas.95.17.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R. Glutamate receptor mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci USA. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sanchez-Gomez M-V. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, et al. Excitotoxic damage to white matter. J Anat. 2007;210:693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996a;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996b;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? J Anat. 2005;207:687–693. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Pende M, Holtzclaw LA, Curtis JL, Russell JT, Gallo V. Glutamate regulates intracellular calcium and gene expression in oligodendrocyte progenitors through the activation of DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1994;91:3215–3219. doi: 10.1073/pnas.91.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Soliven B. Calcium signalling in cells of oligodendroglial lineage. Microsc Res Tech. 2001;52:672–679. doi: 10.1002/jemt.1051. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;207:717–725. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hadzic T, Nishiyama A. Transient upregulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia. 2004;46:311–322. doi: 10.1002/glia.20006. [DOI] [PubMed] [Google Scholar]
- Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]