Abstract
The extracellular matrix of peripheral nerve plays a vital role in terms of normal nerve fibre function and also in the regenerative response following nerve injury. Nerve fibre loss is a major feature of diabetic neuropathy; however, the regenerative response is limited and this may be associated with changes in the composition of the extracellular matrix. Glycoproteins and collagens are major components of the extracellular matrix and are known to be important in terms of axonal growth. This work has therefore examined whether changes in the expression of two major glycoproteins, laminin and tenascin, and three collagen types (IV, V and VI) occur in the endoneurial and perineurial connective tissue compartments of human diabetic nerve. Despite being known to have a positive effect in terms of axonal growth, laminin levels were not elevated in the diabetic nerves. However, the pattern of tenascin expression did differ between the two groups being found in association with axon myelin units in the diabetic samples only. The pattern of collagen IV expression was the same in both tissue groups and was not found to be up-regulated. However, levels of collagen V and VI were both significantly increased in the endoneurium and for collagen VI also in the perineurium.
Keywords: collagen, diabetes, extracellular matrix, glycoproteins
Introduction
Loss of nerve fibres and Wallerian-type degeneration is the major pathological finding in diabetic neuropathic nerve (Thomas & Lascelles, 1966; Dyck et al. 1986; Yagihashi et al. 2007). Currently the precise sequence of events leading to this fibre loss is uncertain. Equally unclear is why the regenerative response is initially intense yet ultimately fails (Bradley et al. 1995). Following nerve injury several elements are known to be required for successful regeneration. Of particular importance is the composition of the extracellular matrix (ECM), with which proliferating Schwann cells must interact (Rummler & Gupta, 2004; Radim & Dubovy, 2006). Matrix molecules provide inhibitory and stimulatory signals that can either promote or deter axonal growth (Dubovy, 2004; Chen et al. 2007). Four categories of components are found within the ECM: glycoproteins, collagens, glycosaminoglycans and proteoglycans (Carbonetto, 1984). The glycoproteins have been shown to be particularly important in terms of nerve regeneration (Tonge et al. 1997; Rummler & Gupta, 2004). For example it is well established that the glycoprotein laminin has a positive effect on axonal elongation (Mėnanger et al. 2004; Rummler & Gupta, 2004; Chen et al. 2007) and following nerve injury its levels are up-regulated (Doyu et al. 1993). During normal development the presence of laminin is thought to be vital to ensure proliferation, differentiation and subsequent survival of the Schwann cells (Rummler & Gupta, 2004). During the regeneration process it is suggested that endoneurial laminin acts either as a substrate for the elongating axon or directly supports the Schwann cell in the repair process (Chen et al. 2007).
Another ECM glycoprotein also important following nerve injury is tenascin (Matsumoto et al. 2002). Tenascin expression is tightly regulated, levels being high during early development, when it acts as a mediator of neuron–glia interactions (Faissner, 1997), but falling once maturity of the nervous system is achieved. Re-expression only occurs in response to wound healing, nerve regeneration and pathological processes (Jones & Jones, 2000).
The composition of the ECM therefore plays an influential role in nerve fibre regeneration, particularly with regard to its glycoprotein composition. However, work to date that has examined endoneurial laminin content in diabetic neuropathic nerve has not confirmed any significant changes, although this may be due to the fact that non-quantitative techniques were used (Bradley et al. 2000). Also, somewhat surprisingly in view of its established role in nerve re-growth, the pattern of tenascin expression has not been examined in diabetic nerve. Work that has been done, on diabetic neuropathic nerve and on cell culture models, has suggested that up-regulation of some of the other components of the ECM in diabetic nerve tissue occurs (Muona et al. 1991; Bradley et al. 2000). Collagen IV is a major component of the ECM and has been shown to have a positive effect in terms of axonal regeneration (Tonge et al. 1997). Although it is thought that collagen IV levels are elevated within the endoneurial compartment of diabetic nerves, this has yet to be quantified in vivo. Similarly, up-regulation of the less abundant collagen types, collagen V and VI, has been reported in the diabetic state but again has been based on visual comparisons (Bradley et al. 2000).
The aim of this current work was therefore to examine the deposition of the major glycoproteins, laminin and tenascin, within the endoneurial and perineurial compartments of diabetic neuropathic nerve using quantitative techniques. In addition, deposition of the collagen types IV, V and VI was determined to detect any changes in expression that might be linked to the diabetic environment.
Main body
Tissue collection
Human sural nerve samples were used in this study. Ethical permission for obtaining and using tissue samples was obtained from Hull and East Yorkshire Ethical and Clinical Trials Committee.
Tissue groups
Samples from both diabetic and control patient groups were treated in an identical manner.
Diabetic nerve samples
In all cases, diabetes was classified as type II, with a range of disease duration from 6 to 20 years and a confirmed history of diabetic neuropathy. Mean age of the group was 71 ± 2.5 years; n = 5.
Control samples
Control samples were obtained at post-mortem having obtained a written consent from the next of kin. In all cases, the tissue was removed within 12 h of death. Patients used in the control group were all free of diabetes, mean age 75 ± 4.3 years; n = 5.
Preparation of tissue for immunohistochemistry
All tissue samples were immediately fixed in formal saline at 10 °C for 24 h and then washed in fresh phosphate buffer for a further 24 h. An ascending ethanol series was used to dehydrate the tissue prior to eventual embedding in paramat wax. Sections that encompassed the complete transverse area of the nerve sample were cut at a thickness of 10 µm and mounted on polylysine-coated slides.
Immunohistochemistry
Slides were de-waxed and immersed into citroclear for 10 min. Rehydration was performed using a descending ethanol series. Hydrogen peroxide 1.2% was used to block any endogenous enzymes. Trypsin 0.1% was used to expose any epitopes masked by the effects of formalin fixation. Sections were blocked using 1 : 100 normal rabbit serum prior to the application of the primary antibody. A dilution of 1 : 100 was used for all three collagen types (collagen IV, Dako; collagens V and VI, Southern Biotech). A monoclonal laminin antibody (Chemicon) that reacts specifically with the B2 laminin chain, the isoform known to be present in the perineurial cell membrane (Aumailley & Smyth, 1998), was applied at a dilution of 1 : 1000. A monoclonal antibody that recognizes all isoforms of human tenascin (Sigma) was used at a dilution of 1 : 4000. Following a 60-min incubation period, slides were rinsed twice in Tris buffer before a biotinylated secondary antibody was applied for 30 min. Epitopes were identified using the avidin-biotin (ABC) complex method and visualized with the use of DAB.
For all nerve samples, a negative control section was included where the primary antibody was replaced with blocking serum before application of the secondary antibody.
Fascicle perimeter measurements
A linear relationship between fascicle size and the width of the perineurial sheath is known to exist (Sunderland & Bradley, 1952). A close linear relationship also exists between perineurial collagen IV content per unit of the perineurium and fascicle perimeter in normal sural nerves (Lowry et al. 1997). It is therefore important to relate the amounts of the different perineurial cell basement membrane components to fascicle size. Fascicle perimeter measurements were therefore made from the immunostained sections using a tracing function on an ImageProPlus image analysis system.
Semi-quantification of ECM components
Semi-quantitative analysis of laminin, tenascin and the individual collagen types within the peripheral nerve compartments was performed using the ImageProPlus analysis system. At least three complete nerve fascicles were analysed from each individual patient in both patient groups. For analysis within the perineurium the method of Lowry et al. (1997) was used. Briefly, sections were visualized and the complete perineurium of each fascicle was viewed using a low power objective. Variation in background staining was corrected prior to thresholding the image. Highlighted pixels that were not part of the perineurium were excluded. A user-defined frame was then drawn around the outermost layer of the perineurium and the area of highlighted pixels within the frame assessed (Lowry et al. 1997). The fascicle perimeter measurements allowed the results to be expressed as area of perineurial laminin, tenascin or collagen type IV, V or VI per unit of the perimeter. The SPSS statistical package was used to perform between-group comparisons of the five components.
Analysis of the endoneurial ECM components was performed by thresholding the image and then excluding all pixels that were not part of the endoneurium and then creating a user-defined frame to assess the highlighted pixels. The results were then expressed as a percentage of the endoneurial area and significance tested for using the SPSS statistical package.
Statistical analysis
Differences in mean values were tested for statistical significance using the Mann–Whitney U-test.
Results
Fascicle perimeter measurements
Fascicle perimeter measurements were compared using the Mann–Whitney U-test. No significant difference between fascicle perimeters between the two groups was detected (P > 0.05).
Immunohistochemistry
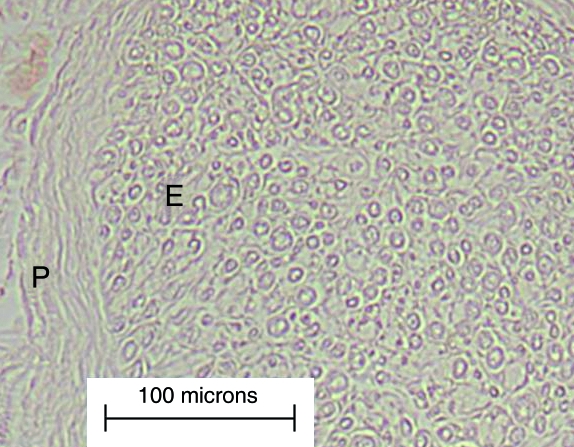
Structural presentation of the nerve samples was found to be very good upon initial observations using all five antibodies. Background staining was minimal and all control sections, where the primary antibody was omitted, were completely devoid of staining (Fig. 1).
Fig. 1.
A light micrograph of part of a fascicle from the negative control of a diabetic nerve sample. Staining is absent in both the perineurium (P) and the endoneurium (E).
Laminin deposition
Both the control and the diabetic nerve samples displayed the same pattern of laminin deposition. Laminin was prominent within the basement membranes of the blood vessels within all connective tissue compartments, it was also present in association with Schwann cells and was particularly obvious within the perineurium. Although the mean value for the perineurial laminin content per unit of the perineurium was greater than that of the control (8.95 ± 3.8 vs. 7.50 ± 2.4) (Table 1), the difference was not significant, (P = 0.257). Similarly no significant difference between the endoneurial laminin content of the diabetic and non-diabetic nerves was found (P = 0.330) (Table 2) (Fig. 2a,b).
Table 1.
Comparisons of mean collagen types IV, V, VI, tenascin and laminin per unit of the perineurium
| Group | Laminin content per unit of the perineurium (mm × 10−2) | Tenascin content per unit of the perineurium (mm × 10−2) | Collagen IV content per unit of the perineurium (mm × 10−2) | Collagen V content per unit of the perineurium (mm × 10−2) | Collagen VI content per unit of the perineurium (mm × 10−2) |
|---|---|---|---|---|---|
| Diabetic (n = 20 fascicles) | 8.95 ± 3.8 | 9.12 ± 6.2 | 7.27 ± 3.01 | 8.0 ± 2.9 | 7.4 ± 1.77* |
| Control (n = 17 fascicles) | 7.50 ± 2.4 | 7.6 ± 4.02 | 5.77 ± 1.91 | 8.8 ± 2.3 | 5.6 ± 1.25 |
Significantly different from the control group (P< 0.001).
Table 2.
Comparisons of endoneurial laminin and collagen types IV, V and VI
| Group | Endoneurial laminin | Endoneurial collagen IV | Endoneurial collagen V | Endoneurial collagen VI |
|---|---|---|---|---|
| Diabetic (n = 20 fascicles) | 18.04 ± 8.9 | 12.3 ± 4.4 | 13.8 ± 5.9* | 21.59 ± 9.36** |
| Control (n = 17 fascicles) | 14.13 ± 4.01 | 11.6 ± 9.92 | 8.5 ± 9.7 | 15.7 ± 7.08 |
Units are not given, as values are relative.
Significantly different from the control group (P < 0.002).
Significantly different from the control group (P < 0.001).
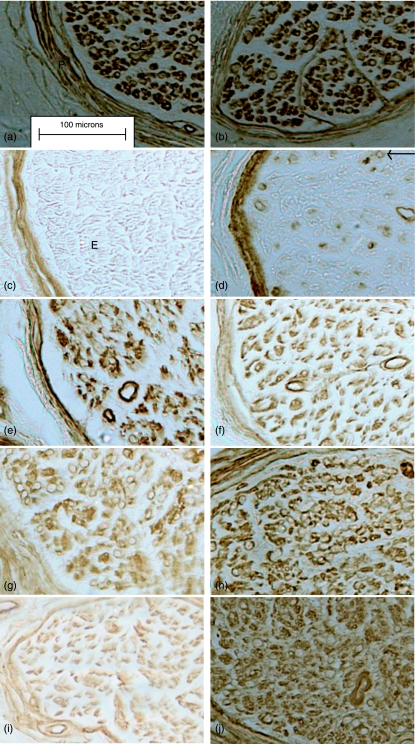
Fig. 2.
Light micrographs of control (left-hand column) and diabetic (right-hand column) immunostained nerve samples. (a,b) Laminin in both the perineurium (P) and endoneurium (E). (c,d) Tenascin staining is present in the perineurium of both groups and is also found in association with axon–myelin units (arrow) in the diabetic group only. (e,f) Collagen IV, (g,h) collagen V and (i,j) collagen VI.
Tenascin deposition
In the control tissue, tenascin expression was confined to the perineurium and the basement membrane of the endoneurial blood vessels. In the diabetic nerve, tenascin expression was also noted within the endoneurial blood vessels and was particularly prominent within the perineurium. A greater overall mean value for perineurial tenascin content was found in the diabetic group (9.12 ± 6.2 vs. 7.62 ± 4.02) but the difference was not significant (P = 0.495) (Table 1). However, expression of tenascin was found within the endoneurium of the diabetic nerve and appeared to be in association with axon–myelin units. No such expression was found in any of the control samples (Fig. 2c,d).
Deposition of collagens IV, V and VI
In the control nerve, collagen IV was clearly evident within the perineurium and also in association with the Schwann cells and endoneurial blood vessels. The same pattern of expression was found within the diabetic nerve (Fig. 2e,f). Semi-quantitation of the perineurial collagen IV showed that although the diabetic group had a greater overall mean value per unit of the perineurium (7.27 ± 3.01 vs. 5.77 ± 1.91) this difference was not significant (P = 0.083) (Table 1). Similarly, comparisons of endoneurial collagen IV content between the two groups showed no significant differences (P= 0.103) (Table 2).
Collagen V was also found around Schwann cells and endoneurial blood vessels both the control and diabetic nerve samples (Fig. 2g,h). It was also prominent within the perineurium in both samples but quantitative analysis found no significant differences between the perineurial collagen V content of the two groups (8.0 ± 2.9 vs. 8.8 ± 2.3) (P= 0.103) (Table 1). However, expression of endoneurial collagen V was found to be significantly increased within the diabetic group (13.8 ± 5.9 vs. 8.5 ± 9.7) (P < 0.002) (Table 2).
At the light microscope level very little collagen VI was obvious in the control group. The greatest expression was within the perineurium. In contrast, collagen VI was found in association with the cellular components of the endoneurium in the diabetic nerve and also within the perineurium (Fig. 2i,j). Semi-quantification of the perineurial collagen VI revealed a significant increase in the diabetic group (7.4 ± 1.77 vs. 5.6 ± 1.25) (P < 0.001). The same was also true for endoneurial collagen VI expression in the diabetic group (21.59 ± 9.36 vs. 15.7 ± 7.08) (P < 0.042) (Table 2).
Discussion
Axonal degeneration of Wallerian type is normally followed by a regenerative response. Axonal sprouting begins from the proximal end of the nerve, whereas Schwann cells proliferate at the distal site, to create columns known as the Bands of Bungner, which ultimately guide the regenerating neurons to the target tissue (Rowshan et al. 2004). Interaction between the Schwann cells and the components of the ECM are crucial for successful nerve regeneration (Rummler & Gupta, 2004; Chen et al. 2007). In diabetic neuropathy, nerve regeneration is almost always unsuccessful and it has been suggested that this may in part be due to changes in the composition of the ECM (Bradley et al. 2000).
This current work has for the first time quantified the deposition of five ECM components in both the endoneurial and perineurial compartments of human diabetic sural nerve samples. The results show that exposure to hyperglycaemia produces significant remodelling of the ECM with increased levels of tenascin, collagen V and collagen VI.
Surprisingly, levels of laminin were not elevated in the endoneurium or perineurium of diabetic nerves. Although laminin is known to have a positive effect in terms of nerve fibre regeneration, the results of this current study are in fact in keeping with those of previous non-quantified studies (Bradley et al. 2000). In contrast, this work has examined tenascin expression for the first time in human diabetic nerve and has demonstrated up-regulation within the endoneurium. This is an important finding in terms of elucidating the effects of diabetes on the nerve microenvironment. Five members of the tenascin glycoprotein family are currently identified. Of these, both TNC and TNR are involved in the early development of the nervous system. Once maturity has been achieved, levels are tightly regulated, particularly of TNC, with altered expression patterns occurring as part of the regenerative response (Jones & Jones, 2000). Work using rodent models of nerve injury suggest that TNC promotes recovery following nerve lesion, whereas TNR is inhibitory (Guntinas-Lichius et al. 2005). It is therefore anticipated that the up-regulation of tenascin found in this work is in the form of TNC; however, further work is required to confirm this, particularly as the regenerative response in diabetic nerve is rarely successful.
In terms of collagen deposition this work has confirmed a generalized increase within the endoneurium of collagen V and also collagen VI, but we found no significant change in the levels of collagen IV. Collagen VI up-regulation in response to hyperglycaemia has been found before in animal models and cultured cells (Muona et al. 1993); however, this is the first study to quantify its deposition in human diabetic nerve. The observed altered levels of collagen V and VI expression and the absence of any increase in collagen IV suggest that preferential remodelling of specific collagen types occurs in response to the diabetic environment. Currently the effects of this in terms of nerve regeneration are unclear. Excess collagen deposition within the endoneurium is thought to be detrimental in terms of nerve fibre regeneration, in that Schwann cell basal laminal tubes, which normally guide elongating axons, may become filled with collagen fibrils that then create a physical barrier preventing nerve fibre growth (Bradley et al. 2000). The precise role of collagen VI within peripheral nerve has not been fully elucidated, but there are suggestions that it acts as an organizer of the ECM, whose expression pattern changes in response to injury (Keene et al. 1988; Peltonen et al. 1991). Further investigation is therefore required to determine if this is an initial response to nerve injury in diabetes and, if so, what its usefulness is in terms of nerve regeneration or, alternatively, if up-regulation of collagen V and VI arises due to the failure of regeneration following prolonged nerve damage and is not unique to the diabetic state. Examination of other components of the ECM is also needed. For example, chondroitin sulfate proteoglycans are found throughout the ECM and can have inhibitory effects in terms of laminin activity (Graham et al. 2007) and so its pattern of deposition within diabetic nerve should be explored.
In terms of the perineurium, only collagen VI deposition was found to be significantly increased. Thickening of the basement membrane of the perineurial cell has been demonstrated several times in the diabetic state (Johnson et al. 1981; King et al. 1989; Bradley et al. 1994; Ghani et al. 1999; Hill & Williams, 2004). Such thickening is thought to be detrimental in terms of the function of the perineurium as both a diffusion barrier and a nutrient delivery system (Powell et al. 1985; Muona & Peltonen 1994). Increased thickness may also contribute to impairment of the microcirculation of the neural tissue by compressing the blood vessels that must traverse it to supply the endoneurium, which may ultimately lead to hypoxia (Tuck et al. 1984; Powell et al. 1985). However, prior to this work the component responsible for this increased thickness had not been identified. The results of this work suggest that collagen VI plays a major part in the altered composition of the perineurium. The significance of this is uncertain. However, the perineurium is in direct contact with the endoneurial environment and interaction between the two may well influence general nerve fibre homeostasis and Schwann cell behaviour.
This work has demonstrated that significant remodelling of the ECM does occur within diabetic nerve. However, it is not clear if this remodelling is beneficial, in terms of the regenerative response, or if it actually impedes axonal elongation. Also it is not certain if the observed changes in collagen V and VI are unique to diabetic nerve or are a general feature in response to other forms of neuropathy. Future work is therefore required to investigate this further.
Acknowledgments
Assistance with this manuscript from Dr Pam Williams is gratefully acknowledged.
References
- Aumailley M, Smyth N. The role of laminin in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JL, Thomas PK, King RMH, Watkins PJ. A comparison of perineurial and vascular basal laminal changes in diabetic neuropathy. Acta Neuropathol. 1994;88:426–432. doi: 10.1007/BF00389494. [DOI] [PubMed] [Google Scholar]
- Bradley JL, Thomas PK, King RHM, et al. Myelinated fibre regeneration in diabetic sensory polyneuropathy: correlation with type of diabetes. Acta Neuropathol. 1995;90:403–410. doi: 10.1007/BF00315014. [DOI] [PubMed] [Google Scholar]
- Bradley JL, King RHM, Muddle JR, Thomas PK. The extracellular matrix of peripheral nerve in diabetic polyneuropathy. Acta Neuropathol. 2000;99:539–546. doi: 10.1007/s004010051158. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. The extracellular-matrix of the nervous system. Trends Neurosci. 1984;7:382–387. [Google Scholar]
- Chen Z, Yu W, Strickland S. Peripheral regeneration. Ann Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Doyu M, Sobue G, Ken E, et al. Laminin A, B1, and B2 chain gene expression in transected and regenerating nerves: regulation by axonal signals. J Neurochem. 1993;60(2):543–541. doi: 10.1111/j.1471-4159.1993.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Dubovy P. Schwann cells and endoneurial extracellular matrix molecules as potential cues for sorting regenerated axons: A review. Anat Sci Int. 2004;79:198–208. doi: 10.1111/j.1447-073x.2004.00090.x. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Karnes JL, O’Brien P, Okazaki H, Lais A, Engelstead J. The spatial distribution of fiber loss in diabetic polyneuropathy suggests ischaemia. Annu Neurol. 1986;19:440–449. doi: 10.1002/ana.410190504. [DOI] [PubMed] [Google Scholar]
- Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res. 1997;290:331–341. doi: 10.1007/s004410050938. [DOI] [PubMed] [Google Scholar]
- Ghani M, Malik RA, Walker D, Sharma AK, Lowrie CT, Schall WD. Perineurial abnormalities in the spontaneously diabetic dog. Acta Neuropathol. 1999;97:98–102. doi: 10.1007/s004010050961. [DOI] [PubMed] [Google Scholar]
- Graham JB, Neubauer D, Xue Q, Muir D. Chondroitinase applied to peripheral nerve repair averts retrograde axonal regeneration. Exp Neurol. 2007;203:185–195. doi: 10.1016/j.expneurol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Angelov DN, Morellini F, et al. Opposite impacts of tenascin-C and tenascin-R deficiency in mice on the functional outcome of facial repair. Eur J Neurosci. 2005;22:2171–2179. doi: 10.1111/j.1460-9568.2005.04424.x. [DOI] [PubMed] [Google Scholar]
- Hill RE, Williams PE. Perineurial cell basement membrane thickening and myelinated nerve fibre loss in diabetic and nondiabetic peripheral nerve. J Neurol Sci. 2004;217:157–163. doi: 10.1016/j.jns.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Brendel K, Meezan E. Human diabetic perineurial cell basement membrane thickening. Lab Invest. 1981;44(3):265–269. [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: Structure, function and regulation during embryonic development and tissue remodelling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988;107:1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RMH, Llwelyn JG, Thomas PK, Gilbey SG, Watkins PJ. Diabetic neuropathy: Abnormalities of Schwann cell and perineurial basal laminae. Implications for diabetic vasculopathy. Neuropathol. 1989;15:339–355. doi: 10.1111/j.1365-2990.1989.tb01234.x. Appl Neurobiol. [DOI] [PubMed] [Google Scholar]
- Lowry A, Wilcox D, Masson EA, Williams PE. Immunohistochemical methods for semiquantitative analysis of collagen content in human peripheral nerve. J Anat. 1997;191:367–374. doi: 10.1046/j.1469-7580.1997.19130367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Hirofumi S, Sato M, Orba Y, Nagashima K, Ariga H. Distribution of extracellular matrix tenascin-X in sciatic nerves. Acta Neuropathol. 2002;104:448–454. doi: 10.1007/s00401-002-0577-x. [DOI] [PubMed] [Google Scholar]
- Mėnanger C, Arimura N, Fukata Y, Kaibuchi K. PIP3is involved in neuronal polarization and axon formation. J Neurochem. 2004;89:109–118. doi: 10.1046/j.1471-4159.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- Muona P, Peltonen J. Connective tissue metabolism in diabetic peripheral nerves. Ann Med. 1994;26:39–43. doi: 10.3109/07853899409147325. [DOI] [PubMed] [Google Scholar]
- Muona P, Peltonen J, Jaakkola S, Uitto J. Increased matrix gene expression by glucose in rat neural connective tissue cells in culture. Diabetes. 1991;40:605–611. doi: 10.2337/diab.40.5.605. [DOI] [PubMed] [Google Scholar]
- Muona P, Jaakkola S, Zhang R, et al. Hyperglycaemic glucose concentrations up-regulate the expression of type VI collagen in vitro. Am J Pathol. 1993;142(5):1586–1597. [PMC free article] [PubMed] [Google Scholar]
- Peltonen J, Hsiao LL, Jaakkola S, et al. Activation of collagen gene expression in keloids: co-localisation of type 1 and VI collagen and transforming growth factor-beta 1 mRNA. J Invest Dermatol. 1991;97:240–248. doi: 10.1111/1523-1747.ep12480289. [DOI] [PubMed] [Google Scholar]
- Powell HC, Rosoff J, Myers RR. Microangiopathy in human diabetic neuropathy. Acta Neuropathol. 1985;68:295–305. doi: 10.1007/BF00690832. [DOI] [PubMed] [Google Scholar]
- Radim J, Dubovy P. Immunohistochemical labeling of components of the endoneurial extracellular matrix of intact and rhizotomized dorsal and ventral spinal roots of the rat – a quantitative analysis. Acta Histochem. 2006;107(6):453–462. doi: 10.1016/j.acthis.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rowshan K, Jones NF, Gupta R. Current surgical techniques of peripheral nerve repair. Oper Tech Orthop. 2004;14:163–170. [Google Scholar]
- Rummler LS, Gupta R. Peripheral nerve repair: a review. Curr Opin Orthop. 2004;15:215–219. [Google Scholar]
- Sunderland S, Bradley KC. The perineurium of peripheral nerves. Anat Rec. 1952;113:125–141. doi: 10.1002/ar.1091130202. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Lascelles RG. The pathology of diabetic neuropathy. Q J Med. 1966;35:489–509. [Google Scholar]
- Tonge DA, Golding JP, Edbladh M, Kroon M, Ekstrom PER, Edstrom A. Effects of extracellular matrix components on axonal outgrowth from peripheral nerves of adult animals in vitro. Exp Neurol. 1997;146:81–90. doi: 10.1006/exnr.1997.6498. [DOI] [PubMed] [Google Scholar]
- Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107:935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Yagihashi S, Yamagishi S, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: Correlation with clinical signs and symptoms. Diabetes Res Clin Pract. 2007;77(Suppl 1):S184–189. doi: 10.1016/j.diabres.2007.01.054. [DOI] [PubMed] [Google Scholar]