Abstract
Mechanical loading is essential for the health and homeostasis of articular cartilage, although the fundamental mechanotransduction pathways are unclear. Previous studies have demonstrated that cyclic compression up-regulates proteoglycan synthesis via an intracellular Ca2+ signalling pathway, mediated by the release of ATP. However, the mechanism(s) of ATP release has not been elucidated. The present study examines expression of the putative mechanosensitive ATP-release channel, connexin 43 and whether it is expressed on the chondrocyte primary cilium, which acts as a mechanosensor in a variety of other cell types. In addition the study characterized the expression of a range of purine receptors through which ATP may activate downstream signalling events controlling cell function. Bovine articular chondrocytes were isolated by sequential enzyme digestion and seeded in agarose constructs. To verify the presence of functional hemichannels, Lucifer yellow (LY) uptake into viable cells was quantified following treatment with a hemichannel agonist (EGTA) and antagonist (flufenamic acid). LY uptake was observed in 45% of chondrocytes, increasing to 83% following EGTA treatment (P < 0.001). Treatment with the hemichannel blocker, flufenamic acid, significantly decreased LY uptake to less than 5% with and without EGTA. Immunofluorescence and confocal microscopy confirmed the presence of primary cilia and the expression of connexin 43. Approximately 50% of bovine chondrocyte primary cilia were decorated with connexin 43. Human chondrocytes in situ within cartilage explants also expressed connexin 43 hemichannels. However, expression was confined to the upper 200 µm of the tissue closest to the articular surface. Immunofluorescence revealed the expression of a range of P2X and P2Y receptor subtypes within human articular cartilage. In conclusion, the expression of functional hemichannels by articular chondrocytes may represent the mechanism through which mechanical loading activates ATP release as part of a purinergic mechanotransduction pathway. Furthermore, the expression of connexin 43 on the chondrocyte primary cilium suggests the possible involvement of the cilium in this pathway.
Keywords: agarose, ATP, calcium, chondrocyte, mechanotransduction, P2X, P2Y, primary cilia
Introduction
Adenosine 5′-triphosphate (ATP), like Ca2+, is now recognized as a second messenger in many tissue types, stimulating various purinergic signalling pathways that lead to changes in cell function (for review see Bodin & Burnstock, 2001). ATP is released by mechanical stimuli as part of mechanotransduction pathways in a diverse range of eukaryotic cell types, including epithelial and endothelial cells, red blood cells, astrocytes, keratinocytes and osteoblasts. Studies have also reported that mechanical loading of articular chondrocytes triggers release of ATP into the pericellular microenvironment, although the precise release mechanisms are unclear (Graff et al. 2000; Millward-Sadler et al. 2004). One putative candidate for mechanosensitive ATP release is through connexin hemichannels (Stout et al. 2002; Bao et al. 2004; Gomes et al. 2005). These are unopposed halves of gap junctions composed of six connexin molecules, providing non-selective permeability to molecules smaller than 1 kDa (Kang et al. 2008).
Once ATP is released into the extracellular milieu, it is hydrolyzed to adenosine monophosphate (AMP) and inorganic pyrophosphate (PPi) by specific ectonucleotidases present in both the cartilage matrix and synovial fluid (Caswell & Russell, 1985). Extracellular ATP and its derivatives may bind to purine receptors on the cell membrane to trigger autocrine or paracrine mechanotransduction signalling cascades, such as those involving intracellular Ca2+.
In addition, chondrocyte mechanosensitivity may also involve the primary cilium. The primary cilium is a single cytoplasmic organelle found in virtually all eukaryotic cells. It consists of a membrane-coated axoneme that projects from the cell surface into the extracellular microenvironment, and an intracellular basal body that comprises the most mature of the two centrioles located within the centrosome. Previous studies have demonstrated the presence of primary cilia in articular chondrocytes and their interaction with integrins and the ECM (Poole et al. 1985, 2001; Jensen et al. 2004; McGlashan et al. 2006a,b,c). In various other cell types, primary cilia function as mechanoreceptors such that deflection of the cilium initiates intracellular Ca2+ signalling as part of a mechanotransduction signalling cascade (Praetorius & Spring, 2001, 2003; Liu et al. 2005). We therefore hypothesize that the primary cilium may be involved in chondrocyte mechanosensitive purinergic Ca2+ signalling such that the cilium acts as a mechanosensor triggering the release of ATP through selective expression of specific mechanoreceptors.
The present study examines expression of the putative mechanosensitive ATP-release channel, connexin 43 and whether it is expressed on the chondrocyte primary cilium. In addition, the study examines the expression of a range of purine receptors through which ATP may activate downstream signalling events controlling cell function.
Materials and methods
Preparation of cartilage explants and chondrocyte/agarose constructs
Studies were conducted using isolated bovine articular chondrocytes cultured in agarose gel, as adopted in numerous previous studies (for review see Lee & Knight, 2004). Bovine metacarpophalangeal joints from 18-month old steers were obtained from a local abattoir within 6 h of slaughter. Full thickness cartilage was removed from the entire proximal surface of the metacarpophalangeal joint from three separate animals. The tissue was finely diced using a scalpel, and digested in 10 mL pronase solution at 37 °C for 1 h (Type E, 700 units mL−1, BDH Industries Ltd, Poole, UK), followed by 16 h in 30 mL collagenase (Type XI, 100 units mL−1). Enzymes were prepared in Dulbecco's Modified Eagle's Medium (DMEM, Gibco Ltd, Paisley, UK) supplemented with 20% (v/v) fetal calf serum (FCS), 2 µm l-glutamine, 5 µg mL−1 penicillin, 5 µg mL−1 streptomycin, 20 mm Hepes buffer and 0.85 µm l-ascorbic acid (DMEM + 20% FCS). The cellular digest was filtered through a 70-µm filter and the resulting cell suspension washed two times in fresh DMEM + 20% FCS. Cell suspensions from the three separate joints were pooled and mixed with an equal volume of 6% agarose type VII prepared in Earle's Balanced Salt Solution (EBSS) to yield a 3% agarose gel containing 10 × 106 cells mL−1. Cell-agarose constructs were gelled at 4 ºC for 20 min in a specially designed mould generating cylindrical constructs, 5 × 5 mm. The constructs were cultured overnight in DMEM + 20% FCS at 37 °C in 5% CO2 before being used for immunofluorescence or Lucifer yellow incorporation studies. Specimens for immunofluorescence were fixed in 3.7% paraformaldehyde for 1 h at 37 °C and then washed in phosphate-buffered saline (PBS). All reagents were obtained from Sigma-Aldrich (Poole, UK) unless otherwise stated.
In addition, further studies of connexin 43 and P2 receptor expression were conducted using human articular cartilage specimens provided by Articular Engineering, USA, from two donors immediately post mortem(one male aged 56 and one female aged 50). Full thickness cylindrical explants, 4 mm in diameter, were taken from the tibial plateau and fixed in 3.7% paraformaldehyde.
Lucifer yellow incorporation assay
To verify the presence of functional hemichannels on the chondrocyte membrane, viable bovine articular chondrocytes in agarose were incubated at 37 ºC for 40 min in Lucifer yellow (LY, Sigma-Aldrich) prepared in PBS (0.4% v/v). Dead cells were simultaneously labelled with ethidium homodimer (5 µm, Sigma-Aldrich). As a positive control, specimens were pretreated with EGTA (5 mm in PBS) for 40 min. EGTA chelates extracellular calcium and activates hemichannels (Quist et al. 2000). In addition, specimens with and without EGTA pre-treatment were incubated for 40 min with LY supplemented with the hemichannel blocker, flufenamic acid (FFA, 500 µm, Sigma-Aldrich) (Gomes et al. 2005;Stout et al. 2002). Following LY incubation, hemichannels were closed by rinsing the specimens in normal DMEM + 20% FCS containing 1.8 mm Ca2+, fixed in 3.7% formaldehyde and then washed repeatedly in PBS. Within 48 h of fixing, the cell agarose constructs were cut transversely to yield a single slice from the central region of the construct, which was mounted on a coverslip. The cells were visualized within the agarose specimens using brightfield and epi-fluorescence microscopy with a ×20 PLAN APO objective. The percentage of viable cells incorporating LY was recorded for 10 separate fields of view across the transverse slice removed from the agarose construct. The procedure was repeated on three separate constructs. Statistical analysis was performed using Student t-tests with a value of P < 0.05 indicating a significant difference.
Immunofluorescence staining and confocal microscopy
Cartilage explants and agarose culture specimens were snap-frozen in the presence of methyl-butane vapour and sectioned at 20 µm thick using a cryostat. The sections were placed into individual wells of a 24-well plate containing PBS. These ‘free-floating’ sections were subsequently incubated in testicular hyaluronidase (2 mg mL−1 in Tris-HCl, pH 5.5; Sigma-Aldrich, Auckland, New Zealand) for 2 h at 37 °C to partially remove matrix proteins and assist antibody penetration. Specimens were then washed in PBS containing 0.1% bovine serum albumin (BSA; Serva, Heidelberg, Germany) and permeabilized for 5 min in 0.5% Triton-X-100 (v/v) in PBS, followed by 30 min in 5% (v/v) goat serum in PBS (Sigma-Aldrich, Auckland, NZ). Sections were labelled with appropriate primary antibody overnight at 4 °C on a rocker plate, washed in PBS + 0.1% BSA and subsequently incubated with the appropriate fluorescent-conjugated Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen, Auckland, NZ) for 2 h at room temperature followed by further washing in PBS + 0.1% BSA. For co-localization studies, a sequential labelling protocol was adopted using antibodies raised in different species with Alexa Fluor 488 and Alexa Fluor 594-conjugated secondary antibodies. Sections were mounted onto slides with Citifluor (Citifluor, London, UK).
The intracellular domain of connexin 43 (Cx 43) was labelled with mouse anti-connexin 43 (1:200; Chemicon, Auckland, NZ) whilst the extracellular or hemichannel domain was labelled at a dilution of 1:10 000 (kind gift from Prof. C. Green, University of Auckland). Primary cilia were labelled using a primary antibody raised against acetylated α-tubulin (C3B9 – 1:5; a generous gift from Dr T Sherwin, University of Auckland) as used in previous studies (McGlashan et al. 2006a,b,c). Purine receptors, P2Y1, P2Y2, P2X2, P2X4 and P2X7 were all labelled individually with appropriate rabbit polyclonal primary antibodies (Alomone, Jerusalem, Israel). Negative controls were prepared without incubation in primary antibody.
Slides were visualized using a confocal laser scanning microscope (Leica SP2). A ×10/0.5 NA objective was used to provide low magnification images of chondrocytes in situ within cartilage sections. A ×100/1.4 NA oil immersion objective was used to generate high resolution confocal z-series (z step size = 0.2 µm), which were then reconstructed to provide 3D-rendered images of individual cells. Simultaneous non-confocal brightfield images were obtained using a transmitted light detector.
Results
Bovine chondrocytes express functional connexin 43 hemichannels
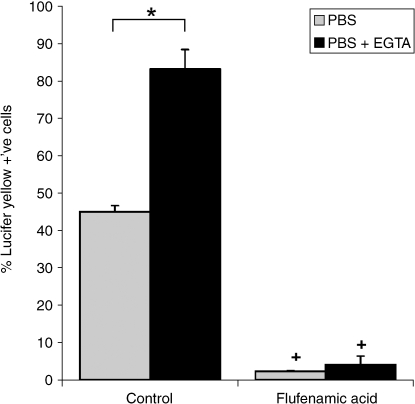
Activation of hemichannels occurred in 45% of bovine articular chondrocytes in agarose, as quantified by Lucifer yellow incorporation over a 40-min period (Fig. 1). Pretreatment with EGTA produced a statistically significant increase in the percentage of Lucifer yellow-positive cells to 83% (P < 0.001). However, treatment with flufenamic acid dramatically reduced Lucifer yellow uptake in cells both with and without EGTA treatment (P < 0.001) (Fig. 1). Consequently there was no statistically significant difference between the FFA-treated specimens with and without EGTA treatment (P > 0.05).
Fig. 1.
Mean percentage of cells showing Lucifer yellow (LY) uptake in the presence of absence of EGTA and with and without pretreatment with flufenamic acid (FFA). Bovine chondrocytes were cultured in agarose for 24 h prior to LY uptake analysis. Error bars represent SEM for n = 3 separate specimens. For each specimen the mean percentage of LY-positive cells was calculated from 10 fields of view. Statistically significant differences based on Student t-tests are indicated at P < 0.05 with and without EGTA (*) and with and without FFA (+).
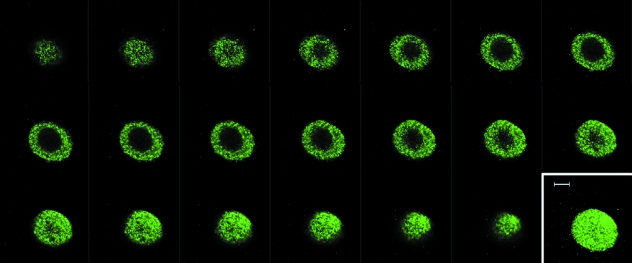
Expression of connexin 43 in isolated bovine articular chondrocytes cultured in agarose gel was confirmed by immunofluorescence. Connexin 43 exhibited punctate staining throughout the cytoplasm in 100% of cells (Fig. 2). Connexin 43 was also expressed by normal bovine patella articular chondrocytes in situ (data not shown).
Fig. 2.
Expression of connexin 43 in the cytoplasm of an isolated bovine chondrocyte cultured for 24 h in agarose gel. Confocal z-series obtained with a ×100 objective. Inset shows 3D serial reconstruction. Scale bar represents 5 µm.
Connexin 43 is expressed on the chondrocyte primary cilium
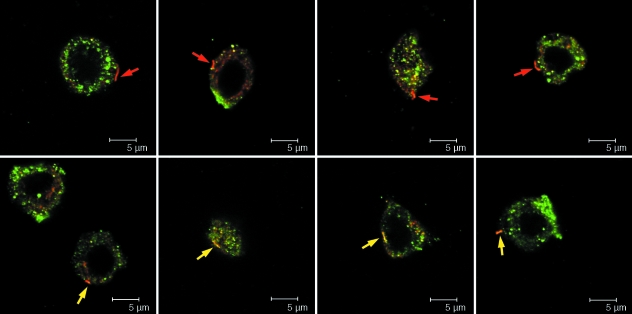
Immunolabelling with α-tubulin primary antibody, C3B9, confirmed that bovine articular chondrocytes express primary cilia. Expression was maintained in isolated bovine chondrocytes cultured in agarose gel, with a primary cilium observed in approximately 30% of cells. Co-labelling studies indicated that connexin 43 was clearly observed on 50% of cilia (Fig. 3).
Fig. 3.
Approximately 50% of bovine chondrocytes expressing a primary cilium showed connexin 43 present on the primary cilium following 24 h in agarose culture. Cells were dual labelled with antibodies for connexin 43 (green) and acetylated α-tubulin (red) and visualized using confocal microscopy with a ×100 objective. Arrows indicate the position of the primary cilium for cells with no co-localization with connexin 43 (top row, red arrow) and for cells with clear connexin 43 expression on the cilium (bottom row, yellow arrow).
Connexin 43 hemichannels are expressed in human cartilage
The cartilage on the tibial plateau of one of the donors showed macroscopic surface fibrillation. Microscopic inspection of this tissue using differential interference contrast (DIC) showed the presence of chondrocyte clusters or clones characteristic of osteoarthritis. Furthermore, immunofluorescence revealed that the cells in the superficial region exhibited numerous extensive cell processes projecting up to 15 µm into the extracellular matrix as previously reported for chondrocytes in osteoarthritic human femoral head cartilage (Holloway et al. 2004). The tissue from this donor was therefore classified as osteoarthritic (OA), in contrast to the tissue from the other donor, which had a normal appearance at both macroscopic and microscopic levels.
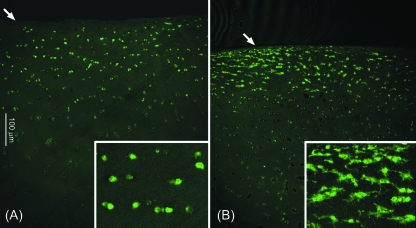
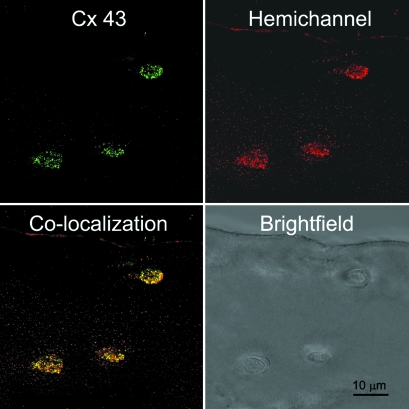
Human articular chondrocytes in situ expressed connexin 43, which exhibited a punctate appearance, both intracellularly and around the cell membrane, similar to that seen in bovine cells. In human cartilage, expression occurred almost exclusively in the superficial region, approximately 100–200 µm below the articular surface (Figs 4 and 5). Connexin 43 expression was similar in both the normal and OA cartilage. Co-localization of the intracellular and extracellular domains revealed the presence of connexin 43 hemichannels on the cell membrane in all cells expressing connexin 43 (Fig. 5).
Fig. 4.
Expression of connexin 43 in normal (A) and osteoarthritic (B) human articular cartilage. Confocal images were obtained with a ×10 objective to show the entire tissue depth. Arrows indicate the articular surface. Boxed inset images show additional ×4 magnification.
Fig. 5.
Expression of connexin 43 hemichannels in chondrocytes within human articular cartilage. Cells were labelled with antibodies to the intracellular domain of connexin 43 (green) and the extracellular or hemichannel domain (red) and visualized using confocal and brightfield imaging with a ×100 objective. Co-localization indicates the presence of uncoupled hemichannels.
Human chondrocytes express P2X and P2Y type purine receptors
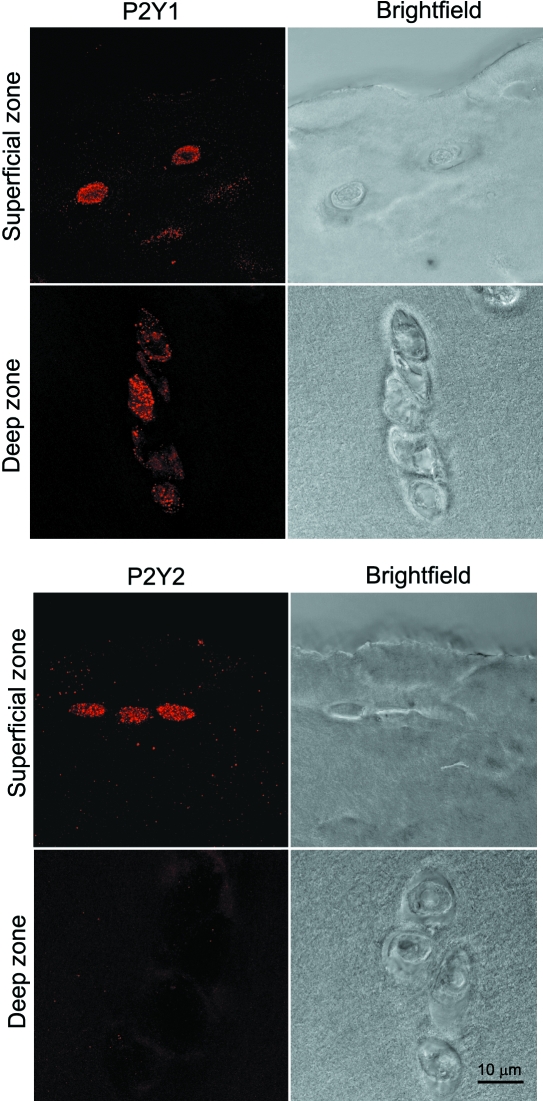
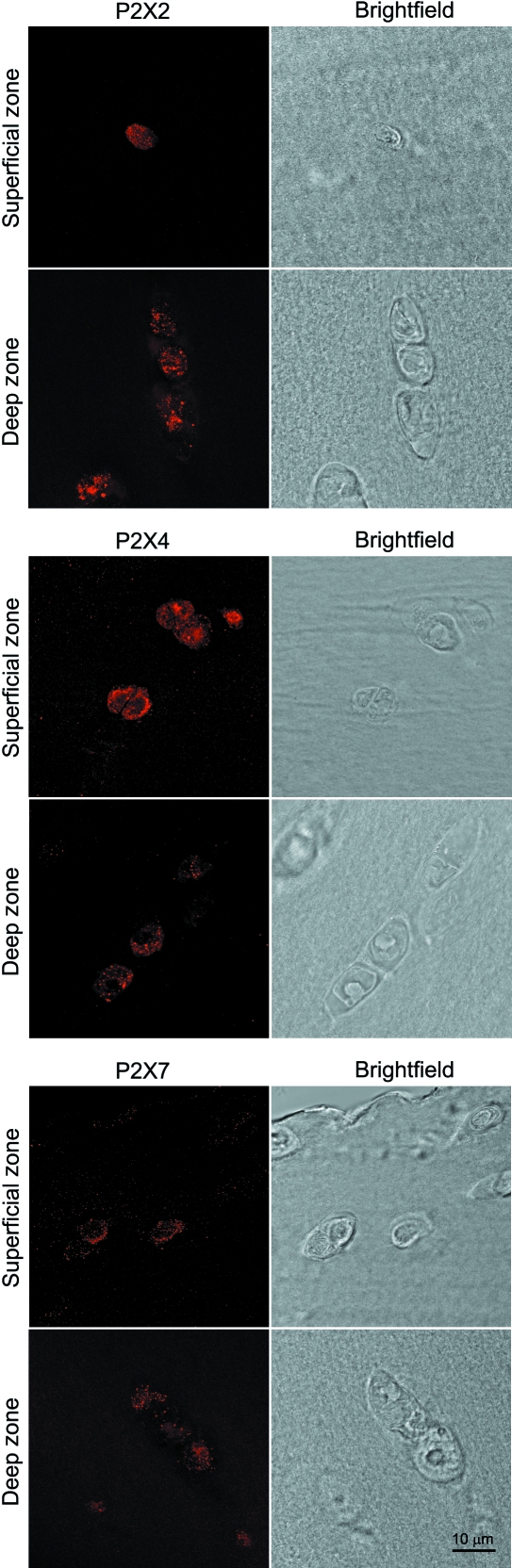
Human articular chondrocytes showed positive immunofluorescence labelling for purine receptors: P2Y1, P2Y2, P2X2, P2X4 and P2X7. Representative confocal images are shown in Figs 6 and 7. There was no labelling visible in the negative controls without primary antibody. The P2 receptors were present in all cells throughout the cartilage depth with the exception of P2Y2 receptors, which were observed only in cells within the superficial zone. There was no difference in the expression of purine receptors between the cartilage of the normal and OA joint.
Fig. 6.
Expression of chondrocyte P2Y1 and P2Y2 purine receptors in the superficial and deep zones of human articular cartilage. Images show 3D serial reconstruction of confocal z-series with corresponding brightfield images, all obtained with a ×100 objective. Note the lack of expression of P2Y2 in the deep zone.
Fig. 7.
Expression of chondrocyte P2X2, P2X4 and P2X7 purine receptors in the superficial and deep zones of human articular cartilage. Images show 3D serial reconstruction of confocal z-series with corresponding brightfield images, all obtained with a ×100 objective.
Discussion
Previous studies have reported that chondrocyte mechanotransduction involves intracellular Ca2+ signalling mediated by extracellular ATP (Chowdhury & Knight, 2006; Pingguan-Murphy et al. 2006). However, although other studies have reported that chondrocytes release ATP in response to mechanical stimuli (Graff et al. 2000), the mechanosensitive mechanisms are far from clear as ATP is membrane impermeant. For all cell types, there is an active debate about the physiological transport mechanisms that facilitate ATP release, with three main putative ATP release mechanisms: (1) hemichannels, (2) anion channels and (3) exocytosis of ATP-filled vesicles. The present study examined whether chondrocytes express connexin 43 hemichannels, which have previously been shown to function as mechanosensitive ATP-release channels in a variety of different cell types (Leybaert et al. 2003;Bao et al. 2004; Gomes et al. 2005). Connexins are membrane proteins that form hemichannels such that the extracellular regions can dock together to produce gap junctions between adjoining cells. There are over 13 different connexins, with some cell types expressing more than one type. Connexin 43 is a widely distributed connexin expressed by a range of cell types including epithelial cells, astrocytes, myocardial cells and smooth muscle cells.
In the present study, immunofluorescence has revealed the presence of connexin 43 within chondrocytes cultured in agarose gel (Fig. 2). In addition, the membrane impermeant dye Lucifer yellow (547 Da) was used to examine the expression of connexin hemichannels as in previous studies (Gomes et al. 2005; Genetos et al. 2007). Lucifer yellow uptake was significantly stimulated in the presence of EGTA and completely abolished by the hemichannel blocker flufenamic acid, in agreement with previous studies (Stout et al. 2002;Cherian et al. 2005; Gomes et al. 2005) (Fig. 1). These results therefore confirm the presence of functional uncoupled hemichannels in bovine articular chondrocytes. Further studies using immunofluorescence confirmed the expression of connexin 43 in situ within both bovine and human cartilage explants. In human cartilage, connexin 43 was confined to cells in the superficial region, down to approximately 200 µm below the articular surface (Fig. 4). The distribution was broadly similar in bovine tissue, although expression was slightly greater in the deep zone compared to that in human tissue (data not shown). Although the human studies were conducted using tissue from only two donors, this is nevertheless the first published evidence of connexin 43 expression in human articular cartilage. Previous studies have reported the presence of connexin 43 in cartilage from newborn and adult rats and mice (Schwab et al. 1998). In this case the expression was also confined to the superficial region; however, the authors concluded that connexin expression was indicative of intercellular gap junction formation, in agreement with similar dye-transfer studies in adult rabbit cartilage (Chi et al. 2004). In contrast, the present study provides strong evidence suggesting that connexin 43 forms uncoupled hemichannels both in intact cartilage (Figs 4 and 5) and in isolated chondrocytes cultured in agarose which show no direct cell–cell contact (Figs 1 and 2). This suggests that connexin 43 hemichannels may function as the mechanosensitive ATP-release channels involved in chondrocyte mechanotransduction. The fact that these potential mechanosensitive cells are primarily in the superficial/middle zone of articular cartilage may indicate that the mechanotransduction pathways in these cells are different to those which may operate in deeper zone cells, as previously suggested (Lee et al. 1998). The loss of expression in deep zone tissue and the enhanced expression in agarose culture may be related to the oxygen environment, as hypoxia is known to regulate connexin 43 dephosphorylation, translocation and proteosomal degradation in other cell types (Tansey et al. 2006).
We have recently shown that in Tg737orpk mice that lack polaris, a protein involved in cilia assembly, chondrocytes have extremely short or missing cilia (McGlashan et al. 2006b). These mice have defects in growth plate and developing articular cartilage and reduced expression of type II collagen, as well as skeletal patterning abnormalities and stunted growth, suggesting that the chondrocyte primary cilium is essential for cartilage health and homeostasis (Zhang et al. 2003;McGlashan et al. 2006b; Koyama et al. 2007; Song et al. 2007). However, the precise function of this fascinating organelle in articular cartilage has not been established. Further studies were therefore conducted to examine the localization of the hemichannels with respect to the primary cilium. Approximately 30% of isolated bovine articular chondrocytes expressed a primary cilium at day 1 of culture, in broad agreement with the authors’ previous studies (McGlashan et al. 2006a,c). Of these, approximately 50% showed expression of the mechanosensitive connexin 43 on the primary cilium (Fig. 3). It is unclear why some cells showed connexin 43 expression throughout the length of the axoneme, but others showed only discreet or nonexistent expression. However, we have found similar variation of integrin expression on the cilium (McGlashan et al. 2006a,c). These differences may be a reflection of the temporal state of the primary cilium of a particular cell. We suggest that those chondrocytes expressing a cilium decorated with connexin 43 may represent a sub-population of mechanosensitive chondrocytes, such that deflection of the cilium activates ATP release via the hemichannels. Indeed, it is interesting to note that the size of this sub-population (∼15%) is very similar to the sub-population of cells that show mechanosensitive purinergic Ca2+ signalling (Pingguan-Murphy et al. 2005, 2006).
Once released from the cell, ATP may activate P2 receptors triggering intracellular Ca2+ signalling cascades. Indeed, the activation of P2 receptors following mechanically induced ATP release or addition of endogenous ATP has been shown to have an anabolic effect, with an up-regulation in proteoglycan and collagen synthesis and cell proliferation (Kaplan et al. 1996; Brown et al. 1997; Croucher et al. 2000; Picher et al. 2003;Millward-Sadler et al. 2004; Chowdhury & Knight, 2006). Furthermore, there is a corresponding down-regulation in MMP expression and nitric oxide release. The present study examined the expression of a range of purine receptors on human articular chondrocytes using confocal immunofluorescence. Purine receptors can be classified into two groups: P1 (adenosine receptors) and P2 (ATP receptors). The latter is divided into two major superfamilies: the intrinsic ATP-gated ion channel-type receptor, P2X, and the G protein-coupled receptor, P2Y. Both P2X and P2Y receptors can be further divided into subtypes dependent on molecular structure and pharmacological properties: P2X1-P2X7 and P2Y1, 2, 4, 6 and 11 (Ralevic & Burnstock, 1998). Studies have reported the expression of P1 and P2 receptor genes in human chondrocytes and in particular the expression of P2Y1 and P2Y2 receptors (Koolpe et al. 1999; Millward-Sadler et al. 2004). Other recent studies suggest that chondrocytes express P2X1, 2, 3 and 5 (Hoebertz et al. 2000; Varani et al. 2008). It is worth noting that the P2X receptor family shows remarkable similarity to the epithelial sodium channel (ENaC) which has previously been implicated in chondrocyte mechanotransduction (Mobasheri & Martin-Vasallo, 1999).
The activation of G protein-coupled P2Y receptors causes mobilization of Ca2+ from intracellular stores via inositol(1,4,5)trisphosphate and phospholipase C. By contrast, channel-type P2X receptors are typically nonselective to monovalent cations, although some are also permeable to Ca2+. However, activation of P2X receptors may also facilitate extracellular Ca2+ entry indirectly via Na+ influx, triggering membrane depolarization and activation of voltage-operated Ca2+ channels (VOCC). The existence of functional P2 receptors in articular chondrocytes has thus been shown by the activation of Ca2+ signalling following the addition of exogenous ATP (Caswell et al. 1991; Kaplan et al. 1996; Koolpe & Benton, 1997; Koolpe et al. 1999; Elfervig et al. 2001). Indirect evidence of P2 receptor expression also comes from studies showing that apyrase or non-specific P2 antagonists such as suramin block mechanically-induced Ca2+ signalling (D’Andrea et al. 2000; Pingguan-Murphy et al. 2006) or membrane hyperpolarization (Millward-Sadler et al. 2004). However, using immunofluorescence the present study provides the first evidence revealing the expression of P2X4, P2X7 and P2Y1 receptor subtypes in human articular chondrocytes, as well as confirming the expression of P2X2 and P2Y2 (Figs 6 and 7). As with connexin 43 expression, the difference in P2Y2 expression between superficial and deep zone cells in human cartilage suggests the possibility that different signalling mechanisms may occur within these two cell populations.
Interesting studies from Millward-Sadler et al. suggest that, in contrast to cells from normal tissue, chondrocytes isolated from osteoarthritic (OA) cartilage do not show mechanically-induced ATP-mediated hyperpolarization (Millward-Sadler et al. 2004). This difference occurs despite the expression of P2Y2 mRNA in both normal and OA cells (Millward-Sadler et al. 2004). The present study found no obvious differences in P2 receptor expression between normal and OA human articular cartilage, although these results were obtained from a very small sample size. Therefore, it has been suggested that changes in purinergic mechanotransduction in OA chondrocytes are not due to differences in P2 receptor expression but may instead involve ATP desensitization as a result of increased levels of ATP found in the OA synovial fluid (Ryan et al. 1991; Millward-Sadler et al. 2000, 2004). Indeed, it has also been suggested that P2 receptor desensitization may explain the temporal up-regulation of Ca2+ signalling in response to continuous cyclic compression (Pingguan-Murphy et al. 2005, 2006).
In conclusion, our previous studies have suggested that chondrocyte mechanotransduction involves a purinergic calcium signalling pathway (Chowdhury & Knight, 2006; Pingguan-Murphy et al. 2006). This study demonstrates that articular chondrocytes express key components of this mechanotransduction pathway, namely mechanosensitive connexin 43 hemichannels and a range of P2X and P2Y purine receptors. Thus we hypothesize that chondrocyte purinergic mechanotransduction involves the release of ATP via connexin 43 hemichannels. ATP may then activate specific purine receptors, initiating a series of downstream events, including Ca2+ signalling, which will ultimately modulate cell function in response to the biomechanical environment. The finding that connexin 43 decorates the primary cilium suggest that the cilium may be involved in this mechanotransduction pathway.
Acknowledgments
This study was largely conducted during a study visit by Dr Knight to the University of Auckland funded by the Royal Society, UK. In addition we acknowledge the financial support of the Whittaker Trust, which has funded Ms Garcia's research within Dr Knight's laboratories at Queen Mary University of London. We are grateful to the meat inspectors at Dawn Cardington for the provision of fresh bovine metacarpophalangeal joints. C. A Poole, C. J. Jensen and S. R. McGlashan were partly funded by the Royal Society of New Zealand Marsden Fund.
References
- Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Caswell AM, Rahman S, Russell RG, Buttle DJ. Proteoglycan breakdown from bovine nasal cartilage is increased, and from articular cartilage is decreased, by extracellular ATP. Biochim Biophys Acta. 1997;1362(2–3):208–220. doi: 10.1016/s0925-4439(97)00080-x. [DOI] [PubMed] [Google Scholar]
- Caswell AM, Russell RG. Identification of ecto-nucleoside triphosphate pyrophosphatase in human articular chondrocytes in monolayer culture. Biochim Biophys Acta. 1985;847:40–47. doi: 10.1016/0167-4889(85)90150-8. [DOI] [PubMed] [Google Scholar]
- Caswell AM, Leong WS, Russell RG. Evidence for the presence of P2-purinoceptors at the surface of human articular chondrocytes in monolayer culture. Biochim Biophys Acta. 1991;1074:151–158. doi: 10.1016/0304-4165(91)90054-k. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SS, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205:363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of. NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209:845–853. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- Croucher LJ, Crawford A, Hatton PV, Russell RG, Buttle DJ. Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochim Biophys Acta. 2000;1502:297–306. doi: 10.1016/s0925-4439(00)00055-7. [DOI] [PubMed] [Google Scholar]
- D’Andrea P, Calabrese A, Capozzi I, Grandolfo M, Tonon R, Vittur F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology. 2000;37(1–2):75–83. [PubMed] [Google Scholar]
- Elfervig MK, Graff RD, Lee GM, Kelley SS, Sood A, Banes AJ. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage. 2001;9:518–526. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Srinivas SP, Van DW, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 2000;43:1571–1579. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR. Expression of P2 receptors in bone and cultured bone cells. Bone. 2000;27:503–510. doi: 10.1016/s8756-3282(00)00351-3. [DOI] [PubMed] [Google Scholar]
- Holloway I, Kayser M, Lee DA, Bader DL, Bentley G, Knight MM. Increased presence of cells with multiple elongated processes in osteoarthritic femoral head cartilage. Osteoarthr Cartilage. 2004;12:17–24. doi: 10.1016/j.joca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Jensen CG, Poole CA, McGlashan SR, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AD, Kilkenny DM, Hill DJ, Dixon SJ. Extracellular nucleotides act through P2U purinoceptors to elevate [Ca2+]i and enhance basic fibroblast growth factor-induced proliferation in sheep chondrocytes. Endocrinology. 1996;137:4757–4766. doi: 10.1210/endo.137.11.8895344. [DOI] [PubMed] [Google Scholar]
- Koolpe M, Benton HP. Calcium-mobilizing purine receptors on the surface of mammalian articular chondrocytes. J Orthop Res. 1997;15:204–212. doi: 10.1002/jor.1100150208. [DOI] [PubMed] [Google Scholar]
- Koolpe M, Pearson D, Benton HP. Expression of both P1 and P2 purine receptor genes by human articular chondrocytes and profile of ligand-mediated prostaglandin E2 release. Arthritis Rheum. 1999;42:258–267. doi: 10.1002/1529-0131(199902)42:2<258::AID-ANR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, et al. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Knight MM. Mechanical loading of chondrocytes embedded in 3D constructs: in vitro methods for assessment of morphological and metabolic response to compressive strain. Methods Mol Med. 2004;100:307–324. doi: 10.1385/1-59259-810-2:307. [DOI] [PubMed] [Google Scholar]
- Lee DA, Noguchi T, Knight MM, O'Donnell L, Bentley G, Bader DL. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res. 1998;16:726–733. doi: 10.1002/jor.1100160615. [DOI] [PubMed] [Google Scholar]
- Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10(4–6):251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- Liu W, Murcia NS, Duan Y, et al. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Chowdhury TT, Joshi P, Knight MM, Poole AC, Jensen CG. Mechanical compression reduces chondrocyte primary cilia expression in vitro. Mol Biol Cell. 2006a;17:54. [Google Scholar]
- McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737(orpk) mice lacking the primary cilia protein polaris. Matrix Biol. 2006b;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006c;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Lee H, Caldwell H, Nuki G, Salter DM. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthr Cartilage. 2000;8:272–278. doi: 10.1053/joca.1999.0301. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41(3–4):567–575. [PubMed] [Google Scholar]
- Mobasheri A, Martin-Vasallo P. Epithelial sodium channels in skeletal cells: a role in mechanotransduction ? Cell Biol Int. 1999;23:237–240. doi: 10.1006/cbir.1999.0405. [DOI] [PubMed] [Google Scholar]
- Picher M, Graff RD, Lee GM. Extracellular nucleotide metabolism and signaling in the pathophysiology of articular cartilage. Arthritis Rheum. 2003;48:2722–2736. doi: 10.1002/art.11289. [DOI] [PubMed] [Google Scholar]
- Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol. 2006;209:389–397. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- Pingguan-Murphy B, Lee DA, Bader DL, Knight MM. Activation of chondrocytes calcium signalling by dynamic compression is independent of number of cycles. Arch Biochem Biophys. 2005;444:45–51. doi: 10.1016/j.abb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5:175–193. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- Poole CA, Zhang ZJ, Ross JM. The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J Anat. 2001;199(Pt 4):393–405. doi: 10.1046/j.1469-7580.2001.19940393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ryan LM, Rachow JW, McCarty DJ. Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. J Rheumatol. 1991;18:716–720. [PubMed] [Google Scholar]
- Schwab W, Hofer A, Kasper M. Immunohistochemical distribution of connexin 43 in the cartilage of rats and mice. Histochem J. 1998;30:413–419. doi: 10.1023/a:1003220225670. [DOI] [PubMed] [Google Scholar]
- Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol. 2007;305:202–216. doi: 10.1016/j.ydbio.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Tansey EE, Kwaku KF, Hammer PE, et al. Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg. 2006;82:1472–1479. doi: 10.1016/j.athoracsur.2006.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K, De MM, Vincenzi F, et al. Pharmacological characterization of P2X(1) and P2X(3) purinergic receptors in bovine chondrocytes. Osteoarthr Cartilage. 2008;16:1421–1429. doi: 10.1016/j.joca.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, et al. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 2003;227:78–90. doi: 10.1002/dvdy.10289. [DOI] [PubMed] [Google Scholar]