Abstract
The phosphoinositide 3-kinase pathway regulates a multitude of cellular processes. Deregulation of PI 3-K signaling is often observed in human cancers. A major effector of PI 3-K is Akt/PKB (protein kinase B). Recent studies have pointed to distinct roles of Akt/PKB isoforms in cancer cell signaling. Studies have shown that Akt1 (PKBα) can attenuate breast cancer cell motility, whereas Akt2 (PKBβ) enhances this phenotype. Here we have evaluated the mechanism by which Akt1 blocks the migration of breast cancer cells through the transcription factor NFAT. A major effector of Akt/PKB is GSK-3β (Glycogen Synthase Kinase-3β), also an NFAT kinase. Inhibition of GSK-3β using shRNA or a selective inhibitor potently blocks breast cancer cell migration concomitant with a reduction in NFAT activity. GSK-3β-mediated inhibition of NFAT activity is due to proteasomal degradation. Experiments using GSK-3β mutants which are unresponsive to Akt/PKB reveal that inhibition of cell migration by Akt/PKB is mediated by GSK-3β. These effects are recapitulated at the levels of NFAT degradation by the proteasome. Our studies demonstrate that activation of Akt/PKB leads to inactivation of the effector GSK-3β and the outcome of this signaling event is degradation of NFAT by the proteasome and subsequent inhibition of cell migration.
Keywords: GSK-3β, cell migration, Akt/PKB, metastasis, NFAT
INTRODUCTION
The phosphoinositide 3-kinase (PI 3-K) signaling pathway controls a variety of biological functions including cell survival, proliferation and migration. Genetic lesions in the PI 3-K pathway are invariably found in all human cancers, either at the levels of PI 3-K itself, or upstream regulators and downstream effectors. To the extent that the PI 3-K pathway is a major determinant of cancer progression, it is now known that the frequency of PI 3-K mutations in cancer is second only to Ras (1). A major effector of the PI 3-K signal in all cells types is the serine/threonine kinase Akt, also known as PKB (Protein Kinase B). Amplifications in one or more of the three mammalian Akt/PKB isoforms (Akt1; PKBα; Akt2, PKBβ; Akt3; PKBγ) as well as mutations in Akt1 are frequently found in human cancers (2). The signaling mechanisms leading to Akt/PKB activation by PI 3-K, and subsequent activation of downstream secondary signaling cascades leading to physiological responses, have been characterized in considerable detail (reviewed in (3)). At the level of cell growth, much is known about the function of Akt/PKB in mediating cell proliferation through regulation of cell cycle progression and cell survival through regulation of pro- and anti-apoptotic transcription factors. Many of these mechanisms have been shown to be causal for cancer progression using mouse models.
A separate but equally important phenotype in carcinoma progression is invasive migration, a process which eventually leads to metastatic dissemination of tumor cells to distant organs. Recent studies from several laboratories have shown that Akt/PKB isoforms have opposing functions at modulating invasive migration of breast cancer cells, both in vitro and in vivo (reviewed in (4)). Our laboratory showed that Akt1 attenuates invasive migration of breast cancer cells in vitro (5). The Brugge laboratory also showed that whereas Akt1 attenuates cell migration in 2D and 3D cell cultures, the Akt2 isoform promotes migration in a growth factor-dependent manner (6), consistent with earlier studies in vivo (7). The Bissell laboratory reported similar results for Akt1 (8). Distinct mechanisms were reported to explain the distinct functions of Akt/PKB isoforms in the regulation of invasive migration, including differential regulation of the ERK (Extracellular-signal Regulated Kinase) pathway (6) and TSC2 (Tuberous Sclerosis Complex 2) (8). Our own studies revealed that Akt1 attenuates the activity of the transcription factor NFAT (5), which we previously showed to function as a pro-migratory and pro-invasive transcription factor in breast cancer cells (9). Other studies have shown that activation of NFAT is concomitant with the induction of genes which promote these phenotypes, such as autotaxin and COX-2 (10, 11). More recently, a number of independent studies have confirmed the distinct functions of Akt/PKB isoforms in modulating cancer cell invasion in vivo, using both Akt/PKB null mice and activated transgenes (12, 13).
The NFAT transcription factor, originally identified and characterized in immune cells, is ubiquitously expressed and functions in a variety of biological settings including in the endothelium, skeletal muscle, cardiac function and neuronal signaling (14, 15). The classical NFAT activation pathway requires an influx of calcium which activates the phosphatase calcineurin, and in turn dephosphorylates cytosolic NFAT unmasking a nuclear localization sequence (NLS) leading to nuclear translocation. Nuclear export of NFAT is mediated by both nuclear and cytosolic NFAT kinases. One of the first identified NFAT kinases is GSK-3β (16).
GSK-3β was originally identified as a negative regulator of glycogen synthase in insulin-responsive tissues (17). GSK-3 is ubiquitously expressed and is basally catalytically active in cells. Following stimulation with agonists inactivation of GSK-3 occurs through phosphorylation of a number of residues and this is mediated by variety of upstream kinases. In this regard, one of the most studied GSK-3β upstream kinases is Akt/PKB (18, 19). Two isoforms of GSK-3 exist in humans, GSK-3α and GSK-3β. In vivo studies using GSK-3β null mice have revealed that GSK-3α cannot compensate for the loss of GSK-3β, again suggesting isoform specificity between these two kinases (20). GSK-3β activity is implicated in many pathophysiological processes including cancer (reviewed in (21)). A major mechanism in the pathophysiology of cancer mediated by GSK-3β is the Wnt signaling axis. In unstimulated cells, GSK-3β facilitates ubiquitination and degradation of β-catenin, through phosphorylation of axin and APC (Adenomatous Polyposis Coli-associated protein) (22–24). Interestingly, mutations of β-catenin at the residues phosphorylated by GSK-3β have been found in numerous cancers (25). A direct link between Akt/PKB, GSK-3β and NFAT is informed by the fact that GSK-3β is an Akt/PKB substrate, and NFAT is a GSK-3β substrate. To this end, studies have shown that phosphorylation of NFAT2 by GSK-3β is associated with reduced NFAT1 activity as well as nuclear export (16). Here, we provide a mechanistic link between Akt/PKB, GSK-3β and NFAT signaling to the phenotype of breast cancer cell migration, and show that signaling through Akt/PKB leads to inactivation of NFAT, and that this occurs in a GSK-3β-dependent manner.
EXPERIMENTAL PROCEDURES
Antibodies and reagents
Anti-HA antibody was purified from the 12CA5 hybridoma in house. Anti-NFAT and COX-2 were from Santa Cruz (Santa Cruz, CA). Anti-GSK3β and Akt pSer473 were from Cell Signaling Technology (Beverly, MA). Anti-β-actin antibody, cycloheximide, insulin and hydrocortisone were from Sigma-Aldrich (St. Louis, MO). SB-415286 was from BioMol International (Plymouth Meeting, PA). Matrigel was from Fischer Scientific (Pittsburgh, PA). Doxycycline was from Clonetech (Palo Alto, CA). ALLN was from Calbiochem (San Diego, CA). Murine HA-tagged NFAT and IL-2-luciferase reporter plasmids have been described (9, 26, 27). The wild-type GSK and mutant GSK.S9A plasmids have also been described (28).
Production of shRNA lentivirus
To silence the expression of GSK3β, the following oligonucleotides were cloned into the pLKO1 lentiviral vector: sequence #1, forward 5′ - CCG GGA AGT CAG CTA TAC AGA CAC TCT CGA GAG TGT CTG TAT AGC TGA CTT CTT TTT G - 3′; reverse 5′ - AAT TCA AAA AGA AGT CAG CTA TAC AGA CAC TCT CGA GAG TGT CTG TAT AGC TGA CTT C - 3′. Sequence #2, forward 5′ - CCG GGA AAG CTA GAT CAC TGT AAC ACT CGA GTG TTA CAG TGA TCT AGC TTT CTT TTT G - 3′; reverse 5′ - AAT TCA AAA AGA AAG CTA GAT CAC TGT AAC ACT CGA GTG TTA CAG TGA TCT AGC TTT C - 3′. Viruses were generated by co-transfecting the cloned pLKO1 plasmids with packaging plasmid pHR’8.2deltaR at an 8:1 ratio and envelope plasmid pCMVVSV-G into HEK-293T cells. Virus was collected 48 h post transfection, passed through a 45 μM filter and kept at −80°C.
Cell lines
MDA-MB-231, HEK-293T and NIH 3T3 cells were obtained from ATCC (Manassas, VA), and maintained in DMEM supplemented with 10% fetal bovine serum at 37°C. The estrogen-independent human breast cancer SUM-159-PT cell line has been described (29). SUM-159-PT cells with inducible NFAT1 expression (SUM-159.N1) have been described (5, 11). NFAT1 expression was induced with 1μg/ml doxycycline for 16 h.
Immunoblotting
Cells were lysed in SDS sample buffer and lysates resolved by SDS PAGE and immunoblotted with the appropriate antibodies.
Migration assays
Migration assays were carried out essentially as previously described, using Transwell chambers (Corning, Acton, MA) with 8 μM pore membranes (5). Cells were treated in order to enhance or downregulate specific signaling pathways, either by co-transfection with the relevant expression plasmids and a pCS2-(n)-β-gal reporter, or by infection of lentiviruses expressing shRNA, or by treatment with chemical inhibitors. Cells were harvested, resuspended in serum-free media containing 0.1 % BSA, added in triplicates to Transwell chambers and allowed to migrate towards NIH 3T3 cell conditioned media for 1.5–16 h at 37°C. Cells that had migrated to the lower surface of the membrane were fixed and stained with either X-gal or with crystal violet.
Luciferase assays
Cells were infected with a lentivirus expressing shRNA against the GSK3β sequence. The infected cells were selected in a medium containing puromycin for 2 days. Cells were then transiently co-transfected with NFAT1-reporter plasmids, pCS2-(n)-β-gal. 24 h after transfection, cells were analyzed for luciferase and β-gal activity using the luciferase assay system (Promega, Madison, WI) and galacton-plus (Tropix, Bedford, MA), and measured on a luminometer. Luciferase activity was normalized against β-gal.
RESULTS
GSK-3β regulates cell migration
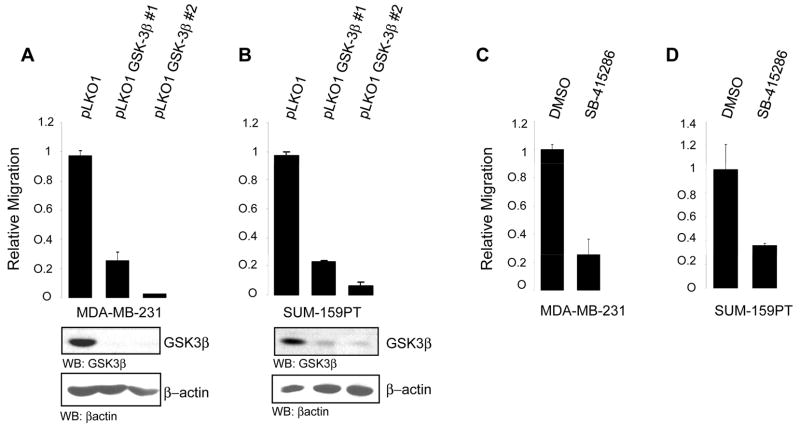
To begin to investigate the role of GSK-3β in cell migration, we first used MDA-MB-231 and SUM-159-PT cells, both highly migratory and invasive breast cancer cell lines which we previously showed express NFAT and where signaling through Akt/PKB attenuates invasive migration (5, 9). Cells were infected with lentiviruses encoding two distinct different GSK-3β shRNA sequences, GSK-3β #1 and GSK-3β #2 or virus control pLKO1. Cells were then selected with puromycin and analyzed for GSK-3β expression. In both conditions GSK-3β expression was either eliminated or dramatically reduced compared to control cells (Fig. 1A, B). We next assessed the effect of GSK-3β silencing on cell motility using Transwell chemomigration assays. In both cell lines, infection with either GSK-3β #1 or with GSK-3β #2 resulted in a potent inhibition of cell migration compared to cell infected with control virus (Fig. 1A, B). To determine whether GSK-3β enzymatic activity is responsible for the effects on migration seen with shRNA, which could be due a loss of a scaffolding function of the protein and not just loss enzymatic activity per se, we also treated cells with SB-415286, a GSK-3 chemical inhibitor. SB-415286 is a potent cell-permeable maleimide compound that is widely used as a selective GSK-3 inhibitor (30). Serum-starved MDA-MB-231 and SUM-159-PT cells were treated with SB-415286 or DMSO control, and the ability of cells to migrate was evaluated as above. As predicted, enzymatic inhibition of GSK-3 activity recapitulated the results with shRNA and almost completely abolished the ability of treated cells to migrate when compared to control cells (Fig. 1C, D). Thus, GSK-3β activity is required for the highly migratory capacity of two distinct breast cancer cell lines, and reduction of GSK-3β activity results in a near complete loss of the motile phenotype.
FIGURE 1. GSK-3β regulates cell migration.
MDA-MB-231 cells (A) and SUM-159-PT cells (B) were infected with lentiviruses directing expression of two distinct shRNA sequences against human GSK3β (GSK-3β #1 and GSK-3β #2). 24 h after infection cells were selected for a further 48 h with puromycin and then subjected to chemomigration assays. Cell lysates from each infection were immunoblotted with anti-GSK3β and control anti-actin. MDA-MB-231(C) and SUM-159-PT cells (D) treated for 16 h with the GSK3β inhibitor SB-415286 (25μM) or DMSO as control were subjected to chemomigration assays. All results are representative of three independent experiments and were performed in triplicates. Error bars indicate ± SD.
Signaling through Akt/PKB and GSK-3β regulates cell migration
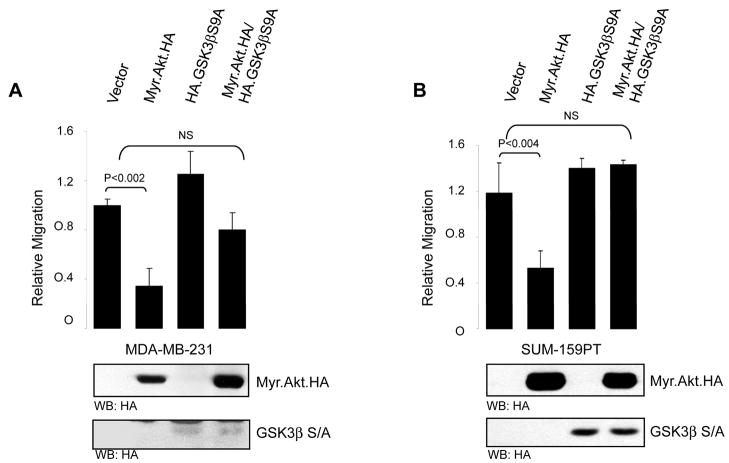
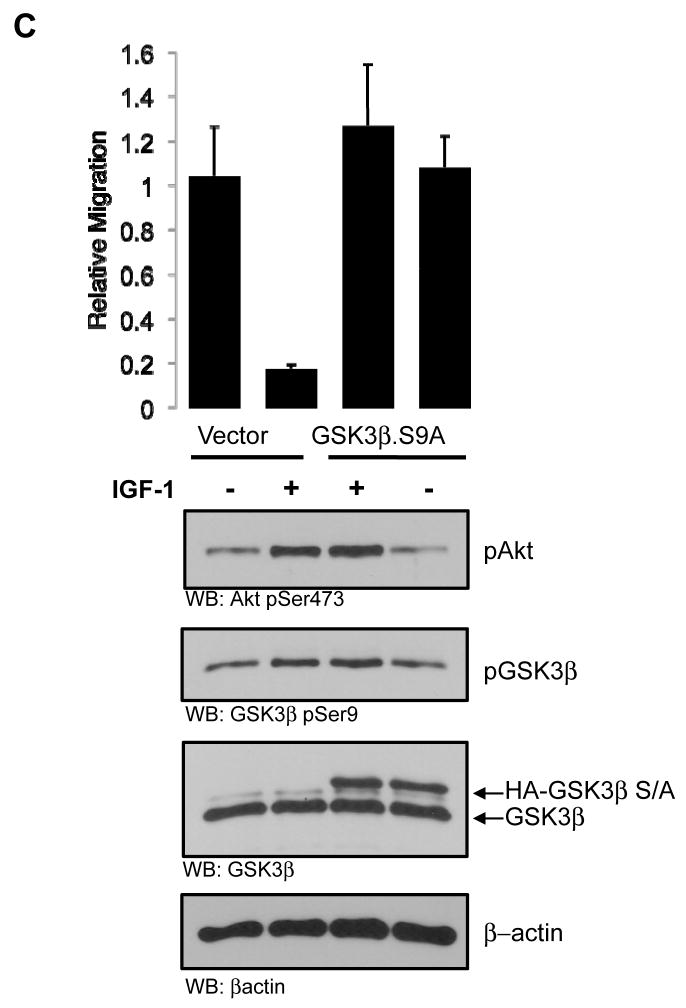
We next sought to implicate Akt/PKB in the modulation of invasive migration through GSK-3β. Since GSK-3β is a well-characterized Akt/PKB substrate, we took advantage of a mutant of GSK-3β which is unresponsive to Akt/PKB, GSK-3β.Ser9Ala. Phosphorylation of GSK-3β at Ser9 by Akt/PKB effectively inhibits the high basal and constitutive activity of GSK-3β (19). Since we and others have shown that Akt1 blunts invasive migration of breast cancer cells (5, 6, 8), we devised an experiment to determine whether one mechanism which accounts for this phenotype is signaling through GSK-3β. As previously shown, introduction of a conditionally active Myr.Akt/PKB allele potently blocked breast cancer cell migration in both MDA-MB-231 and SUM-159-PT cells (Fig. 2A, B). Expression of the Akt/PKB unresponsive GSK-3β.S9A mutant had little or no effect on cell migration. However, co-expression of the GSK-3β.S9A mutant along with Myr.Akt/PKB either partially (Fig. 2A) or completely (Fig. 2B) rescued the inhibition of cell migration induced by activated Akt/PKB. We also evaluated the ability of a physiological ligand of the Akt/PKB and GSK-3β pathway to control cell migration. We previously showed that stimulation of cells with IGF-1, which potently activates Akt/PKB, results in inhibition of cell migration, an effect which could be reversed with Akt1 shRNA (5). If this effect is mediated through Akt/PKB and GSK-3β, then a GSK-3β mutant resistant to Akt/PKB phosphorylation should phenocopy Akt1 shRNA. Consistent with this notion, SUM-159-PT cells stimulated with IGF-1 showed a marked inhibition of cell migration, and this was completely rescued in cells expressing the GSK-3β S9A mutant which cannot be inhibited by Akt/PKB. Thus, either with genetic (Myr.Akt/PKB) or physiological (IGF-1) means, inactivation of GSK-3β by the Akt/PKB pathway regulates cell migration. Taken together, the interpretation here is that GSK-3β activity is required for the high basal migratory phenotype of MDA-MB-231 and SUM-159-PT breast cancer cells, and inhibition of GSK-3β activity, either by shRNA (Fig. 1) or physiologically by Akt/PKB (Fig. 2) blocks this phenotype.
FIGURE 2. Akt1 blocks invasion through GSK-3β.
MDA-MB-231 (A) and SUM-159-PT (B) cells were transfected with either vector control, constitutively active Akt1 (Myr.Akt.HA) along with vector control or mutant GSK-3β (HA.GSK3β.S9A), or mutant GSK-3β alone. 16 h after transfection the ability of the cells to migrate was assessed by a chemomigration assays. A portion of the transfected cells were immunoblotted with anti-HA. (C) SUM-159-PT cells were transfected with either vector control, or mutant GSK-3β (HA.GSK-3β.S9A). Cells were then serum starved for 19 h, then stimulated with IGF-1 for 18 h (100 ng/ml), followed by chemomigration assays. All results are representative of three independent experiments, and were performed in triplicate. Error bars indicate ± SD, with p values as indicated (NS, not significant).
NFAT1 activity is regulated by GSK3β
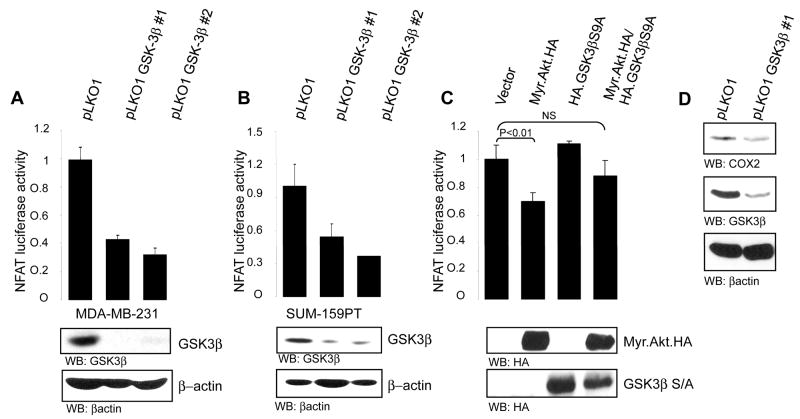
We next sought to determine the mechanism by which attenuation of GSK-3β activity blocks breast cancer cell migration. We focused our attention on the transcription factor NFAT, because we previously showed that NFAT is a pro-invasive migration factor (9), and because NFAT activity is potently blocked by positive signaling through Akt/PKB, to the extent that, for example, constitutively active Akt/PKB completely abrogates NFAT transcriptional activity (5). To begin to investigate the model that GSK-3β promotes enhanced migration through effects on NFAT activity, we first used shRNA. Consistent with the results on cell migration, lentiviral infection of both MDA-MB-231 and SUM-159-PT cells with two distinct shRNA sequences targeting GSK-3β significantly decreased NFAT transcriptional activity as measured using an NFAT-luciferase reporter (Fig. 3A, B). As already shown, this was accompanied by a substantial reduction in GSK-3β protein.
FIGURE 3. GSK-3β regulates NFAT1 transcriptional activity.
MDA-MB-231 (A) and SUM-159-PT (B) cells and were infected with lentiviruses expressing shRNA against GSK-3β and selected as in the legend to Fig. 1. Cells were transfected with IL-2-luciferase reporter plasmid and pCS2-(n)-β-gal immediately after selection. 16 h after transfection cells were lysed and luciferase assays were performed. Lysates were immunoblotted with anti-GSK-3β and anti-actin. SUM-159-PT cells (C) were transfected with either vector control, constitutively active Akt (Myr.Akt.HA), Myr.Akt.HA and mutant GSK-3β (HA.GSK-3β.S9A) or with the GSK-3β mutant alone together with the IL-2-luciferase reporter plasmid and pCS2-(n)- β-gal. 16 h after transfection luciferase assays were performed. (D) MDA-MB-231 cells were infected with lentivirus expressing shRNA against GSK-3β and selected with puromycin (1μg/ml) for 48 h then lysed and immunoblotted with anti-COX-2, anti-GSK-3β or anti-actin. All assays were performed in triplicate. Error bars indicate ± SD, with p values as indicated (NS, not significant).
To provide a causal and direct demonstration of the effects of GSK-3β on NFAT activity, we once again made use of the GSK-3β.S9A mutant which is Akt/PKB unresponsive. Again, and as previously published (5), expression of constitutively active Myr.Akt/PKB significantly reduced NFAT activity as measured in reporter assays (Fig. 3C). Expression of the GSK-3β.S9A mutant had little or no effect on NFAT activity. In contrast, co-expression of GSK-3β.S9A with Myr.Akt/PKB rescued the inhibition of NFAT activity induced by activated Akt/PKB. Finally, we used a distinct reporter of NFAT activity, COX-2. We and others have shown that COX-2 is an NFAT-induced gene in breast cancer cells (11). Consistent with the luciferase reporter assays, depletion of GSK-3β with shRNA also reduced total levels of COX-2 protein (Fig. 3D). These results essentially phenocopy the migration assays in Fig. 2. Again, the interpretation of these results is that phosphorylation of GSK-3β by Akt/PKB at Ser9, which inhibits GSK-3β activity, is required for the down regulation of NFAT activity. Thus, the model here is that under physiological conditions, the high basal migratory capacity of these breast cancer cells is maintained by constitutive GSK-3β activity, and that removal of this activity by either Akt/PKB acting on GSK-3β through Ser9 phosphorylation, or artificially using shRNA, reverses the phenotype.
NFAT1 Stability is Regulated by the Akt/PKB and GSK-3β Pathway
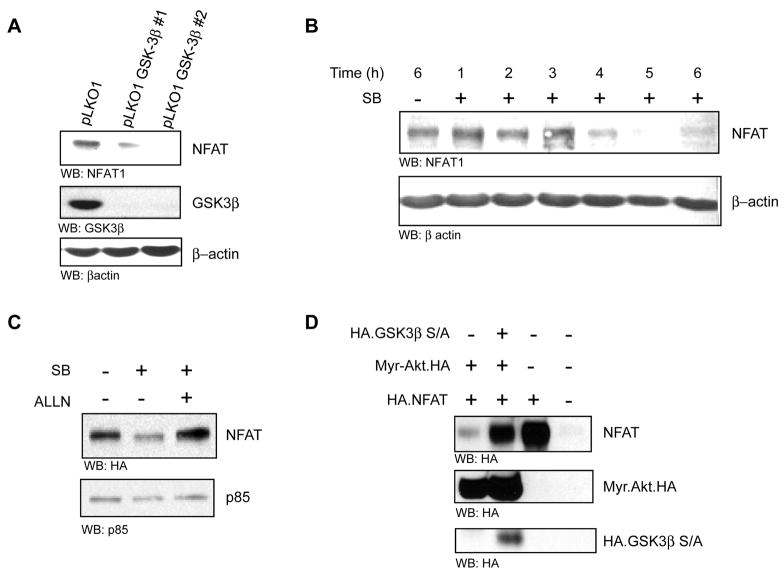
Finally, we investigated the mechanism by which the Akt/PKB and GSK-3β signaling axis attenuates NFAT activity. Consistent with the results in Fig. 3A and B, shRNA-mediated silencing of GSK-3β in MDA-MB-231 cells results in a marked reduction of NFAT protein under steady state conditions (Fig. 4 A). This effectively explains the diminution of NFAT activity under the same conditions.
FIGURE 4. GSK-3β stabilizes NFAT.
(A) MDA-MB-231 cells were infected with lentiviruses directing expression of two distinct sequences targeting human GSK-3β. Cells were selected in the presence of 1μg/ml puromycin for 48 h then lysed and separated by SDS PAGE and immunoblotted with anti-NFAT, anti-GSK-3β and anti-actin. (B) MDA-MB-231 cells were serum starved for 1 h then treated with cycloheximide (100μg/ml) together with the SB-415286 (25μM) over time, as indicated. Total cell lysates were immunoblotted with anti-NFAT and anti-actin. (C) SUM-159-PT.N1 clone #16 cells harboring tetracycline-inducible HA.NFAT were induced with doxycycline (0.5 μg/ml) for 16 h in the presence of SB-415286 (25μM) or DMSO control and with ALLN (50μM) as indicated. Cells were lysed and immunoblotted with anti-HA and anti-p85 as control (D). SUM-159-PT cells were transiently transfected with HA.NFAT, constitutively active Akt (Myr.Akt.HA) and GSK-3β mutant (HA.GSK-3β.S9A) as indicated. 24 h following transfection cells were lysed and lysates were immunoblotted with anti-HA, anti-Akt and anti-GSK-3β. All experiments were performed in triplicate.
We also evaluated the half-life of NFAT1 protein in cells treated with the GSK-3β inhibitor SB-415286. Cells were serum-starved and treated with the protein synthesis inhibitor cycloheximide to prevent de novo NFAT1 protein synthesis, then treated over a period of 6 h with the GSK-3β inhibitor. The total levels of NFAT1 and control actin were measured by immunoblotting. There were little or no detectable changes in total NFAT1 protein during the first 3 hours of drug administration (Fig. 4B). However, by 4 hours the levels of NFAT1 had declined to near undetectable levels, whereas levels of control actin were unchanged. As we had previously shown that NFAT is subject to proteasomal degradation by the Akt/PKB pathway, we next investigated if GSK-3β is part of this mechanism. Again, exposure of cells to SB-415286 resulted in a marked reduction of NFAT1 protein, but this was completely reversed to control levels when cells were co-treated with the proteasome inhibitor ALLN (Fig. 4C). To provide genetic evidence for the regulation of NFAT1 proteasomal degradation downstream of GSK-3β, we again used the GSK-3β.S9A mutant. Expression of constitutively active Myr.Akt/PKB led to marked reduction in totals levels of NFAT1 (Fig. 4D). Again, this was rescued to near control levels when cells were co-transfected with the Akt/PKB resistant GSK-3β.S9A mutant (Fig. 4D). Thus, signaling through Akt/PKB blocks NFAT1 activity and it does so by promoting the proteasomal degradation of the transcription factor. GSK-3β is an intermediary in this pathway and specifically mediates the effects of Akt/PKB on NFAT. The net effect of this mechanism is attenuated cell migration.
DISCUSSION
Hyperactivation of the PI 3-K pathway invariably leads to malignant transformation and tumor progression in humans. This can occur as a result of genetic alterations at the level of PI 3-K itself, as well as upstream regulators such as Ras, PTEN and PDK-1. Similarly, amplifications and mutations in downstream effectors such as Akt/PKB, HDM2, S6K and TSC2 are often observed and have been shown in various in vivo models to promote tumorigenesis (1). For this very reason numerous small molecule inhibitors targeting one or more kinases in this pathway are currently being evaluated in clinical trials as cancer therapeutics. The Akt/PKB family of kinases have been shown to play a major role in PI 3-K signaling, both at the level of signal relay as well as malignant transformation in a variety of settings. However, recent studies concerning the specific function of individual Akt/PKB isoforms and their effects on carcinoma invasive migration have demonstrated that while the Akt2 isoform can promote tumor cell invasion and migration in vitro and in vivo, curiously the Akt1 isoform does not phenocopy this response (4). In fact, studies from our laboratory and others have shown that Akt1 can actually function as an inhibitor of breast cancer cell invasive migration (5, 6, 8), an observation originally made by the Muller laboratory who showed that in a mouse model of ErbB2/Neu-driven mammary tumorigenesis expression of activated Akt1 can promote proliferation but also leads to a reduction in the number of metastatic lesions (12). It is also noteworthy that the function of Akt isoforms in modulating invasive migration differs depending on the cell type. For example, while Akt1 inhibits or does not promote migration in breast epithelial cell lines, in fibroblasts Akt1 clearly functions to promote motility, at least in vitro (31). Thus, the genetic background of distinct cell types is likely to govern the specific mechanisms which Akt isoforms use to modulate this phenotype.
In our recent study, we showed that the ability of Akt1 to block breast cancer cell migration is due in part to the inactivation of a crucial pro-invasive migration transcription factor, NFAT1 (5). NFAT1 can promote this phenotype through the induction of genes which can enhance the motility and invasiveness of cells, including autotaxin/ENPP2 and COX-2 (10, 11). The ability of Akt1 to inactivate NFAT1 is due to the proteasomal degradation of the transcription factor, mediated by the E3 ubiquitin ligase HDM2, also an Akt/PKB substrate. In the present study, we have extended our investigation to delineate role of GSK-3β in modulating cell migration in response to Akt1 signaling and the contribution of NFAT in this response. Using specific shRNA’s as well as the chemical inhibitor SB-415286, we demonstrate that inhibition of GSK-3β in two distinct breast cancer cells lines results in a marked reduction of cell migration. We then used a genetic rescue approach to provide a causal demonstration that the inactivation of GSK-3β by Akt/PKB is responsible for the inhibition of cell migration. This was possible through the use of a GSK-3β mutant, S9A, which is unresponsive to the Akt/PKB signal and therefore cannot be inactivated by phosphorylation. Indeed, this GSK-3β mutant effectively rescued the ability of Akt/PKB to blunt cell migration (Fig. 2). Thus the first conclusion to be drawn from these studies is that signaling through Akt/PKB leads to inactivation of GSK-3β, and this results in inhibition of cell migration in vitro, an effect that is phenocopied by loss of GSK-3β activity using pharmacological or genetic means. It is however worth noting that there exist other mechanisms by which Akt can blunt cell migration in a manner that is independent of GSK-3β. This is highlighted by the fact that in certain experiments, expression of Myr.Akt/PKB in the context of activated GSK-3β.SA does not elicit a complete rescue of migration (Fig. 2A). In this context, other studies have already demonstrated that, for example, Akt1 can attenuate migration through ERK and TSC2 signaling (6, 8).
Previous studies have investigated the role of GSK-3β in modulating cell migration in distinct models, albeit with different results. For example, inactivation of GSK-3β by Akt/PKB was shown to regulate the recycling of integrins thus promoting cell migration in fibroblasts (32). Similarly, genetic or pharmacological inactivation of GSK-3β was shown to lead to a less of epithelial architecture and induction of a more mesenchymal phenotype of non-tumorigenic breast epithelial cells, suggestive of a role for GSK-3β in preventing the acquisition of a motile phenotype (33). In contrast, other studies using tumorigenic cell lines have shown that GSK-3β is required for maintaining cell motility and invasion in vitro, consistent with our own studies. For example, GSK-3β has been shown to cooperate with h-prune to promote cell migration through modulation and phosphorylation of focal adhesion kinase (34). Similarly, in human keratinocytes inhibition of GSK-3β results in attenuated levels of the small GTPase Rac at lamellapodia, thus inhibiting wound closure (35). Moreover, paxillin has been identified as a GSK-3β substrate by a dual ERK/GSK-3β mechanism, and this was shown to play an important role in cytoskeletal rearrangement of various cell lines (36). Thus, depending on the cell origin and precise genetic background, GSK-3β can play both positive and negative roles in modulating cell migration and invasion, and in many cases this is modulated through the Akt/PKB pathway. In the context of aggressive breast cancer cells, GSK-3β clearly functions as an enhancer of the motile phenotype, whereas in other cell types of the surrounding stroma such as fibroblasts, GSK-3β may function in an opposing manner.
We next turned our attention to the mechanism by which inactivation of GSK-3β can attenuate cell migration in the Akt/PKB pathway. Because we recently showed that Akt1 can blunt cell migration in an NFAT1-dependent manner, and because GSK-3β has been identified as an NFAT kinase, we asked the most logical question: does GSK-3β inactivation lead to inactivation of NFAT, and does this then explain the consequence of GSK-3β inhibition on cell migration? As predicted, inhibition of GSK-3β by chemical or genetic means also potently blocked NFAT transcriptional activity in cells, and again we were able to show that this was specifically mediated by Akt/PKB signaling through the use of the GSK-3β mutant which is Akt/PKB unresponsive (Fig. 3). To extend this observation, we showed that the inactivation of NFAT activity by loss of GSK-3β is due to proteasomal degradation of the transcription factor, a result that essentially recapitulates what is observed in cells expressing activated Akt/PKB, where NFAT1 is also degraded by the proteasome (ref. (5) and Fig. 4D). Although we showed that HDM2 is in part required for the proteasomal degradation of NFAT1 by the Akt/PKB pathways, whether GSK-3β functions in the same, or a distinct but parallel pathway remains to be determined.
The ability of GSK-3β to control ubiquitination and protein stability of its substrates through phosphorylation has been studied in some detail, again revealing differences on the mechanism depending on the pathway in question. For example, in the Wnt pathway GSK-3β phosphorylates axin and increases its stability (23). In contrast, GSK-3β phosphorylation of β-catenin induces its ubiquitin and subsequent proteolysis. Although the detailed mechanism remains to be determined, GSK-3β signaling to NFAT is associated with stabilization, as loss of GSK-3β activity leads to a robust degradation of NFAT1 by the proteasome.
Given that opposing functions for Akt/PKB and GSK-3β in modulating cell migration have been reported and shown to be dependent on the cellular context, it is perhaps not surprising to find that contrasting functions for GSK-3β in the regulation of NFAT1 activity are also evident. In the original report where GSK-3β was identified as an NFAT kinase, it was shown that GSK-3β inactivates the transcription factor by promoting nuclear export (16). Thus, inactivation of GSK-3β would be expected to retain NFAT in the nucleus, and be reflected by increased transcriptional activity. These results were obtained in COS cells. However, in our own studies in breast cancer cells, GSK-3β appears to function in an opposing manner, because inactivation of GSK-3β results in a loss of transcriptional activity, which would not be predicted to be associated with nuclear retention. Instead, it appears that GSK-3β is required to maintain active NFAT1 in the nucleus, presumably through phosphorylation, and that loss of phosphorylation at one or more sites promotes proteasomal degradation. Considering the function of Akt/PKB in this response, our studies are consistent to those of Patra et al, who showed that expression of activated Akt/PKB results in cytoplasmic accumulation of NFAT, and therefore inhibition of transcriptional activity (37). Regardless of the mechanisms, these findings highlight the importance of addressing specific mechanisms of signaling to phenotypic responses such as migration, which are often found to be opposing in distinct cell types.
To date, numerous other NFAT upstream kinases have been identified which affect NFAT cellular localization, and in turn transcriptional activity. For example, casein kinase 1 (CK1) was recently shown to phosphorylate NFAT1 in T cells and promote NFAT1 nuclear export, in a manner dependent on subsequent GSK-3β phosphorylation of the transcription factor (38). One extrapolation from these findings is that CK1 phosphorylates NFAT1 thus promoting nuclear export, as well providing a priming site for subsequent GSK-3β-mediated phosphorylation. This latter event may promote nuclear export of NFAT1, and also protect it from proteasomal degradation, a predominantly cytoplasmic event (5). Whether this or other mechanisms are operate to control NFAT localization, activity and stability have yet to be determined.
In summary, our studies have added a new layer in the mechanism by which Akt1 functions as an inhibitor of breast cancer cell migration, specifically by inactivation of GSK-3β leading to destabilization of NFAT. This has important consequences for the multitude of genes which are induced by NFAT in cancer cells, which are likely to impact phenotypes such as cell motility, survival and proliferation.
Acknowledgments
We thank Frank McCormick for providing the GSK-3β constructs and members of the Toker laboratory for insightful discussions and advice. This work was supported by National Institutes of Health Grants CA096710 and CA122099 (A.T.), T32HL007893 (C.H.), the Dan David Prize of Tel Aviv University (M. Y.-L.) and a fellowship from the Susan G. Komen Breast Cancer Foundation (Y. R. C.).
Abbreviations
- CK1
Casein Kinase 1
- COX-2
Cyclooxygenase-2
- ERK
Extracellular signal-Regulated Kinase
- GSK-3β
Glycogen Synthase Kinase 3β
- HA
hemagglutinin
- HDM2
Human Double Minute 2
- PDK-1
phosphoinositide-dependent kinase-1
- PI 3-K
phosphoinositide 3-kinase
- PKB
Protein Kinase B
- NFAT
Nuclear Factor of Activated T cells
- shRNA
short hairpin RNA
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer cell. 2007;12(2):104–7. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends in Cell Biology. 2006;16(9):461–6. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20(4):539–50. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Irie HY, Pearline RV, Grueneberg D, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. The Journal of cell biology. 2005;171(6):1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arboleda MJ, Lyons JF, Kabbinavar FF, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer research. 2003;63(1):196–206. [PubMed] [Google Scholar]
- 8.Liu H, Radisky DC, Nelson CM, et al. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4134–9. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4(7):540–4. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, O’Connor KL. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene. 2005;24(32):5125–30. doi: 10.1038/sj.onc.1208729. [DOI] [PubMed] [Google Scholar]
- 11.Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. The Journal of biological chemistry. 2006;281(18):12210–7. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer research. 2004;64(9):3171–8. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 13.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer research. 2007;67(1):167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 (Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 15.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Molecules and cells. 2004;18(1):1–9. [PubMed] [Google Scholar]
- 16.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275(5308):1930–4. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 17.Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788(3):339–47. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 18.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 19.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303 (Pt 3):701–4. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 21.Hardt SE, Sadoshima J. Glycogen Synthase Kinase-3{beta}: A Novel Regulator of Cardiac Hypertrophy and Development. Circ Res. 2002;90(10):1055–63. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 22.Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. The Journal of biological chemistry. 2000;275(44):34399–406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. The Journal of biological chemistry. 1999;274(16):10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 24.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 25.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 26.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. Embo J. 2000;19(17):4783–95. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Northrop JP, Ho SN, Chen L, et al. NF-AT components define a family of transcription factors targeted in T- cell activation. Nature. 1994;369(6480):497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 28.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. The Journal of biological chemistry. 2000;275(42):32475–81. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan L, Van Weelden K, Ammerman C, Ethier SP, Welsh J. SUM-159PT cells: a novel estrogen independent human breast cancer model system. Breast Cancer Res Treat. 1999;58(3):193–204. doi: 10.1023/a:1006331716981. [DOI] [PubMed] [Google Scholar]
- 30.Coghlan MP, Culbert AA, Cross DA, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & biology. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. The Journal of biological chemistry. 2006;281(47):36443–53. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- 32.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24(4):1505–15. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. The Journal of cell biology. 2005;168(1):29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi T, Hino S-i, Oue N, et al. Glycogen Synthase Kinase 3 and h-prune Regulate Cell Migration by Modulating Focal Adhesions. Mol Cell Biol. 2006;26(3):898–911. doi: 10.1128/MCB.26.3.898-911.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koivisto L, Alavian K, Hakkinen L, Pelech S, McCulloch CA, Larjava H. Glycogen synthase kinase-3 regulates formation of long lamellipodia in human keratinocytes. J Cell Sci. 2003;116(Pt 18):3749–60. doi: 10.1242/jcs.00693. [DOI] [PubMed] [Google Scholar]
- 36.Cai X, Li M, Vrana J, Schaller MD. Glycogen Synthase Kinase 3- and Extracellular Signal-Regulated Kinase-Dependent Phosphorylation of Paxillin Regulates Cytoskeletal Rearrangement. Mol Cell Biol. 2006;26(7):2857–68. doi: 10.1128/MCB.26.7.2857-2868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patra AK, Na SY, Bommhardt U. Active protein kinase B regulates TCR responsiveness by modulating cytoplasmic-nuclear localization of NFAT and NF-kappa B proteins. J Immunol. 2004;172(8):4812–20. doi: 10.4049/jimmunol.172.8.4812. [DOI] [PubMed] [Google Scholar]
- 38.Okamura H, Garcia-Rodriguez C, Martinson H, Qin J, Virshup DM, Rao A. A Conserved Docking Motif for CK1 Binding Controls the Nuclear Localization of NFAT1. Mol Cell Biol. 2004;24(10):4184–95. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]