Abstract
Caveolae are involved in clathrin-independent endocytosis, transcytosis, signal transduction, and tumor suppression – all of which depend on their main constituent protein caveolin families. The periodontal Ruffini ending has been reported to develop a caveola-like structure on the cell membrane of both the axon terminals and Schwann sheaths, suggesting the existence of an axon–Schwann cell interaction in the periodontal Ruffini endings. However, little information is available concerning the functional significance of these caveolae. The present study was undertaken to examine the immunolocalization of caveolin-1, -3 (Cav-1, Cav-3) and Ca2+-ATPase in the periodontal Ruffini endings of the rat incisor. Decalcified sections of the upper jaws were processed for immunocytochemistry at the levels of light and electron microscopy. Some immunostained sections were treated with histochemistry for nonspecific cholinesterase (nChE) activity. Observations showed the periodontal Ruffini endings were immunopositive for Cav-1, but not Cav-3. Immunoreactive products for Cav-1 were confined to caveola-like structures in the cell membranes of the cytoplasmic extensions and cell bodies of the terminal Schwann cells associated with the periodontal Ruffini endings. However, the axonal membranes of the terminals did not express any Cav-1 immunoreaction. Double staining with Ca2+-ATPase and either protein gene product 9.5 (PGP 9.5) or S-100 protein disclosed the co-localization of immunoreactions in the axonal branches of the periodontal Ruffini endings, but not in the terminal Schwann cells. As Ca2+ plays an important role in mechanotransduction, these characteristic immunolocalizations show Cav-1/Ca2+-ATPase might be involved in the quick elimination of intracellular Ca2+ in mechanotransduction.
Keywords: calcium pump, caveolin, mechanoreceptor, periodontal ligament
Introduction
Caveolae are omega-shaped plasmalemmal invaginations which are considered subtypes of lipid rafts enriched with cholesterol, glycosphingolipids, signaling molecules, and caveolins. They are involved in clathrin-independent endocytosis, transcytosis (for review, see Stan, 2002, 2005), signal transduction (for a review, see Quest et al. 2004), and tumor suppression (Lee et al. 1998) which depend on their main constituent proteins; caveolin-1, -2, and -3 (Cav-1, -2, and -3) (for review see Cohen et al. 2004). Cav-1 has been considered a biochemical marker for caveolae because of its localization in most cells and the absence of caveolae formation in Cav-1-deficient mice (for review, Quest et al. 2004). Cav-2 was first discovered in adipocytes with a peptide sequence similar to that of Cav-1, as confirmed by its co-localization of Cav-1 (for review see Cohen et al. 2004). Cav-3 is essentially restricted to muscle cells (Tang et al. 1996). In the case of neuronal tissue, several studies have been conducted on cultured cells (Cameron et al. 1997, 2002; Galbiati et al. 1998), glioma cells (Silva et al. 2005), and brain tissue (Ikezu et al. 1998). In the peripheral nervous system, Schwann cells in the sciatic nerve express Cav-1 and its levels are down-regulated after axotomy (Mikol et al. 1999). However, the current analysis of the Cav-1 expression in the peripheral nervous system remains insufficient.
The periodontal ligament, a dense collagenous tissue between the tooth and alveolar bone, contains rich mechanoreceptors which are involved in the control of mastication. Many reports on various kinds of mechanoreceptors have revealed that the Ruffini ending, a low-threshold slowly adapting type II stretch mechanoreceptor (Chambers et al. 1972; Biemesderfer et al. 1978), is the primary mechanoreceptor in the periodontal ligament (for review, Maeda et al. 1999). The periodontal Ruffini endings are characterized by extensive arborizations of thick axon terminals and by an association with specialized Schwann cells called terminal Schwann cells (Byers, 1985; Maeda et al. 1989). Ultrastructurally, the periodontal Ruffini endings develop caveola-like invaginations on the cell membranes of both the axon terminals and covering Schwann sheaths (for review, see Maeda et al. 1999), suggesting the existence of axon–Schwann cell interaction in the periodontal Ruffini endings. However, little information is available on the functional significance of these caveola-like structures.
Many neural functions are mediated by Ca2+ (Greenberg, 1987). Previous studies have shown that Cav-1 is important for Ca2+ homeostasis, suggested by the immunocytochemical findings of co-localizations of Cav-1 and various Ca2+ regulatory proteins (Daniel et al. 2001; Jung et al. 2005). A series of immunocytochemical studies have revealed abundant calcium binding proteins – S-100 protein, calbindin, calretinin, and parvalbumin – in the axon terminals of the periodontal Ruffini endings (Sato et al. 1988; Ichikawa et al. 1997; Ochi et al. 1997a,b; Asahito et al. 1999). It has been reported that Meissner's corpuscles fixed under mechanical stimulation contain high concentrations of Ca2+ in the terminal axoplasm and in the caveolae of the lamellar plates (Tachibana et al. 1992), and that high affinity Ca2+-ATPase, i.e. Ca-pump, is located in the plasma membrane of its axon terminals (Tachibana & Nawa, 1992), suggesting the involvement of Ca2+ in the mechanotransduction. These findings imply a possibility that Cav-1 and Ca2+-ATPase play important roles in the periodontal Ruffini endings. Accordingly, the present study examined immunolocalizations of Cav-1, -3, and the calcium pump of the plasma membrane (PMCA) – which is one of the Ca2+-ATPases – in the rat periodontal Ruffini endings by immunocytochemistry at the levels of both light and electron microscopy.
Materials and methods
All experiments were performed under the guidelines of the Niigata University Institutional Animal Use and Care Committee (approval number #41).
Western blotting
Three male Wistar rats (8 weeks of age, 240–260 g) were anesthetized by an intraperitoneal injection of 8% chloral hydrate (400 mg kg−1), and then decapitated. The trigeminal ganglia, epididymis (control tissue for Cav-1), and skeletal muscle (control tissue for Cav-3) were removed and rapidly frozen in liquid nitrogen. Frozen tissues were homogenized in a standard RIPA buffer (Tris-HCl 50 mm, 1% Triton X-100, NaCl 150 mm, 0.1% SDS, and EDTA 2 mm, pH 7.4) containing a protease inhibitor cocktail (Sigma, St. Louis, MO, USA) in a Multibeads shocker (Yasui Kikai, Osaka, Japan) for 2800 r.p.m. for 20 s. After centrifugation at 16 000 g for 15 min at 4 °C, 30- or 90-µg proteins were separated in 10% SDS-PAGE gel and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. After being blocked with 5% milk, the membrane was incubated with monoclonal antibodies against Cav-1 (1 : 500, BD Transduction Laboratories, San Diego, CA, USA), Cav-3 (1 : 2500, BD Transduction Laboratories), and GAPDH (1 : 20 000, Abcam, Tokyo, Japan) overnight at 4 °C, and then with horseradish peroxidase-conjugated anti-mouse IgG (1 : 1000, Vector Lab., Burlingame, CA, USA). Final visualization used 0.04% 3,3′-diaminobenzidine tetrahydrochloride (DAB) and 0.0125% H2O2 in a 0.05 m Tris-HCl buffer, pH 7.6.
Immunocytochemistry for Cav-1, Cav-3, and PMCA
An additional eight male Wistar rats were perfused with a fixative containing 4% paraformaldehyde and 0.0125% glutaraldehyde in a 0.1 m phosphate buffer (pH 7.4). The removed heads were decalcified with a 5% ethylene diamine tetraacetic acid disodium (EDTA-2Na) solution for 8 weeks at 4 °C. Frozen sections of demineralized upper jaws were cut at a thickness of 35 µm with a freezing microtome (Yamato Koki, Tokyo, Japan). Additionally, cryostat sections of the trigeminal ganglion were sectioned at a thickness of 10 µm in a cryostat (CM 3050S; Leica, Nussloch, Germany). Frozen or cryostat sections were primarily incubated with monoclonal antibodies to Cav-1 (1 : 500), Cav-3 (1 : 1000), and PMCA (1 : 10 000, Affinity BioReagents Inc., Golden, CO, USA), which recognize the high affinity of Ca2+-ATPase, for 12 h at 4 °C. The origin and characterizations of these antibodies have been reported previously (Borke et al. 1989; Caride et al. 1996; Filoteo et al. 1997; Breton et al. 1998; Rybin et al. 2000). The incubated sections were reacted with consecutive two incubations with biotinylated anti-mouse IgG (1 : 100, Vector Lab.) and avidin-conjugated peroxidase (ABC kit; Vector Lab.). Final visualization employed 0.04% DAB and 0.0125% H2O2 in a 0.05 m Tris-HCl buffer (pH 7.6). Some immunostained sections were counterstained with 0.03% methylene blue.
Immunoelectron microscopy
Immunostained sections with DAB-development were osmificated, dehydrated in ascending ethanols, and finally embedded in an epoxy resin (Epon 812; Taab, Berkshire, UK). Plastic sections 1 µm thick were stained with 0.1% methylene blue. Ultrathin sections 70 nm thick were briefly double-stained with uranyl acetate and lead citrate and examined in an H-7000 transmission electron microscope (Hitachi Co. Ltd., Tokyo, Japan).
Histochemistry for nonspecific cholinesterase (mChE) activity
Some sections with Cav-1, which was visualized using Cy™3-conjugated anti-mouse IgG (1 : 400, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), were processed for enzymatic histochemistry for nChE activity, a marker for terminal Schwann cells (Maeda et al. 1990) according to a method of Karnovsky & Roots (1964). The specificity of reactions for the nChE has been reported previously (Maeda et al. 1990).
Double staining with PMCA and either PGP 9.5 or S-100 protein
Some sections were incubated with a cocktail of antibodies to PMCA and either PGP 9.5 (1 : 10 000; Ultraclone Co. Ltd., Cambridge, UK) or S-100 protein (1 : 500, Immunotech, Marseille, France). PMCA was recognized by Cy™3-conjugated mouse IgG (1 : 400, Jackson ImmunoResearch Lab.), and both S-100 and PGP 9.5 were incubated with FITC-conjugated anti-rabbit IgG (1 : 250, Jackson ImmunoResearch Lab.). They were examined in a confocal laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany).
Results
Western blotting
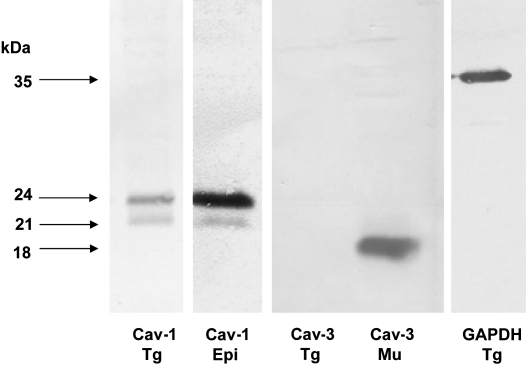
Western blotting analysis demonstrated two bands for Cav-1 in the samples of both the trigeminal ganglia and epididymis around the expected sizes (21 and 24 kDa) as shown in the manufacturer's data sheet. However, bands for Cav-3 were detectable around 18 kDa in the skeletal muscle but not in the trigeminal ganglia (Fig. 1).
Fig. 1.
Western blotting of trigeminal ganglion (Tg), epididymis (Epi), and skeletal muscle (Mu) using monoclonal antibodies for caveolin-1 (Cav-1) and -3 (Cav-3). In the trigeminal ganglion and epididymis, two bands for Cav-1 are detected at 24 kDa and 21 kDa, which recognizes the α- and β-isoforms of the protein. The Cav-3 immunoreaction, which is found in a single band around 18 kDa, is detected in skeletal muscle but not in the trigeminal ganglion.
Immunoreaction for Cav-1 and Cav-3 in the periodontal ligament
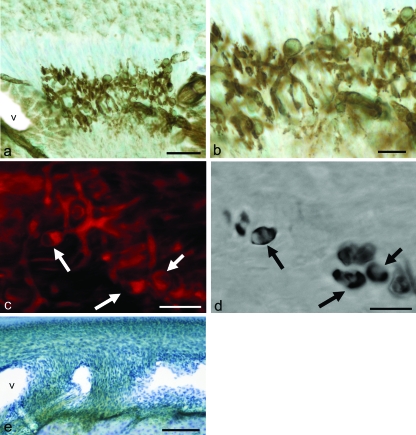
The lingual periodontal ligament of the rat incisors contained an intense Cav-1 immunoreaction in various kinds of cellular elements (Fig. 2a) but lacked Cav-3 immunoreactivity (Fig. 2e). In addition to the periodontal fibroblasts and endothelial cells, numerous Cav-1 immunoreactive structures were restricted to the alveolar half of the periodontal ligament, referred to as the alveolus-related part by Beertsen et al. (1974). They were comparatively thick structures with dendritic profiles. In addition, several rounded cells with immunoreaction were situated near them (Fig. 2b). Judging from their morphology and location, these immunoreactive structures were considered to be the periodontal Ruffini endings. Double staining with Cav-1 and nChE activity (Fig.2c,d) demonstrated their co-localizations in the cytoplasm of the cell bodies of the terminal Schwann cells associated with the periodontal Ruffini endings. Furthermore, their cytoplasmic extensions which covered the axon terminals – the Schwann sheath covering the axon terminals – also exhibited Cav-1 immunoreaction.
Fig. 2.
Immunostaining for caveolin-1 (Cav-1) (a,b) or -3 (e) and double staining with Cav-1 (c) and nonspecific cholinesterase (nChE) reactivity (d) of the periodontal ligament. (a) Numerous immunoreactive elements are restricted to the alveolus-related part. Cav-1 immunopositive structures extend in the periodontal ligament to form the Ruffini endings, whose positive axon terminals have dendritic expansions. (b) Higher magnification of (a). The terminal Schwann cells, round in profile, are also positive in the Cav-1 immunoreaction. (c,d) The Cav-1 positive terminal Schwann cells (arrows in c) contain reaction products for nChE (arrows in d) in their cytoplasm. (e) No Cav-3 positive element is recognized in the periodontal ligament. Scale bars: 50 µm (a), 10 µm (b–d), 150 µm (e).
In the trigeminal ganglion, Cav-1 immunoreaction was found in the satellite cells around neurons and the endothelial cells of microvessels but not in the trigeminal neurons (data not shown).
Immunoelectron microscopy for Cav-1
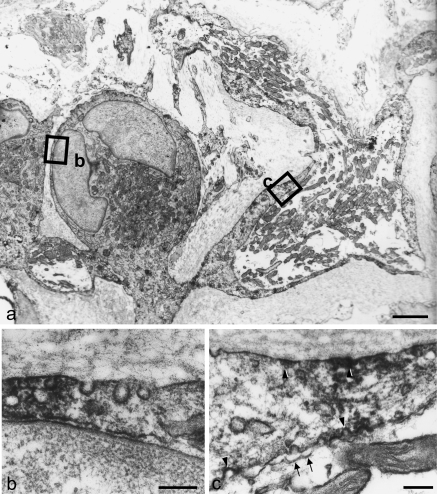
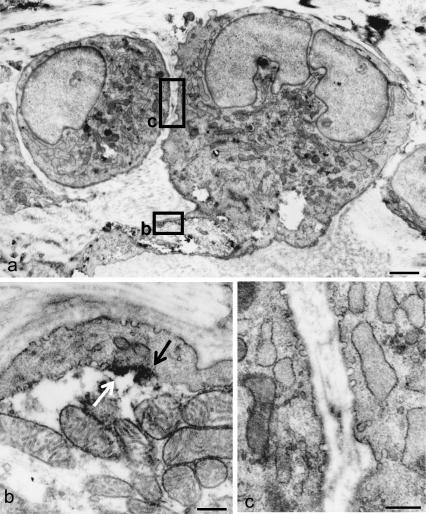
Under the electron microscope, it was easy to identify the terminal Schwann cells associated with the periodontal Ruffini endings because they were situated in the alveolus-related part between the blood vessels and because they have characteristic ultrastructural features which have been reported previously (for review, see Maeda et al. 1999). Immunoreactive products for Cav-1, observed as electron-dense deposits, were localized on the cell membrane of the cytoplasmic extensions and cell bodies of the terminal Schwann cells (Fig. 3a). More highly magnified observations revealed that immunoreactive products for Cav-1 were confined to the caveola-like structure in the cell membranes of their cell bodies (Fig. 3b).
Fig. 3.
Immunoelectron micrographs for Cav-1 of the Ruffini ending. Pre-embedding method using DAB development. (a) A terminal Schwann cell extends its long projection toward the mitochondria-rich axon terminals. Both the cell body and Schwann sheath contain many electron-dense immunoproducts along their cell membranes. (b) Magnification of the view box in (a). Immunoreactive materials assemble in the flask-like invaginations of the cell membrane. (c) Higher magnification of the box in (a). At the Schwann sheath, the immunopositive caveola-like structures (arrowheads) face both sides of the basal membrane and axon. However, no immunopositive caveola-like structure (arrows) is found in the axon terminal. Scale bars: 5 µm (a), 0.5 µm (b,c).
At the periaxonal Schwann sheaths extending from the cell bodies of the terminal Schwann cells, well-developed caveola-like structure also showed intense immunopositivity for Cav-1 at the surface facing both the basal lamina and the axon terminals (Fig. 3c, arrowheads). Although the axonal membranes of the nerve endings also constructed caveola-like structures, they did not express any Cav-1 immunoreaction (Fig. 3c, arrows).
Immunoreaction for Ca2+-ATPase
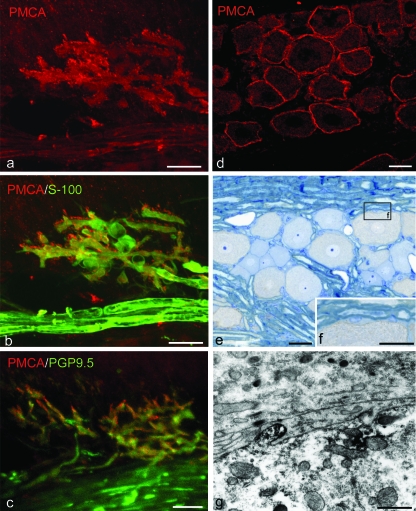
Immunocytochemistry for the calcium pump of the plasma membrane (PMCA) was employed for the detection of Ca2+-ATPase in the rat periodontal ligament and trigeminal ganglion. Immunostaining with antibody against PMCA demonstrated intense immunoreactions in the periodontal Ruffini endings. Double staining with PMCA and PGP 9.5 disclosed the co-localization of immunoreactions in the axonal branches of the periodontal Ruffini endings, but not in the nerve bundles in the periodontal ligament (Fig. 4a–c). Double labeling with PMCA and S-100 protein showed a lack of immunoreaction for PMCA in the terminal Schwann cells (Fig. 4b). Under the electron microscope, electron-dense immunopositive reactions for PMCA were restricted to the axoplasm near the axonal membranes of the periodontal Ruffini endings; they did not appear in the covering Schwann cells (Fig. 5a,b). Despite careful observation, we failed to find any positive reactions in the cell bodies of the terminal Schwann cell with well-developed caveolae (Fig. 5c).
Fig. 4.
Immunolocalization of PMCA-ATPase in the periodontal ligament (a–c) and trigeminal ganglion (d–g). (a,b) Double labeling with PMCA and S-100 protein shows that the axon terminals have both PMCA and S-100 immunoreactions; however, the cell bodies do not express any co-localization. (c) PGP9.5 positive axonal processes are detected with PMCA immunoreaction. (d–f) In the trigeminal ganglion, the PMCA-positive reactions exist along the outlines of neuron cells. These reactions are found in the cell membrane of the neuronal cell. (g) Immunoelectron micrograph of the trigeminal ganglion. Electron-dense immunopositive products gather on the cell membrane, especially in a finger-like projection. Scale bars: 25 µm (a–e), 20 µm (f), 0.15 µm (g).
Fig. 5.
Electron microscopic views of the localization of PMCA in the periodontal Ruffini ending. The cell body of the terminal Schwann cell with developed cell organellae extends cytoplasmic processes to cover the axon terminal, which is filled with mitochondria (a). At a higher magnification of the axon–Schwann cell interface (the boxed area in a), electron-dense immunoreactive products (arrows) are seen to assemble on the axoplasm near the axon membrane (b). However, the covering Schwann sheath does not express any obvious immunoreaction for PMCA (b). Similarly, the cell bodies of the terminal Schwann cells also develop caveola-like structures, but they do not contain any specific immunoreaction (c: magnified view of the boxed area in a). Scale bars: 5 µm (a), 0.3 µm (b,c).
In the trigeminal ganglion, PMCA immunoreaction was recognizable in the perimeter of the neurons (Fig. 4d). Observations of plastic sections showed immunoreactions for PMCA along the cell membrane of the medium-sized and large neurons (Fig. 4e,f). However, satellite cells around the neurons were devoid of PMCA-immunoreaction. Electron microscopy revealed immunoreactive products for PMCA in the cell membrane of the neurons (Fig. 4g). In particular, they were localized in the axoplasm, which frequently showed finger-like projections.
Discussion
The Ruffini ending, a primary mechanoreceptor in the periodontal ligament, develops caveola-like invaginations of cell membranes both in the axon terminals and the associated terminal Schwann cells, though its functional significance remains unclear. The present study was able to demonstrate the immunolocalization of Cav-1, but not muscle-specific Cav-3, in the terminal Schwann cells. In contrast to many studies on central nervous system (Cameron et al. 1997, 2002; Ikezu et al. 1998; Silva et al. 2005), there are few reports on the peripheral nervous system. Cav-1 immunoreaction was found in the caveola-like structure in Schwann cells associated with the rat sciatic nerve. Although the detailed function of Cav-1 has remained unclear in the peripheral nervous system, three possible roles have been suggested. Firstly, this protein may play a role in the biology of myelinating Schwann cells (Mikol et al. 1999, 2002; Kawahara, 2004). However, the terminal Schwann cells associated with the periodontal Ruffini endings are categorized by a type of non-myelinating Schwann cell (Byers, 1985; Maeda et al. 1990). Second, as Cav-1 is a cholesterol-binding protein (Murata et al. 1995), its involvement in cholesterol transport/homeostasis has also been suggested (Mikol et al. 1999, 2002). However, no information is available on the role of cholesterol in the terminal Schwann cells. Finally, Cav-1 may be involved in the trafficking and retrograde transport of neurotrophin. In cultured sympathetic neurons, Hibbert et al. (2006) recently revealed that the p75 neurotrophin receptor (p75-NGFR)/brain-derived neurotrophic factor (BDNF) is associated with the β isoforms of Cav-1, which can be detected by the antibody used in this study. As the terminal Schwann cells displayed intense and constant immunoreactions for p75-NGFR (Byers, 1990) and trkB (Ochi et al. 1997c; Atsumi et al. 1999), both of which can bind BDNF, it is more likely that Cav-1 expression in the terminal Schwann cells is involved in neurotrophin transport.
Previous reports have shown that caveolae had concentrated Ca2+-ATPase and inositol 1,4,5-triphosphate (IP3) receptors (Fujimoto et al. 1992; Rothberg et al. 1992; Dupree et al. 1993; Fujimoto, 1993). The present immunocytochemical observations revealed a characteristic localization pattern of Ca2+-ATPase immunoreaction – a positive reaction in caveola-like structures of the axon terminals vs. a negative one in that of terminal Schwann cells. This immuno-expression pattern is consistent with previous reports that high affinity Ca2+-ATPase, i.e. the Ca-pump, was located in the plasma membrane of axon terminals of Meissner's corpuscles (Tachibana et al. 1992). According to another report by Tachibana & Nawa (1992), Meissner's corpuscles fixed during mechanical stimulation contained a high content of Ca2+ in the axoplasm and the caveolae of lamellar plates, whereas in the non-stimulated condition, the corpuscles fixed a high content of Ca2+ in the cytoplasm of the lamellar plates. These findings indicate Ca2+ influx into the axon terminals of the mechanoreceptors during mechanical stimulation, suggesting the involvement of Ca2+ in mechanotransduction. Therefore, it is reasonable to consider that Ca2+-ATPase in the periodontal Ruffini endings might be involved in the quick elimination of intracellular Ca2+ in mechanotransduction.
One of the ultrastructural features of cutaneous mechanoreceptors is the existence of characteristic cell organellae in axon terminals, called the receptoplasm (Andres & von Düring, 1972). The receptoplasm contains a cluster of mitochondria, various kinds of vesicles, smooth endoplasmic reticulum, and poor cytoskeletal filaments, all of which have also been found in the axon terminals of periodontal Ruffini endings. A series of studies investigating the immunoreaction of the synaptosomal-associated protein of 25 kDa (SNAP25) showed this to be discernible around vesicle membranes, which are usually located near the axonal membrane, of the periodontal Ruffini endings. Braun & Madison (2000) reported that Cav-1 was detected in a complex with SNAP25, and suggested that the Cav-1/SNAP25 complex regulated neurotransduction. The cholesterol-binding property of Cav-1 (Murata et al. 1995) has suggested its role in membrane remodeling (Gaudreault et al. 2005), indicating that Cav-1 in caveolae may participate in the remodeling of the periodontal Ruffini endings. Indeed, the active tissue remodeling of the periodontal ligament due to exposure to occlusal force has been thought to cause a constant adaptation of the periodontal Ruffini endings (Maeda et al. 1999).
Acknowledgments
We thank Mr Masaaki Hoshino and Kiichi Takeuchi for their technical assistance. This study was supported by a grant from the Japan Society for the Promotion of Science (JSPS) (No. 20390464 to T. M.).
References
- Andres KH, von Düring M. Morphology of cutaneous receptors. In: Iggo A, editor. Handbook of Sensory Physiology, Vol. II. Somatosensory System. Berlin: Springer-Verlag; 1972. pp. 3–28. [Google Scholar]
- Asahito T, Ohshima H, Hanada K, Wakisaka S, Maeda T. Postnatal expression of calretinin-immunoreactivity in periodontal Ruffini endings in the rat incisor: a comparison with protein gene product 9.5 (PGP 9.5)-immunoreactivity. Arch Histol Cytol. 1999;62:57–69. doi: 10.1679/aohc.62.57. [DOI] [PubMed] [Google Scholar]
- Atsumi Y, Hayashi S, Nakakura-Ohshima K, Maeda T, Kurisu K, Wakisaka S. Heterogeneous localizations of Trk B among individual periodontal Ruffini endings in the rat incisor. Arch Histol Cytol. 1999;62:435–440. doi: 10.1679/aohc.62.435. [DOI] [PubMed] [Google Scholar]
- Beertsen W, Everts V, van den Hoof A. Fine structure and possible function of cells containing leptomeric organelles in the periodontal ligament of rat incisor. Arch Oral Biol. 1974;19:1099–1100. doi: 10.1016/0003-9969(74)90236-2. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D, Munger BN, Binck J, Dubner R. The pilo-Ruffini complex: a non-sinus hair and associated slowly adapting mechanoreceptor in primate facial skin. Brain Res. 1978;142:197–222. doi: 10.1016/0006-8993(78)90631-5. [DOI] [PubMed] [Google Scholar]
- Borke JL, Caride A, Verma AK, Penniston JT, Kumar R. Plasma membrane calcium pump and 28kDa calcium binding protein in cells of rat kidney distal tubules. Am J Physiol Renal Physiol. 1989;257:F842–F849. doi: 10.1152/ajprenal.1989.257.5.F842. [DOI] [PubMed] [Google Scholar]
- Braun JE, Madison DV. A novel SNAP25-caveolin complex correlates with the onset of persistent synaptic potentiation. J Neurosci. 2000;20:5997–6006. doi: 10.1523/JNEUROSCI.20-16-05997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D. Basolateral distribution of caveolin-1 in the kidney: absence from H+-ATPase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem. 1998;46:205–241. doi: 10.1177/002215549804600209. [DOI] [PubMed] [Google Scholar]
- Byers MR. Sensory innervation of periodontal ligament of rat molars consists of unencapsulated Ruffini-like mechanoreceptors and free nerve endings. J Comp Neurol. 1985;231:500–518. doi: 10.1002/cne.902310408. [DOI] [PubMed] [Google Scholar]
- Byers MR. Segregation of NGF receptor in sensory receptors, nerves and local cells of teeth and periodontium demonstrated by EM immunocytochemistry. J Neurocytol. 1990;19:765–775. doi: 10.1007/BF01188044. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci. 1997;17:9520–9535. doi: 10.1523/JNEUROSCI.17-24-09520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron PL, Liu C, Smart DK, Hantus ST, Fick JR, Cameron RS. Caveolin-1 expression is maintained in rat and human astroglioma cell lines. Glia. 2002;37:275–290. doi: 10.1002/glia.10036. [DOI] [PubMed] [Google Scholar]
- Caride AJ, Filoteo AG, Enyedi A, Verma AK, Penniston JT. Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies. Biochem J. 1996;316:353–359. doi: 10.1042/bj3160353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MR, Andres KH, von Düring M, Iggo A. Structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972;57:417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Jury J, Wang YF. nNOS in canine lower esophageal sphincter: colocalized with Cav-1 and Ca2+-handling proteins? Am J Physiol Gastrointest Liver Physiol. 2001;281:G1101–G1114. doi: 10.1152/ajpgi.2001.281.4.G1101. [DOI] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo AG, Elwess NL, Enyedi A, Caride A, Aung HH, Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem. 1997;272:23741–23747. doi: 10.1074/jbc.272.38.23741. [DOI] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Gil O, et al. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc Natl Acad Sci U S A. 1998;95:10257–10262. doi: 10.1073/pnas.95.17.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault SB, Blain JF, Gratton JP, Poirier J. A role for caveolin-1 in post-injury reactive neuronal plasticity. J Neurochem. 2005;92:831–839. doi: 10.1111/j.1471-4159.2004.02917.x. [DOI] [PubMed] [Google Scholar]
- Greenberg DA. Calcium channels and calcium channel antagonists. Ann Neurol. 1987;21:317–330. doi: 10.1002/ana.410210402. [DOI] [PubMed] [Google Scholar]
- Hibbert AP, Kramer BM, Miller FD, Kaplan DR. The localization, trafficking and retrograde transport of BDNF bound to p75NTR in sympathetic neurons. Mol Cell Neurosci. 2006;32:387–402. doi: 10.1016/j.mcn.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jacobowitz DM, Sugimoto T. Coexpression of calretinin and parvalbumin in Ruffini-like endings in the rat incisor periodontal ligament. Brain Res. 1997;770:294–297. doi: 10.1016/s0006-8993(97)00826-3. [DOI] [PubMed] [Google Scholar]
- Ikezu T, Ueda H, Trapp BD, et al. Affinity-purification and characterization of caveolins from the brain: differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998;804:177–192. doi: 10.1016/s0006-8993(98)00498-3. [DOI] [PubMed] [Google Scholar]
- Jung SY, Kwak JO, Kim HW, et al. Calcium sensing receptor forms complex with and is up-regulated by caveolin-1 in cultured human osteosarcoma (Saos-2) cells. Exp Mol Med. 2005;37:91–100. doi: 10.1038/emm.2005.13. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A ‘Direct-coloring’ thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kawahara T. Caveolae localization and caveolin expressions in Schwann cells of mature rat spinal nerves. Kurume Med J. 2004;51:263–271. doi: 10.2739/kurumemedj.51.263. [DOI] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sato O, Kobayashi S, Iwanaga T, Fujita T. The ultrastructure of Ruffini endings in the periodontal ligament of rat incisors with special reference to the terminal Schwann cells (K-cells) Anat Rec. 1989;223:95–103. doi: 10.1002/ar.1092230114. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kannari K, Sato O, Kobayashi S, Iwanaga T, Fujita T. Cholinesterase activity in terminal Schwann cells associated with Ruffini endings in the periodontal ligament of rat incisors. Anat Rec. 1990;228:339–344. doi: 10.1002/ar.1092280313. [DOI] [PubMed] [Google Scholar]
- Maeda T, Ochi K, Nakakura-Ohshima K, Youn SH, Wakisaka S. The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: its morphology, cytochemical features, regeneration, and development. Crit Rev Oral Biol Med. 1999;10:307–327. doi: 10.1177/10454411990100030401. [DOI] [PubMed] [Google Scholar]
- Mikol DD, Hong HL, Cheng HL, Feldman EL. Caveolin-1 expression in Schwann cells. Glia. 1999;27:39–52. [PubMed] [Google Scholar]
- Mikol DD, Scherer SS, Duckett SJ, Hong HL, Feldman EL. Schwann cell caveolin-1 expression increases during myelination and decreases after axotomy. Glia. 2002;38:191–199. doi: 10.1002/glia.10063. [DOI] [PubMed] [Google Scholar]
- Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K, Wakisaka S, Youn SH, Hanada K, Maeda T. Calretinin-like immunoreactivity in the Ruffini endings, slowly adapting mechanoreceptors, of the periodontal ligament of the rat incisor. Brain Res. 1997a;769:183–187. doi: 10.1016/s0006-8993(97)00847-0. [DOI] [PubMed] [Google Scholar]
- Ochi K, Wakisaka S, Youn SH, Hanada K, Maeda T. Immunohistochemical localization of calbindin D28k in the periodontal Ruffini endings of rat incisors. Neurosci Lett. 1997b;228:195–198. doi: 10.1016/s0304-3940(97)00398-4. [DOI] [PubMed] [Google Scholar]
- Ochi K, Saito I, Hanada K, Maeda T. Expression of TrkB-like immunoreactivity in non-neural cells of rat periodontal ligament. Arch Oral Biol. 1997c;42:455–464. doi: 10.1016/s0003-9969(97)00030-7. [DOI] [PubMed] [Google Scholar]
- Quest AF, Leyton L, Párraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rybin Vo, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Sato O, Maeda T, Kobayashi S, Iwanaga T, Fujita T, Takahashi Y. Innervation of periodontal ligament and dental pulp in the rat incisor: an immunohistochemical investigation of neurofilament protein and glia-specific S-100 protein. Cell Tissue Res. 1988;251:13–21. doi: 10.1007/BF00215442. [DOI] [PubMed] [Google Scholar]
- Silva WI, Maldonado HM, Velázquez G, et al. Caveolin isoform expression during differentiation of C6 glioma cells. Int J Dev Neurosci. 2005;23:599–612. doi: 10.1016/j.ijdevneu.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57:350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Nawa T. Ultrastructural localization of Ca++-ATPases in Meissner's corpuscle of the Mongolian gerbil. Arch Histol Cytol. 1992;55:375–379. doi: 10.1679/aohc.55.375. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Nawa T, Mizuhira V, Yoshida Y. Ultrastructural localization of calcium in mechanoreceptors of the oral mucosa. J Neurocytol. 1992;21:745–753. doi: 10.1007/BF01181589. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]