Abstract
Internal elastic lamina (IEL) hole (fenestration) characteristics and myoendothelial gap junction (MEGJ) density were examined in selected resistance and conduit arteries of normal and diseased rat and mouse models, using conventional, ultrastructural and confocal microscopy methods. Selected vessels were those commonly used in functional studies: thoracic aorta, proximal and distal mesenteric, caudal, saphenous, middle-cerebral and caudal cerebellar artery. Rat and mouse strains and treatment groups examined were Dahl, Sprague Dawley, Wistar Kyoto, Wistar, spontaneously hypertensive (SHR), deoxycorticosterone (DOC) treated rat; and apolipoprotein E knockout, C57/BL6 and BALB/c mice. Vessel size (as IEL circumference), IEL hole and MEGJ density were quantified. In mesenteric arteries, the width of IEL holes and the percent of IEL occupied by holes were also determined. IEL hole density varied significantly within and between mesenteric artery beds, even among normotensive rat strains. Among the hypertensive rats (SHR and DOC), hole density in some vessels was higher in the normotensives than in the hypertensives within each strain, whereas in Dahl rats, hole density was similar between hypertensives and normotensives. Hole density was not correlated with the formation of intimal lesions in superior mesenteric artery. There was no positive general correlation between IEL hole and MEGJ density in resistance and conduit vessels. However, there was a positive correlation between the size of some resistance arteries and MEGJ density, although such a relationship did not hold for conduit vessels or during development, and there was no such relationship between vessel size and IEL hole density. Whilst IEL holes are obviously required for MEGJ communication, their presence is not an indication of contact-mediated communication, but rather may be related to the presence of sites for the low resistance passage of diffusion-mediated release of vasoactive endothelial and smooth muscle substances.
Keywords: artery, endothelium, gap junction, hypertension, internal elastic lamina hole (fenestration), morphology, myoendothelial communication, smooth muscle
Introduction
Arterial internal elastic lamina (IEL) and its associated holes (fenestrations) are present in human and animal arteries (Osborne-Pellegrin, 1978; Osborne-Pellegrin, 1979; Campbell & Roach, 1981; Song & Roach, 1984; Capdeville et al. 1989; Dunmore et al. 1990). The IEL represents a flexible barrier between the endothelium and inner smooth muscle cell layer and may have a role in atherogenesis via its modulation of diffusion across the artery wall (Hutchison & Sanders, 1990; Osborne-Pellegrin, 1986). Holes in the IEL are implicated in myoendothelial signalling; enabling the passage of endothelial and smooth muscle cell projections and subsequent contact between these cell layers in some vessels, which is integral for local vasodilator activity enabling the passage of endothelium-derived hyperpolarization (EDH), as well as for the conduction of vasomotor responses over distance (Sandow et al. 2002; Sandow, 2004; De Wit et al. 2006; Sandow & Tare, 2007). However, a significant proportion of such holes do not have cellular contents and the functional significance of these holes is unknown, although various possibilities have been suggested. A possible role for these holes is to act as low-resistance pathways for the diffusion of substances between intima and media. This possibility suggests that an IEL with a low density of holes may be causally related to the passage of atherogenic molecules to accumulate in the intima. The IEL is an almost total barrier to higher molecular weight lipid so that increased plasma low density lipoprotein (LDL) results in a large increase in medial LDL concentration only if the IEL is damaged (Smith & Staples, 1980). A more permeable IEL would allow high molecular weight atherogenic chemicals to dissipate from the intima.
The IEL has also been suggested to play a role in modulating smooth muscle cell migration from the media to the intima. For such smooth muscle cell migration to occur, it was suggested that IEL holes must be greater than 3–4 µm wide (Sims, 1985). Furthermore, IEL holes have been suggested to be necessary to ‘nourish’ the media (Hassler, 1962). Thus, if the holes are too small or if they are blocked, it is possible that fibrosis or atheromatous medial degeneration may occur. Conversely, enlarged IEL holes have also been suggested to represent a weakness in the IEL which may contribute to the initiation of cerebral aneurysms (Campbell & Roach, 1981).
IEL hole incidence is, at least in part, likely genetically determined, as in normotensive rat strains (Brown Norway, Long Evans, Sprague Dawley and Wistar), caudal and renal arteries and abdominal aorta exhibit a different IEL hole incidence among them (Osborne-Pellegrin, 1985; Capdeville et al. 1989). Such differences are also found between sexes (Osborne-Pellegrin, 1985). Studies of pig thoracic aorta suggest that IEL holes may be associated with atherosclerosis (Dunmore et al. 1990; Kwon et al. 1998), although there is no known relationship between the two. However, as IEL hole size is independent of age, at least in the pig aorta (Dunmore et al. 1990), and thus not likely associated with atherosclerotic development, as atherosclerosis develops with increasing age, the role of IEL holes in this process is speculative, but worthy of further examination.
Here we determined IEL hole density and size in different strains and treatment groups of rats: normotensive Wistar and Wistar-Kyoto (WKY), spontaneously hypertensive (SHR), Dahl (salt-sensitive and salt-resistant), and deoxycorticosterone-NaCl treated (DOC/NaCl). Intimal lesion number and size were also measured in the superior mesenteric artery of these rats. In addition, IEL hole and myoendothelial gap junction (MEGJ) density were measured in selected resistance and conduit arteries of Sprague Dawley (SD), Wistar, WKY, SHR; and apolipoprotein E knockout (ApoE−/–), C57/BL6 and BALB/c mice. The hypothesis of the study was that IEL hole density is related to arterial development, disease and MEGJ incidence.
Materials and methods
Male rats and mice were used in this study. SHR and WKY were obtained from the breeding colonies maintained at McMaster University and the University of New South Wales (UNSW). Hypertension was induced in a group of rats by deoxycorticosterone (DOC) injection and salt was added to their drinking water (DOC/NaCl, Lee et al. 1989). Male Dahl rats, both salt-sensitive (DS) and salt-resistant (DR), were obtained from the Brookhaven National Laboratories. DS and DR were divided into two groups which were maintained on a low or high salt diet (Lee & Triggle, 1986). Additional rat and mouse strains were obtained from UNSW colonies.
Procedures for isolation, perfusion and fixation were as previously described (Lee, 1985a; Sandow et al. 2006). Of note, arteries were maximally relaxed (via the addition of a maximal concentration of sodium nitrite or sodium nitroprusside in the clearance solution) prior to fixation so that the IEL would be at its maximum circumference for accurate relative measurements. For light microscopy cross-section analysis of mesenteric vessels, the number of arteries measured for each rat was two for the superior and six for the primary mesenteric artery. Vessels were sectioned at 90o perpendicular to the long vessel axis to obtain optimal accurate cross-sections. Alternatively, where vessels were not sectioned at 90o perpendicular to the long vessel axis, correction for the eccentricity of the sections due to an oblique sectioning angle was applied to compare the IEL circumferences of the various groups (Lee et al. 1983). Cross-section vessel profiles 1 µm thick were projected onto a digitizing board and IEL circumference and the width of holes were measured using an LCS Micro-Planimeter System (Laboratory Computer Systems, Inc., Cambridge, MA, USA). The average number of holes, hole width and the percent of the total IEL occupied by holes were measured and calculated. In addition, measurements were made of the lesions that occurred sporadically in the rat superior mesenteric artery. The number of lesions, lesion length and number of cells present in the lesion were recorded. The averages for each rat group were calculated and compared.
At the confocal microscope level, the IEL was visualized from autofluorescence at 488 nm and images acquired using an Olympus FV1000 with appropriate filter sets. The mean number of IEL holes (visualized as dark spots; Fig. 1), from four 103-µm2 regions from each vessel was collated. Ultrastructural preparation and analysis were conducted as previously described, including IEL, medial and MEGJ characteristics (Sandow et al. 2002, 2006). Morphological analysis was undertaken using Olympus CellR software. For scanning electron microscopy, the vessels were treated with NaOH to remove cellular elements (Dunmore et al. 1990).
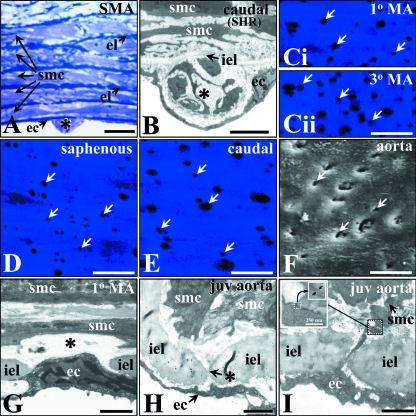
Fig. 1.
Intimal lesion, internal elastic lamina (IEL) hole, myoendothelial gap junction lesion characteristics in selected male rat and mouse vessels. Intimal lesions (*) in rat superior mesenteric (SMA), with endothelial (ec), elastic laminae (el) and smooth muscle cells (smc), and SHR caudal artery (A,B, respectively). Autofluorescence of IEL with IEL holes as intermittent dark spots (arrows); adult male rat mesenteric (primary, Ci; and tertiary, Cii branch); lateral saphenous (D), and distal caudal (E) artery, as potential sites for passage of ec and smc projections associated with heterocellular gap junction or low resistance communication pathways between ecs and smcs. Scanning electron microscopy shows such holes as pits in the luminal IEL surface (F, arrows, rat aorta). Transmission electron microscopy of IEL holes shows the gap in the IEL typical for such holes (*) in resistance and conduit vessels (G,H, rat mesenteric and juvenile (2 wk) male mouse aorta, respectively). Myoendothelial gap junction from juvenile mouse thoracic aorta (I) with typical pentalaminar gap junction membrane density (inset, arrowed). Bars: A, 10 µm; B, 5 µm; C–F, 20 µm; G, 1 µm; H,I, 2 µm.
Data were analysed with the program Statsgraphic using two-tailed Student t-tests. P < 0.05 was considered significant.
Results
Mesenteric artery: light microscopy section analysis
SHR and WKY
In primary or large mesenteric arteries (LMA), at 3 weeks of age, the proportion of IEL occupied by holes was lower in SHR than in WKY; this was due to a smaller number of IEL holes, because the average hole width was similar between SHR and WKY (Table 1). These differences were maintained in the 28-week-old group, except that the average IEL hole width became smaller in SHR than in WKY. There was an age-dependent increase in the percent of IEL occupied by IEL holes in both SHR and WKY that was associated with the increase in the number and width of the holes. There was no difference in the circumference of IEL between SHR and WKY in these two age groups, showing that the difference observed was not due to a difference in IEL circumference (Table 1).
Table 1.
Comparisons among different vessels from different age groups of SHR and WKY
| Large mesenteric arteries | Superior mesenteric arteries | |||
|---|---|---|---|---|
| Group | 3 weeks | 28 weeks | 3 weeks | 28 weeks |
| Percent hole | ||||
| SHR | 2.07 ± 0.21* (11) | 4.04 ± 0.16*‡ (6) | 7.82 ± 0.39* (10) | 7.23 ± 1.0* (5) |
| WKY | 2.93 ± 0.22 (8) | 7.96 ± 0.35‡ (5) | 5.28 ± 0.42 (9) | 9.55 ± 0.7‡ (5) |
| Hole number | ||||
| SHR | 10.9 ± 1.2* (11) | 13.2 ± 0.4* (6) | 24.5 ± 1.6(10) | 37.3 ± 3.0*‡ (5) |
| WKY | 14.3 ± 0.7 (8) | 22.2 ± 0.7‡ (5) | 20.9 ± 1.4(9) | 47.2 ± 2.6‡ (5) |
| Hole width (µm) | ||||
| SHR | 0.84 ± 0.07 (11) | 2.08 ± 0.09*‡ (6) | 3.21 ± 0.15* (10) | 3.23 ± 0.44 (5) |
| WKY | 0.95 ± 0.08 (8) | 2.40 ± 0.07‡ (5) | 2.69 ± 0.11 (9) | 3.15 ± 0.12‡ (5) |
| IEL circumference (µm) | ||||
| SHR | 438 ± 17 (11) | 667 ± 34‡ (6) | 996 ± 22 (10) | 1683 ± 58‡ (5) |
| WKY | 460 ± 15 (8) | 673 ± 24‡ (5) | 1064 ± 34 (9) | 1555 ± 47‡ (5) |
Significantly different from WKY;
significantly different from SHR;
significant difference between 3- and 28-week age groups. Numbers in brackets signify nvalue (number of animals) for each group.
In the superior mesenteric arteries (SMA), the proportion of IEL occupied by holes (percent hole) was higher in SHR than WKY due to wider holes at 3 weeks of age (Table 1). At 28 weeks, the relative proportion of IEL as holes became higher in the WKY than SHR, due to a smaller number of IEL holes in the SHR than WKY (Table 1). There was an age-dependent increase in percent hole in WKY but not in SHR. In the WKY, this increase was due to an increase in hole number and hole width, whereas in the SHR, there was a small increase in hole number but not in hole size. Comparison between LMA and SMA showed that percent IEL area occupied by holes was higher in SMA than in LMA in both SHR and WKY at 3 weeks (P < 0.05), but at 28 weeks, percent hole was higher in SMA than in LMA only in SHR (P < 0.007), but not in WKY (P = 0.07).
DOC/NaCl
Three rat groups were examined: untreated normotensive Wistar, rats treated with DOC, but remaining normotensive (DOC-N), and hypertensive rats (DOC-H). There was no difference between the two normotensive groups in either LMA or SMA in the proportion of IEL as IEL holes and hole number and size (Table 2). A significant difference between the hypertensive and normotensives was found only in the SMA where the proportion of IEL as holes was lower in the hypertensive than in the normotensives, due to smaller hole size in the hypertensives (Table 2). Percent hole was higher in SMA than LMA in all the three groups of rats (P < 0.05).
Table 2.
Comparisons among the IEL holes from the mesenteric arteries of DOC/NaCl-treated and control Wistar rats
| Group | LMA | SMA |
|---|---|---|
| Percent hole | ||
| Wistar | 3.11 ± 0.24 (6) | 17.0 ± 1.2* (5) |
| DOC-N | 2.59 ± 0.21 (5) | 15.6 ± 2.0* (4) |
| DOC-H | 3.44 ± 0.33 (6) | 9.8 ± 0.8 (6) |
| Hole number | ||
| Wistar | 12.8 ± 1.5 (6) | 53.6 ± 2.7 (5) |
| DOC-N | 11.2 ± 0.9 (5) | 52.8 ± 3.6 (4) |
| DOC-H | 16.8 ± 2.0 (6) | 44.0 ± 3.8 (6) |
| Hole width (µm) | ||
| Wistar | 1.77 ± 0.09 (6) | 4.72 ± 0.13* (5) |
| DOC-N | 1.50 ± 0.09 (5) | 4.79 ± 0.37* (4) |
| DOC-H | 1.55 ± 0.08 (6) | 3.96 ± 0.18 (6) |
| IEL circumference (µm) | ||
| Wistar | 688 ± 62 (6) | 1577 ± 55 (5) |
| DOC-N | 626 ± 9 (5) | 1610 ± 42 (4) |
| DOC-H | 766 ± 49 (6) | 1663 ± 19 (6) |
Significantly different from DOC-H. Numbers in brackets signify n value (number of animals) for each group.
LMA, large mesenteric artery; SMA, superior mesenteric artery.
Dahl rats
The Dahl salt-sensitive (DS) and salt-resistant (DR) rats were maintained on a low salt or a high salt diet. Only DS rats given a high salt diet developed hypertension (Lee & Triggle, 1986). There was no difference in IEL hole density between DS and DR rats given either a high or a low salt diet in LMA or SMA characteristics (Table 3). However, percent hole was higher in SMA than LMA in all four groups of rats (P < 0.05).
Table 3.
Comparisons among the IEL holes of the mesenteric arteries from Dahl salt-sensitive and salt-resistant rats, on high and low salt diets
| Large mesenteric arteries | Superior mesenteric arteries | |||
|---|---|---|---|---|
| Group | High salt diet | Low salt diet | High salt diet | Low salt diet |
| Percent hole | ||||
| Salt-sensitive | 3.80 ± 0.33 (6) | 3.25 ± 0.26 (6) | 8.10 ± 0.65 (6) | 10.20 ± 0.50 (6) |
| Salt-resistant | 4.28 ± 0.30 (6) | 4.14 ± 0.42 (6) | 10.50 ± 1.00 (6) | 13.60 ± 1.70 (6) |
| Hole number | ||||
| Salt-sensitive | 18.6 ± 1.1 (6) | 16.4 ± 1.0(6) | 48.5 ± 14.3 (6) | 52.5 ± 1.8 (6) |
| Salt-resistant | 23.0 ± 1.8 (6) | 21.7 ± 1.1* (6) | 50.5 ± 3.9 (6) | 58.2 ± 5.6 (6) |
| Hole width (µm) | ||||
| Salt-sensitive | 1.54 ± 0.13 (6) | 1.19 ± 0.07† (6) | 3.04 ± 0.08 (6) | 3.66 ± 0.13† (6) |
| Salt-resistant | 1.26 ± 0.10 (6) | 1.45 ± 0.08* (6) | 3.46 ± 0.34 (6) | 4.19 ± 0.18* (6) |
| IEL circumference (µm) | ||||
| Salt-sensitive | 761 ± 31 (6) | 617 ± 38 (6) | 1797 ± 45 (6) | 1869 ± 64 (6) |
| Salt-resistant | 670 ± 46 (6) | 775 ± 50 (6) | 1570 ± 71 (6) | 1706 ± 63 (6) |
Significant difference between corresponding salt-sensitive group;
significant difference between high and low salt. Numbers in brackets signify n value (number of animals) for each group.
Normotensive rats
Among the normotensive rats (WKY, Wistar and Dahl salt-resistant, DR), significant differences were observed for the proportion of IEL as holes in both LMA and SMA (Table 4). In LMA, the proportion of IEL as holes was higher in WKY than in Wistar and DR rats, partly due to larger hole size in WKY than the other two groups. Hole number was similar between WKY and DR rats, but higher than in Wistar rats. In contrast, in SMA, the proportion of IEL as holes was lower in WKY than Wistar or DR rats, due to smaller hole width in the WKY. There was also a difference between Wistar and DR rats in the proportion of IEL as holes and hole width.
Table 4.
Comparisons of IEL holes from the mesenteric arteries of normotensive rats
| Group | LMA | SMA |
|---|---|---|
| Percent hole | ||
| WKY | 7.96 ± 0.35 (5) | 9.55 ± 0.72 (5) |
| Wistar | 3.11 ± 0.24* (6) | 17.01 ± 1.2* (5) |
| DR | 4.21 ± 0.24*† (12) | 12.10 ± 1.0*† (12) |
| Hole number | ||
| WKY | 22.2 ± 0.7 (5) | 47.2 ± 2.6 (5) |
| Wistar | 12.8 ± 1.5* (6) | 53.6 ± 2.7 (5) |
| DR | 22.3 ± 1.0† (12) | 54.3 ± 3.4 (12) |
| Hole width (µm) | ||
| WKY | 2.40 ± 0.07 (5) | 3.15 ± 0.12 (5) |
| Wistar | 1.77 ± 0.09* (6) | 4.72 ± 0.13* (5) |
| DR | 1.36 ± 0.07*† (5) | 3.82 ± 0.21*† (12) |
| IEL circumference (µm) | ||
| WKY | 667 ± 29 (5) | 1696 ± 44 (5) |
| Wistar | 688 ± 62 (6) | 1577 ± 55 (5) |
| DR | 723 ± 36 (12) | 1638 ± 50 (12) |
Significantly different from WKY;
significantly different from Wistar. Numbers in brackets signify n value (number of animals) for each group. LMA, large mesenteric artery; SMA, superior mesenteric artery.
Intimal lesions
Intimal lesions consisting of neointimal smooth muscle cells were often found in the SMA (Fig. 1A). It is possible that the incidence of intimal lesions associated with hypertension may show a direct correlation with the size and number of holes in the IEL. However, comparison among the various groups showed that the number of lesions, the average number of smooth muscle cells inside each lesion, and the length of IEL occupied by each lesion were similar among the hypertensive rats (SHR, DSS ad DOCA-H) and between their corresponding normotensive rats (Table 5). Intimal lesions were also present in SHR distal caudal artery (Fig. 1B).
Table 5.
Comparisons of intimal lesions in the superior mesenteric arteries from different rat strains
| Group | Number of lesions | No. of Cells | Lesion width (µm) |
|---|---|---|---|
| SHR (5) | 2.10 ± 0.60 | 2.65 ± 0.50 | 25.8 ± 6.7 |
| WKY (4) | 0.38 ± 0.24 | 0.63 ± 0.38 | 12.2 ± 8.2 |
| DSS (12) | 1.50 ± 0.27 | 1.91 ± 0.46 | 34.7 ± 7.8 |
| DSR (12) | 0.75 ± 0.24 | 1.38 ± 0.38 | 16.9 ± 5.1 |
| DOC-H (6) | 2.25 ± 0.82 | 1.87 ± 0.43 | 25.8 ± 5.6 |
| DOC-N (4) | 1.75 ± 0.75 | 1.75 ± 0.25 | 25.4 ± 6.9 |
| Wistar (5) | 1.20 ± 0.40 | 1.46 ± 0.54 | 28.4 ± 8.4 |
Numbers in brackets signify n value (number of animals) for each group. Age of the rats was 28 weeks for SHR and WKY, 11–12 weeks for DSS and DSR, and approximately 15 weeks for DOC-H, DOC-N and Wistar rats.
Comparison of vessel IEL and myoendothelial gap junction incidence: confocal and ultrastructural analysis
Under confocal microscopy, IEL holes appeared as intermittent dark spots (Fig. 1C–E), as compared with their appearance as pits with scanning electron microscopy (Fig. 1F) and gaps in IEL with transmission electron microscopy (Fig. 1G,H). Hole density in the thoracic aorta of SD rats was high compared to other rat vessels, but no MEGJs were found (Table 6), even with serial section ultrastructural analysis. In the distal caudal artery of SHR and WKY, hole density and MEGJ incidence were similar between the two groups (Table 6). In the mesenteric vascular bed, hole density was higher in the smaller distal branch than in the larger proximal branch. High hole density was also associated with more MEGJs in the distal vessels than in the proximal vessels, which had less hole density and fewer MEGJs. Therefore there was an inverse relationship between vessel size and IEL hole and MEGJ density in rat mesenteric artery: larger vessel (proximal) has less IEL hole and lower MEGJ density than smaller (distal) vessel. Comparison between saphenous artery from 2- and 16-week WKY showed a lower hole density (P < 0.05), but more MEGJs (P < 0.05) in the young than in the old rats (Table 6), indicating developmental changes. In the cerebral arteries from Wistar rats, even though the number of holes was similar between the middle and caudal cerebellar artery, more MEGJs were found in the smaller caudal cerebellar artery (P < 0.05). Hole density was also higher in the cerebral vessels than in the systemic vessels (P < 0.05).
Table 6.
Selected male rat resistance and conduit artery characteristics
| Age/vessel | IEL circumference (µm) | #SMC layers | #IEL holes/103µm2 IEL | #MEGJs /103µm2 IEL |
|---|---|---|---|---|
| 12 weeks SD thoracic aorta | 5089 ± 370 (4) | 6.7 ± 0.3 (4) | 9.4 ± 1.4 (4) | 0 (4) |
| 11–13 weeks SHR distal caudal | 889 ± 18 (3)1 | 8.8 ± 0.5 (3)1 | 4.2 ± 0.8 (3) | 1.5 ± 0.1 (3)1 |
| 11–13 weeks WKY distal caudal | 1088 ± 51 (3)1 | 7.0 ± 0.3 (3)1 | 4.6 ± 0.6 (3) | 0.9 ± 0.1 (3)1 |
| 16 weeks Wistar distal mesenteric* | 488 ± 53 (3) | 3.4 ± 0.1 (3) | 16 ± 1.9 (3) | 9.2 ± 1.1 (3) |
| 16 weeks Wistar proximal mesenteric* | 648 ± 57 (3)2 | 4.8 ± 0.3 (3)2 | 7.7 ± 2.2 (3) | 2.3 ± 0.4 (3)2 |
| 2 weeks WKY saphenous branch† | 390 ± 28 (8)3 | 3.5 ± 0.4 (4)3 | 3.0 ± 1.5 (3)3 | 5.6 ± 0.1 (5)3 |
| 16 weeks WKY saphenous branch† | 745 ± 36 (4)3 | 7.2 ± 0.1 (4)3 | 8.7 ± 1.0 (5)3 | 0.3 ± 0.1 (4)3 |
| 12 weeks Wistar middle cerebral | 465 ± 28 (4) | 3.6 ± 0.3 (4) | 19 ± 2.3 (4) | 4.8 ± 0.9 (4) |
| 2 weeks Wistar caudal cerebellar | 234 ± 14 (4) | 2.4 ± 0.2 (6)4 | 18 ± 1.4 (4) | 8.3 ± 2.3 (6)4 |
,
indicates significant difference (P < 0.05) for all parameters for proximal-distal and age-related comparisons of mesenteric and saphenous artery, respectively; number in brackets signifies nvalue (number of animals) for each group. IEL, internal elastic lamina; MEGJ, myoendothelial gap junction; SD, Sprague Dawley; SHR, spontaneously hypertensive rat; SMC, smooth muscle cell; WKY, Wistar Kyoto.
Data respectively from Sandow et al. 2003, 2002, 2004, and Haddock et al. 2006.
In C57/BL6 mice, a normal strain, MEGJs were found only in the thoracic aorta of young mice (2 weeks; Fig. 1I) and no MEGJs were found in older (12 weeks) mouse aorta, despite the fact that hole density was similar (Table 7). In contrast, some MEGJs were found in adult ApoE−/– mouse aorta, an animal model of atherosclerosis. In the BALB/c mice, another normal strain, MEGJs were found only in the mesenteric artery, but not in the saphenous artery (Table 7).
Table 7.
Selected male mouse resistance and conduit artery characteristics
| Age/vessel | IEL Circumference (µm) | #SMC layers | #IEL holes/103µm IEL | #MEGJs/103µm2 IEL |
|---|---|---|---|---|
| 2 weeks C57/BL6 mouse thoracic aorta | 1548 ± 88 (4) | 4.4 ± 0.3 (4) | 9.0 ± 0.7 (4) | 3.3 ± 0.7 (4) |
| 12 weeks C57/BL6 mouse thoracic aorta | 2453 ± 66 (4) | 6.5 ± 0.3 (4) | 9.3 ± 1.8 (4) | 0 (4) |
| 12 weeks ApoE−/– mouse thoracic aorta | 2434 ± 308 (4) | 6.4 ± 0.2 (4) | 9.8 ± 1.5 (4) | 0.8 ± 1.5 (4) |
| 10–12 weeks BALB/c mouse saphenous artery branch | 546 ± 27 (3) | 4.5 ± 0.4 (3) | 14 ± 2.2 (3) | 0 (3) |
| 10–12 weeks BALB/c mouse 1st order mesenteric artery | 452 ± 43 (4) | 2.3 ± 0.2 (4) | 16 ± 2.2 (4) | 7.0 ± 0.8 (3) |
n(in brackets), number of animals. ApoE−/–, apolipoprotein E knockout; IEL, internal elastic lamina; MEGJ, myoendothelial gap junction; SM, smooth muscle cell.
Discussion
For the animals and vascular beds examined in the present study, with the exception of SMA from adult WKY, percent IEL occupied by holes and hole density were generally higher in larger vessels such as aorta or SMA than smaller arteries (e.g. LMA). However, in the mesenteric bed of Wistar rats, higher hole density was found in smaller distal segment than in larger proximal segment. Thus, overall there is no general relationship of IEL hole density and vessel size. IEL hole density also varied with animal strain, vessel type, animal age and disease state. In a similar manner, there was no general relationship between IEL hole and MEGJ density in resistance and conduit vessels. However, there was a general positive correlation between the size of some resistance arteries and MEGJ density, although such a relationship did not hold for conduit vessels or during development.
In LMA, even though IEL hole density was less in two hypertensive models (SHR and DOC-H) than their corresponding comparative normotensive strains, no such difference was found in the Dahl hypertensive model. In addition, significant variation was found among the different normotensive groups. Thus, these data suggest that there is a genetic component involved in IEL hole density, as previously described in caudal and renal arteries and abdominal aorta of Brown Norway, Long Evans, SD and Wistar rat strains (Capdeville et al. 1989). LMA was examined in the present study, as most studies of vascular morphology in hypertension reported this vessel as having significant changes in morphology, such as a large increase in medial wall thickness (Lee & Smeda, 1985; Lee, 1987). In SHR, a smaller hole density was present at 3 weeks of age due to a smaller number of IEL holes, and this difference was maintained in older SHR with established hypertension. Blood pressure of SHR at 3 weeks is similar to that of WKY (Lee, 1985b). Furthermore, although neonatal sympathectomy results in a lowered blood pressure to normal in SHR, there were still significantly fewer holes than in the mesenteric artery of sympathectomized SHR compared to WKY (Lee & Gzik, 1991). These results show that differences in hole density between SHR and WKY in the mesenteric artery were independent of blood pressure. In contrast, there was no difference in hole density in the caudal artery between SHR and WKY, showing that the difference in hole density between SHR and WKY also depended on the type of vessel studied. In the other two hypertensive models (DOC/NaCl and Dahl), there was no difference in hole density in LMA of hypertensives and normotensives, suggesting that the difference in hole density in this vessel type is not associated with hypertension.
At least to some extent, the IEL serves as a barrier between the arterial intimal and medial layers (Smith & Staples, 1980; Svendsen & Tindall, 1988). The function of IEL holes is in part for cell contact and communication between the endothelium and smooth muscle (Rhodin, 1968; Sandow et al. 2002). There were differences in the relationship of IEL hole and MEGJ density in vessels from normotensive and hypertensive rats. As SHR had a lower proportion of the IEL as holes, this suggests a potential lower efficiency/patency for cellular communication between the endothelium and smooth muscle, and this was examined in this study. In the distal caudal artery of WKY and SHR, IEL hole density was similar, whilst there was a significant (∼70%) increase in SHR MEGJ density. This situation is in contrast to IEL hole and MEGJ density in the mesenteric artery of WKY and SHR (Ellis et al. 2008), where MEGJ and IEL hole density were decreased in SHR compared with WKY. These data thus show that there is heterogeneity in remodeling of WKY and SHR vessels. Various substances released from the endothelium can affect the structure and function of the smooth muscle. In hypertensive animals, the activity of substances such as nitric oxide and EDH, which cause smooth muscle relaxation (Shimokawa, 1998), and heparin-like substance, which inhibits vascular smooth muscle proliferation (Castellot et al. 1982), may be reduced, thus being potentially causally related to some of the functional changes found in vascular disease. Furthermore, as suggested by Hassler (1962), IEL holes may serve to transport nutrients from the lumen to the media as the compact nature of the IEL probably has low permeability. Atherosclerosis is partly associated with smooth muscle cell migration from the media to the intima, which has been thought to occur through IEL holes. In these regards, our results showing the presence of more area occupied by holes in the IEL of large vessels such as SMA than in smaller vessels such as LMA in rats (Tables 1–3), and the presence of intimal lesions in SMA, do support the idea that IEL holes in large arteries serve as a conduit for nutrient transport and smooth muscle migration.
Intimal lesions are seldom found in LMA, but are often found in larger vessels such as the SMA and aorta, and indeed were found in the SHR caudal artery (this study). The presence of intimal lesions may be related to IEL hole size. It has been suggested that IEL holes must be at least 3–4 µm in diameter to allow the growth of mesenchymal cells into the intima from the media (Sims, 1985). In this regard, it is interesting that the average width of IEL holes in the LMA examined in this study was around 2 µm or smaller. In larger vessels such as SMA, where intimal lesions are usually found, the average IEL hole width was between 3 and 5 µm. However, in this study we could not find a correlation between the percent of holes in IEL with the incidence of intimal lesions in the SMA. In the SHR and DOC-H rats, the percent of IEL occupied by holes was lower in the hypertensives than in the normotensives, but the number of lesions was similar, even though there was a tendency for the lesion number to be higher in the hypertensives than the normotensives. Among the Dahl rats, there was no difference in the percent of holes in the IEL or hole number in DS and DR rats on high or low salt diet, and the number of lesions was also similar. In the Dahl model, the extent of intimal damage including intimal lesion number and size was found to correlate with high salt diet and hypertension (Lee & Triggle, 1986). It is clear that other factors besides hole density are involved in intimal lesion formation in hypertension in such vessels.
Myoendothelial gap junctions obviously require IEL holes for endothelial cells to make contact with smooth muscle cells, and thus it is logical to assume that there may be a positive correlation between hole and MEGJ density, and that such a relationship may be altered in disease states such as hypertension or atherosclerosis. These possibilities were examined in the present study. We found that the relationship between hole density and MEGJs was highly dependent on animal strain, vessel type, animal age and disease state and that this relationship is complex, as discussed below.
In the thoracic aorta of the adult SD rat, in spite of a relatively high IEL hole density, no MEGJs were found. IEL hole density in juvenile and adult mouse aorta was roughly the same as in the SD rat aorta, whilst MEGJs were absent in the adult but present in the juvenile mouse aorta. These data show that IEL hole presence is not an indication of MEGJ presence, and further demonstrate a developmental association of IEL holes and MEGJs in typical conduit vessels. In saphenous artery, a developmental relationship between juvenile and adult rats was also found with regard to MEGJ-related EDH function (Sandow et al. 2004). In the cerebral arteries, IEL hole density was similar in juvenile and adult vessels, although this does not reflect MEGJ density. In contrast, in adult vessels there was a significant decrease in MEGJ density (∼40%), thus further demonstrating a lack of relationship between IEL hole and MEGJ density, and demonstrating considerable heterogeneity in aspects of vessel morphology within the same vascular bed during development. Interestingly, development-related altered connexin (gap junction protein) expression has been described in vascular, cardiac and neural tissue (Sandow et al. 2004; Bruzzone & Dermietzel, 2006), implicating altered gap junction activity during system differentiation and development.
We also found that IEL hole density and MEGJ number were generally higher in the cerebral vessels than in the systemic arteries in the rats irrespective of age or strain. The reason for increased IEL hole density in cerebral vessels is unknown. Of interest, EDH is correlated with MEGJ density in a number of arteries, including within the same vascular beds, such as in proximal and distal mesenteric arteries (Sandow & Hill, 2000; Sandow, 2004). Results from proximal and distal mesenteric arteries suggest that there was a similar increased density of IEL holes in smaller distal mesenteric vessels, thus in this case suggesting a relationship to function. Indeed, in the aorta (Sandow, 2004; Feletou & Vanhoutte, 2007; Sandow & Tare, 2007) the presence or absence of MEJGs shows an apparent correspondence with the presence of EDH, this mechanism being present in rat aorta of juvenile (Martinez-Org et al. 1999) and disease models (hypercholesterolemic, diabetic and hypertensive and with altered estrogen levels), but absent in healthy adult aorta (Wu et al. 1993; Endo et al. 1995; Kagota et al. 2000; Shimamura et al. 2000; Matsumoto et al. 2004; Woodman & Boujaoude, 2004; Malakul et al. 2008). In many vascular beds, the vasodilator EDH mechanism is dependent on MEGJs, with correlative and concomitant modification in development, ageing and disease (Griffith, 2004; Feletou & Vanhoutte, 2007; Sandow & Tare, 2007).
Further changes in IEL and MEGJ characteristics are seen in mouse models of disease. In ApoE−/– mice which develop atherosclerotic lesions when put on a special diet (Zhou et al. 2001), MEGJs were present in the aorta of adult mice as compared with an absence in adult C57/BL6 mice, whereas IEL hole density was similar to such C57/BL6 mice. Whether the presence of MEGJs in the aorta of ApoE−/– mice was associated with the pathological background of the mice remains to be determined. In another strain of normal mice (BALB/c), MEGJs were found in the mesenteric arteries, but not in the saphenous arteries, even though the vessels were of comparable size, with comparable IEL hole density. Such variation highlights the heterogeneity of MEGJs and IEL holes among different vessel types within the same strain of mice. These results raise some cautionary issues for relating IEL holes to MEGJs. Although IEL holes are required for MEGJ contact, the presence of holes alone, as mentioned above, is not indicative of such contacts. An additional cautionary note relates to the use of in vitro cell culture results. Co-culture of mouse aortic endothelial and smooth muscle cells can form MEGJs (Isakson & Duling, 2005). However, as shown in this study, MEGJs were absent in the aorta of adult mice. Formation of MEGJs in cell co-culture may represent a developmental or diseased aortic model, as MEGJs were found in the aorta of juvenile and ApoE−/– mice, but such co-culture does not reflect the state of the normal adult aorta.
In summary, here we found that IEL hole density varied significantly among resistance and conduit vessels; regardless of species, strain, vascular bed and age. Among the hypertensive rats (SHR, and DOC-H), hole density was higher in the normotensives than in the hypertensives within each strain in some vessels, whereas in Dahl rats, hole density was similar between hypertensives and normotensives. At face value, a lower hole density in the hypertensives suggests a limited prospect for myoendothelial contact formation, which would thereby potentially lower the inhibitory effect of endothelium on smooth muscle cell proliferation, and decrease relaxation due to a potential for reduced influence of hole-dependent endothelium-derived relaxation mechanisms. Indeed, although there is a general positive correlation between selected resistance artery size and MEGJ density, such a relationship does not hold for conduit vessels or during development, and there is no such relationship between vessel size and IEL hole density.
In conclusion, we found that IEL hole density is not related to hypertension or intimal lesion formation. Although IEL holes are obviously required for MEGJ communication, their presence is not an indication of contact-mediated communication.
Acknowledgments
This study was supported by a National Health and Medical Research Council of Australia Project Grant and RD Wright Fellowship (455243, 401112, respectively) to S.L.S., and Heart and Stroke Foundation of Ontario to R.M.K.W.L. R.M.K.W.L. was supported by a Human Frontier Science Program Short term Fellowship. We thank Shane Thomas, Centre for Vascular Research, UNSW for supplying the ApoE−/– tissue.
References
- Bruzzone R, Dermietzel R. Structure and function of gap junctions in the developing brain. Cell Tissue Res. 2006;326:239–248. doi: 10.1007/s00441-006-0287-0. [DOI] [PubMed] [Google Scholar]
- Campbell GJ, Roach MR. Fenestrations in the internal elastic lamina at bifurcations of human cerebral arteries. Stroke. 1981;12:489–496. doi: 10.1161/01.str.12.4.489. [DOI] [PubMed] [Google Scholar]
- Capdeville M, Coutard M, Osborne-Pellegrin MJ. Spontaneous rupture of the internal elastic lamina in the rat: the manifestation of a genetically determined factor which may be linked to vascular fragility. Blood Vessels. 1989;26:197–212. doi: 10.1159/000158768. [DOI] [PubMed] [Google Scholar]
- Castellot JJ, Favreau LV, Karnovsky MJ, Rosenberg RD. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. J Biol Chem. 1982;257:11256–11260. [PubMed] [Google Scholar]
- De Wit C, Hoepfl B, Wolfle SE. Endothelial mediators and communication through vascular gap junctions. Biol Chem. 2006;387:3–9. doi: 10.1515/BC.2006.002. [DOI] [PubMed] [Google Scholar]
- Dunmore PJ, Song SH, Roach MR. A comparison of the size of fenestrations in the internal elastic lamina of young and old porcine aortas as seen with the scanning electron microscope. Can J Physiol Pharmacol. 1990;68:139–143. doi: 10.1139/y90-022. [DOI] [PubMed] [Google Scholar]
- Ellis A, Goto K, Brackenbury TD, Falck JR, Hill CE. Gap junction-dependent and independent mechanisms underlie EDHF dilation in mesenteric arteries from normotensive and hypertensive rats. J Vasc Res. 2008;45(Suppl. 1):4. [Google Scholar]
- Endo K, Abiru T, Machida H, Kasuya Y, Kamata K. Endothelium-derived hyperpolarizing factor does not contribute to the decrease in endothelium-dependent relaxation in the aorta of streptozotocin-induced diabetic rats. Gen Pharmacol. 1995;26:149–153. doi: 10.1016/0306-3623(94)00159-k. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: Past beliefs and present facts. Ann Med. 2007;39:1–22. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, et al. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- Hassler O. The windows of the internal elastic lamella of the cerebral arteries. Virchows Arch A [Pathol Anat] 1962;335:127–132. doi: 10.1007/BF02438699. [DOI] [PubMed] [Google Scholar]
- Hutchison KJ, Sanders EJ. Patterns of internal elastic lamina morphology in the canine common carotid artery. Blood Vessels. 1990;27:1–13. doi: 10.1159/000158791. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Kagota S, Yamaguchi Y, Nakamura K, Kunitomo M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen Pharmacol. 2000;34:201–209. doi: 10.1016/s0306-3623(00)00061-6. [DOI] [PubMed] [Google Scholar]
- Kwon HM, Kim D, Hong BK, et al. Ultrastructural changes of the internal elastic lamina in experimental hypercholesterolemic porcine coronary arteries. J Korean Med Sci. 1998;13:603–611. doi: 10.3346/jkms.1998.13.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RMKW. Preparation methods and morphometric measurements of blood vessels in hypertension. Prog Appl Microcirc. 1985a;8:129–134. [Google Scholar]
- Lee RMKW. Vascular changes at the prehypertensive phase in the mesenteric arteries from spontaneously hypertensive rats. Blood Vessels. 1985b;22:105–126. doi: 10.1159/000158589. [DOI] [PubMed] [Google Scholar]
- Lee RMKW. Structural alterations of blood vessels in hypertensive rats. Can J Physiol Pharmacol. 1987;65:1528–1535. doi: 10.1139/y87-241. [DOI] [PubMed] [Google Scholar]
- Lee RMKW, Gzik DJ. Sympatholytic intervention and vascular remodelling. Basic Res Cardiol. 1991;86(Suppl. 1):55–64. [PubMed] [Google Scholar]
- Lee RMKW, Smeda JS. Primary versus secondary structural changes of the blood vessels in hypertension. Can J Physiol Pharmacol. 1985;63:392–401. doi: 10.1139/y85-070. [DOI] [PubMed] [Google Scholar]
- Lee RMKW, Triggle CR. Morphometric study of mesenteric arteries from genetically hypertensive Dahl strain rats. Blood Vessels. 1986;23:199–224. doi: 10.1159/000158642. [DOI] [PubMed] [Google Scholar]
- Lee RMKW, Garfield RE, Forrest JB, Daniel EE. Morphometric study of structural changes in the mesenteric blood vessels of spontaneously hypertensive rats. Blood Vessels. 1983;20:57–71. doi: 10.1159/000158460. [DOI] [PubMed] [Google Scholar]
- Lee RMKW, Richardson M, McKenzie R. Vascular changes associated with deoxycorticosterone-NaCl-induced hypertension. Blood Vessels. 1989;26:137–156. doi: 10.1159/000158763. [DOI] [PubMed] [Google Scholar]
- Malakul W, Thirawarapan S, Suvitayavat W, Woodman OL. Type 1 diabetes and hypercholesterolaemia reveal the contribution of endothelium-derived hyperpolarizing factor to endothelium-dependent relaxation of the rat aorta. Clin Exp Pharmacol Physiol. 2008;35:192–200. doi: 10.1111/j.1440-1681.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Org, Gonzalez R, Alonso MJ, Marin J. Nitric oxide-dependent and -independent mechanisms in the relaxation elicited by acetylcholine in fetal rat aorta. Life Sci. 1999;64:269–277. doi: 10.1016/s0024-3205(98)00562-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Alterations in vascular endothelial function in the aorta and mesenteric artery in type II diabetic rats. Can J Physiol Pharmacol. 2004;82:175–182. doi: 10.1139/y04-002. [DOI] [PubMed] [Google Scholar]
- Osborne-Pellegrin MJ. Some ultrastructural characteristics of the renal artery and abdominal aorta in the rat. J Anat. 1978;125:641–652. [PMC free article] [PubMed] [Google Scholar]
- Osborne-Pellegrin MJ. ‘Spontaneous’ lesions of the intima in the rat caudal artery. Principal morphologic characteristic and occurrence as a function of age and sex. Lab Invest. 1979;40:668–677. [PubMed] [Google Scholar]
- Osborne-Pellegrin MJ. Natural incidence of interruptions in the internal elastic lamina of caudal and renal arteries of the rat. Acta Anat (Basel) 1985;124:188–196. doi: 10.1159/000146117. [DOI] [PubMed] [Google Scholar]
- Osborne-Pellegrin MJ. Spontaneous arterial lesions involving breaks in the internal elastic lamina in the rat: Effects of β-aminopropionitrile and familial distribution. Exp Mol Pathol. 1986;45:171–184. doi: 10.1016/0014-4800(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2004;31:563–570. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Bramich NJ, Bandi HP, Rummery NM, Hill CE. Structure, function, and endothelium-derived hyperpolarizing factor in the caudal artery of the SHR and WKY rat. Arterioscler Thromb Vasc Biol. 2003;23:822–828. doi: 10.1161/01.ATV.0000067425.06019.D7. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556:875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KC) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Yamamoto K, Sekiguchi F, et al. Transmural field stimulation-induced relaxation in the rat common hepatic artery. J Smooth Muscle Res. 2000;36:137–144. doi: 10.1540/jsmr.36.137. [DOI] [PubMed] [Google Scholar]
- Shimokawa H. Endothelial dysfunction in hypertension. J Atheroscler Thromb. 1998;4:118–127. doi: 10.5551/jat1994.4.118. [DOI] [PubMed] [Google Scholar]
- Sims FH. Discontinuities in the internal elastic lamina: a comparison of coronary and internal mammary arteries. Artery. 1985;13:127–143. [PubMed] [Google Scholar]
- Smith EB, Staples EM. Distribution of plasma proteins across the human aortic wall. Atherosclerosis. 1980;37:579–590. doi: 10.1016/0021-9150(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Song SH, Roach MR. Comparison of fenestrations in internal elastic laminae of canine thoracic and abdominal aortas. Blood Vessels. 1984;21:90–97. [PubMed] [Google Scholar]
- Svendsen E, Tindall AR. The internal elastic membrane and intimal folds in arteries: important but neglected structures? Acta Physiol Scand. 1988;133(Suppl. 572):1–71. [PubMed] [Google Scholar]
- Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein and 17 beta-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. Br J Pharmacol. 2004;141:322–328. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Chen SJ, Yen MH. Different responses to acetylcholine in the presence of nitric oxide inhibitor in rat aortae and mesenteric arteries. Clin Exp Pharmacol Physiol. 1993;20:405–412. doi: 10.1111/j.1440-1681.1993.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Moller J, Danielsen CC, et al. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]