Abstract
Objective
Repetitive transcranial magnetic stimulation (rTMS) has shown promising results in treating Parkinson’s disease (PD), but the best values for rTMS parameters are not established. 50 Hz rTMS may be superior to ≤ 25 Hz rTMS investigated so far. The objective of this study was to determine if 50 Hz rTMS could be delivered safely in PD patients since current safety limits are exceeded.
Methods
50 Hz rTMS was applied with a circular coil on the primary motor cortex (M1). Stimulation intensity was first tested at 60% rest motor threshold [RMT] and 0.5 sec train duration and then increased in 0.5 sec steps to 2 sec, and by 10% steps to 90% RMT. Multi-channel electromyography (EMG) was recorded to control for signs of increasing time-locked EMG activity including correlates of the spread of excitation and after-discharges, or an increase of M1 excitability. Pre- and post-50 Hz rTMS assessments included EEG, Unified Parkinson Disease Rating Scale (UPDRS), Grooved Pegboard Test, Serial Reaction Time Task (SRTT), Folstein Mini-Mental Status Examination (MMSE) and Verbal Fluency to control for motor and cognitive side effects.
Results
Ten PD patients were investigated. Multi-channel EMG showed no signs of increased time-locked EMG activity including correlates of the spread of excitation and after-discharges, or increased M1 excitability in 9 patients. A PD patient with bi-temporal spikes in the pre-testing EEG had clinical and EMG correlates of spread of excitation at 90% RMT, but no seizure activity. Pre- and post-50 Hz assessment showed no changes. No adverse events were observed. 50 Hz rTMS was well tolerated except by one patient who wished to terminate the study due to facial muscle stimulation.
Conclusion
50 Hz rTMS at an intensity of 90% RMT for 2 sec appears safe in patients with PD, but caution should be taken for patients with paroxysmal EEG activity. For this reason, comprehensive screening should include EEG before higher-frequency rTMS is applied.
Significance
This is the first study to investigate safety of 50 Hz rTMS in humans.
Keywords: repetitive transcranial magnetic stimulation (rTMS), safety, Parkinson’s disease, 50 Hz rTMS
Introduction
High-frequency (>1 Hz) repetitive transcranial magnetic stimulation (rTMS) has been successfully applied as a potential therapy in Parkinson’s disease (PD). A wide range of high-frequencies up to 25 Hz rTMS has been safely investigated in PD patients, and 25 Hz rTMS has shown improved gait which often only partially responds to dopaminergic therapy (Lomarev et al., 2006). Non-invasive brain stimulation using rTMS might represent a powerful addition to conventional therapy in PD. Twenty-five Hz rTMS has been postulated to be superior to 10 Hz rTMS (Khedr et al., 2006), and, thus, higher frequencies may yield a greater therapeutic effect. Before therapeutic effects of higher frequencies up to 50 Hz rTMS, which are out of range of frequencies known to be safe (Wassermann, 1998), can be investigated, their safety must be established.
The purpose of this study was to test the safety of longer and higher-intensity 50 Hz rTMS of the motor cortex (M1) in PD patients which exceeds currently approved frequency and intensity limits for healthy subjects and to establish new safety guidelines for future higher-frequency rTMS therapeutic trials in PD.
The most serious side effect of high-frequency rTMS is the induction of a seizure. To minimize seizure risk, the study was designed to test the safety of 50 Hz rTMS by stepwise increasing intensity and duration while simultaneously monitoring for warning signs of an impending seizure. In addition, motor and cognitive tests were performed to exclude other potential adverse effects.
In establishing guidelines for the safe combination of rTMS frequency, intensity and train duration to stimulate the M1 in healthy subjects, we looked specifically for two phenomena suggestive for potentially dangerous increased cortical excitability: (a) brief EMG bursts (BEB) which persist after the rTMS train has ceased, and (b) spread of excitation (SE) to muscles not activated by single-pulse TMS during the 50 Hz rTMS train (Wassermann, 1998). BEB is considered an EMG correlate of an after-discharge in the EEG, which is a recognized sign of epileptic activity (Ajmone Marsan, 1972; Wassermann, 1998). SE has been observed preceding TMS-induced seizures (unpublished observation), but the mechanism is unknown. SE is postulated to indicate a breakdown of lateral inhibition in the cortex which might predispose to seizure generation (Pascual-Leone et al., 1993b; Pascual-Leone et al., 1994). An alternative explanation is that “recruitment” of more proximal muscles with higher threshold than those of the hand occurs later when a certain “amount” of stimulation has been applied which could appear like a propagation of excitation. We also looked for increased MEP amplitudes in the target muscle to single pulse (supra-threshold) TMS between 50 Hz rTMS trains indicating increased M1 excitability which was previously documented after supra-threshold high-frequency rTMS (Pascual-Leone et al., 1994). At present, low-intensity 50 Hz rTMS by means of short bursts of 5–15 stimuli and triple-bursts repeated at 5 Hz (Theta-Burst Stimulation [TBS]) have only been investigated; these were safe in healthy subjects (Huang and Rothwell, 2004; Huang et al., 2005) and subsequently were applied in various disease conditions without reports of seizures or other adverse events.
Methodology
Study population
Ten patients (5 women and 5 men, mean age 62.6 ± 9.6 years, range 50–77 years, 9 right-handed and 1 ambidextrous) with mild to moderate PD (Hoehn and Yahr stage mean 2.3 ± 0.4 in “on” and 2.7 ± 0.3 in “off” medication state) were investigated (see Table 1). An eligible patient withdrew for personal reasons after signing the consent. Inclusion criteria were men and women aged 40 to 80 years with PD in a Hoehn and Yahr (HY) stage of 2 to 4 while “off” medication. The same inclusion criteria were used in a published 25 Hz rTMS trial (Lomarev et al., 2006) which would also be applied when selecting the target PD population for future rTMS trials. Baseline assessment included a detailed medical history, neurological examination and electroencephalography (EEG). Exclusion criteria were significant medical or psychiatric illness, dementia, pregnancy, history of seizures, epilepsy or epileptiform discharges in the EEG, concurrent use of tricyclic antidepressants, neuroleptics or other drugs with strong contraindications for rTMS because of their seizure threshold lowering potential. In contrast, drugs considered a relative contraindication according to a recent Consensus Conference and commonly used in PD such as selective serotonin reuptake inhibitors (n=3), anticholinergics (n=1) or occasional antihistamines (n=1) were not excluded.
Table 1. Motor and cognitive tests before and after 50 Hz rTMS testing.
Results (mean ± standard deviation [SD]) of cognitive and motor tests before and after 50 Hz rTMS testing and change of post-50 Hz rTMS testing to baseline (median, range, in percentage).
| Before (mean ± SD) | After (mean ± SD) | Change (median, range, in %) | p-value | ||
|---|---|---|---|---|---|
| MMSE | 29 ± 1.1 | 29.4 ± 0.7 | 0, −3.5 – 7.1 | 0.26 | |
| Verbal Fluency | letters | 13.7 ± 4.2 | 14.4 ± 4.1 | 0.9, −15.2 – 41.4 | 0.41 |
| category | 19.5 ± 6 | 21.8 ± 6 | −5.2, −21.2 –114.3 | 0.86 | |
| SRTT | visuomotor speed | 691 ± 240 ms | 645 ± 157 ms | 2.5, −12.9 – 24.5 | 0.21 |
| Grooved Pegboard | right hand | 117 ± 39.5 sec | 114.9 ± 38.7 sec | −1.3, −22.2 – 32.8 | 0.96 |
| left hand | 127.9 ± 48.2 sec | 134 ± 63 sec | −7.4, −26.1 – 19.7 | 0.44 | |
| Combined Movement | right hand | 12.4 ± 5.2 sec | 11.7 ± 5.0 sec | 3.4, −13.9 – 19.3 | 0.09 |
| left hand | 12.9 ± 5.6 sec | 12.4 ± 5.7 sec | 4.9, −30.9 – 13.7 | 0.09 | |
| 10-m walk | time | 8.2 ± 1.7 sec | 7.7 ± 2 sec | 5.5, −6.7 – 23.3 | 0.11 |
| steps | 15 ± 2.7 | 14.7 ± 2.4 | 0, −7.7 – 12.5 | 0.33 | |
| UDPRS III | motor score | 21.1± 5.9 | 21.7 ± 7.4 | −5.1, −16.7 – 27.8 | 0.48 |
MMSE Folstein: Mini-mental status examination; UDPRS III: Unified Parkinson’s disease rating scale, motor part; SRTT: serial reaction time test
We also excluded persons with pacemakers, brain stimulators, medication pumps or any type of metal object in the head including eyes except for dental appliances or fillings which might pose a physical hazard during rTMS.
The study was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board and the Food and Drug Administration (FDA) from whom an IDE was obtained. Written informed consent was obtained from all study patients.
Repetitive TMS procedures
Fifty Hz rTMS of the left primary motor cortex (M1) was performed using a circular 90-mm coil connected to a Magstim Rapid magnetic stimulator (Whitland, Dyfed, UK). A coil holder (Magstim, Whitland, Dyfed, UK) kept the coil in a constant position with reference to the patient’s head. The patient was seated in a comfortable chair in a reclined position; a head restraint was applied to prevent movements. The coil was placed at the optimal position (“hot spot”) over the left motor cortex for eliciting motor-evoked potentials (MEPs) in the right abductor pollicis brevis (APB), the target muscle, while avoiding stimulation of proximal arm muscles as much as possible. The position of the stimulating coil was marked on a cap which was tightly fixed and monitored throughout the experiment. Patients were tested while on medication and asked to relax during the testing. We refrained from testing when patients experienced significant dyskinesias or tremor precluding fixed positioning or relaxation. Patients wore ear plugs during the testing.
Individual resting motor threshold (RMT) of the APB was determined to the nearest 1% of the maximum stimulator output and defined as the minimal stimulus intensity required to elicit MEPs of >50 μV in at least 5 of 10 consecutive trials.
EMG activity was recorded from the target muscle APB, and also the extensor carpi radialis (ECR), biceps brachii (BB), and the deltoid (DEL) muscles of the right arm using silver-silver chloride surface EMG electrodes placed over these muscles in a belly-tendon montage. EMG amplitude was amplified using a conventional EMG machine (Nicolet Viking, Skovlunde, Denmark) and filtered with a bandpass between 100 Hz and 2500 Hz. The signal was digitized at a frequency of 5 kHz and exported into a computer for further off-line analysis.
Safety testing (EMG and EEG recording)
The testing consisted of consecutive 50 rTMS trains of increasing stimulation intensity and duration (60%, 70%, 80% and 90% of individual RMT intensity and 0.5, 1.0, 1.5, and 2 sec train duration corresponding to 16 combinations total and 1000 stimuli), starting at 60% RMT intensity and a train duration of 0.5 sec. Patients were closely monitored for any clinical signs which could indicate a seizure; EMG was continuously monitored for spread of excitation (SE) to more proximal muscles which were not activated by single-pulse TMS (ECR, BB and DEL) during rTMS trains, for post-rTMS EMG activity in the form of brief EMG bursts immediately after each rTMS trains and for increase in (single pulse) MEP amplitude between rTMS trains, which might indicate a significant increase of cortical excitability or even epileptic activity. We defined SE to be present if at least 4 MEPs emerged in the proximal muscles (ECR, BB and DEL) during the 50 Hz rTMS train when no visible MEP was identified after the first TMS pulse in those muscles. Since it was at times impossible to evoke MEPs from the APB muscle without inducing small MEPs in the ECR, in rare cases in BB and even in DEL, SE was considered present if MEP amplitude in ECR, BB and/or DEL increased by more than 100% of the baseline (Chen et al., 1997). On the other hand, MEPs in the target (APB) and other muscles which were activated by single-pulse TMS during the course of sub-threshold stimulation were considered to reflect facilitation which is present in normal subjects and not considered unsafe. Post-TMS EMG activity was considered pathological, i.e., to be a possible correlate of after-discharges, if one or more brief EMG bursts (BEB) of less than 50 ms and more than 100 μV in the 950 ms following a train were observed. These criteria were thought helpful in differentiating epileptic activity from rTMS-unrelated EMG activity resulting from tremor, rigidity, dyskinesias, poor muscle relaxation, or voluntary activity.
Before the first train of 50 Hz rTMS, baseline M1 excitability was determined with single- pulse TMS at 120% individual RMT (0.1 Hz for 5 min yielding 30 MEPs for averaging). Only if SE and BEBs were excluded by investigators’ consensus, and mean MEP amplitudes (at 120% RMT and 0.1 Hz) remained or, if increased, returned to baseline within 5 min after the preceding train, would the next rTMS train be applied. The first 50 Hz rTMS train was tested at 60% RMT and 0.5 sec train duration and then increased in 0.5 sec steps to 2 sec before increasing intensity by 10% and testing all durations from 0.5 to 2 seconds. Maximal stimulation intensity and duration was 90% RMT for 2 sec. SE, BEB and/or increased MEP amplitudes for more than 5 min were considered to indicate that stimulation exceeded safety limits. The safety limit would then be established as the set of parameters just below this and would not be exceeded in future experiments in the next patients.
An EEG was repeated within 10–30 min after the last 50 Hz rTMS train and reviewed by an experienced electroencephalographer to exclude epileptiform discharges, other pathological EEG phenomena or any changes compared to the pre-testing EEG which was done at study enrollment.
Assessment of cognitive and motor performance
To detect possible side effects, the following investigations were performed before and after the 50 Hz rTMS testing on the same day while on medication including the Unified Parkinson’s Disease Rating Scale (UDPRS). Bradykinesia was assessed with the timed Combined Movements Test which consists of performing 10 movements combining flexion-extension in the elbow joint with clenching and unclenching of the fist and a 10-meter walk. The neuropsychological test battery consisted of the Folstein Mini-Mental Status Examination (MMSE) and the Verbal Fluency Test (in the phonetic category with letters: F, A and S, or C, J, and M, and in the semantic category with animals, groceries, or tools; each for 60 sec and in a randomized and counterbalanced order across patients and conditions [pre- and post-testing]). The Grooved Pegboard Test was performed to test manipulative dexterity and complex visuomotor coordination with each hand, and the Serial Reaction Time Test (SRTT) to test visuomotor speed and (implicit) procedural learning. During the SRTT, the patient was instructed to press the corresponding key to the number (1–4) appearing on a screen as quickly and accurately as possible. The test consisted of seven blocks each with 120 numbers. The first and last blocks contained a random series of numbers, while the 2nd through 6th blocks contained the same sequence of 12 numbers which was repeated 10 times in each block. The reaction time (RT) and errors were recorded. For the analysis, the median of RT was chosen to minimize potential skewing within the results and the error rate (ER) calculated in relation to the total of responses (in percentage). Visuomotor speed was determined by the median RT during blocks 1 and 7 (random blocks). Procedural learning was measured by computing learning rate, defined as the reduction of RT in the repeated sequences during blocks 2–6, and the sequence-specific learning, defined as the difference in RT between blocks 7 (random) and 6 (last block with repeated sequences) (Muslimovic et al., 2007). Correspondingly, ER was determined as a measure of accuracy to look for a possible trade-off between speed and accuracy.
A 30% decrease in Verbal Fluency or a 30% increase in time to perform the Grooved Pegboard Test, the Combined Movements Test or the 10-m walk, in visuomotor speed (SRTT), or of the motor UPDRS (part III) score after the 50 Hz rTMS testing was considered a significant worsening and, therefore, a side effect of rTMS and required a re-testing within 24 hours, while the opposite finding might indicate a beneficial effect. A ≥ 30% pre- to post-testing change was determined significant taking into account variability in performance of tests and inherent in PD due to wearing-off, fluctuations and fatigue after testing for 2–3 hours. Procedural learning in the SRTT was considered present or absent.
Statistical analysis
Statistical analysis of clinical data was carried out with SPSS Version 12.0.1 for Windows. Data were checked for normal distribution (p>0.05 in the Kolmogorov-Smirnov test). Paired t-test was used to compare parametric data before and after 50 Hz rTMS testing, and Mann-Whitney U test for non-parametric data. Learning rate in the SRTT was analyzed using linear mixed effects models with autoregressive (1) covariance structure to take into account the correlations among the five measurements (blocks 2–6) per patient which precluded the comparison before and after 50 Hz rTMS testing. The threshold for significance was set at p <0.05.
Results
Safety testing
Mean individual RMT was 65.9 ± 13.9 % of the maximum stimulator output, ranging between 49 and 92 %. Since maximal output of the Magstim Rapid magnetic stimulator (Whitland, Dyfed, UK) is approximately 70 % when stimulating at 50 Hz for 2 seconds, higher individual RMT in a patient (92% of stimulator output) precluded testing at 80% RMT and instead, maximal intensities of 75% RMT was tested. Otherwise, all possible combinations of 50 Hz rTMS trains up to 90% of RMT intensity and 2 sec duration were consecutively tested except in a patient who withdrew after testing at 60% RMT intensity because of ipsilateral stimulation of the facial nerve caused by single-pulse TMS (before and between trains), but not by the 50 Hz rTMS trains which were well tolerated.
The 50 Hz rTMS testing was performed without any significant adverse event. Testing was performed at rest, but some background EMG activity in the APB, ECR, biceps and deltoid muscles, most commonly rest tremor (n=6) and lack of relaxation, were recorded in all patients for a variable duration during the continuous 2 – 3 hours of testing. This concurrent muscular activity may have facilitated occasional MEPs during sub-threshold 50 Hz rTMS trains in the APB and ECR and more rarely in the biceps. Facilitation only occurred in those muscles which were also stimulated by single-pulse TMS over the hot spot for the target muscle (APB) which were the ECR in all and the biceps in some, but never in the deltoid muscle.
Continuous EMG recording showed no correlates of either spread of excitation or of after-discharges which could indicate increased M1 excitability or even epileptic activity. Additionally, we neither observed sustained increase nor decrease of MEP amplitudes of the target muscle APB. Mean amplitudes at baseline of 1.9 ± 1.8 mV remained within the same range over the entire testing.
An exception to the lack of warning signs was a patient who would have normally been excluded because of bi-temporal spikes in the pre-testing EEG if they had not been erroneously misinterpreted as wicket spikes and considered a normal variant. During rTMS at 90% RMT intensity for 1 sec, the patient experienced a few clonic contractions of the contralateral hand, arm and leg muscles which stopped after cessation of stimulation while manifesting EMG correlates of SE in the biceps and deltoid muscles.
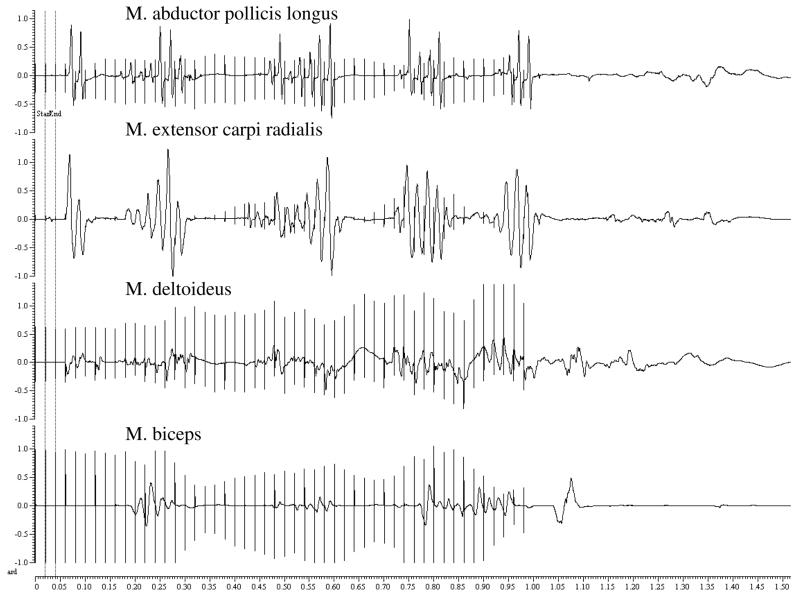
There were no BEBs after the stimulation train (see Figure 1), but residual muscle activity followed clonic contractions and superimposed EMG recording. In this patient, RMT of 60% corresponded to the median RMT (61%) in the group, and no increase of MEP amplitudes was observed between and after the 50 Hz rTMS trains, which would have indicated increased cortical excitability. Clinical observation strongly suggested SE also to leg muscles which were not monitored by EMG. We observed no any clinical nor EMG activity (BEBs) of seizure activity. A slight increase in frequency of left (side of the stimulation) temporal spikes was noted in the immediate post-testing EEG which returned to baseline activity with occasional bi-temporal spikes (left > right) 24 h later. This patient had initially reported no particular medical history and denied head trauma and seizures, and only later, mentioned a prior car accident some years ago with a blunt head trauma and possible loss of consciousness. At that time, brain injury had been excluded by a CT scan, and no sequelae were noted or further medical attention needed. Further testing was declined by the patient.
Figure 1.
Electromyographic (EMG) recording during 50 Hz rTMS at 90% RMT for 1 second in the patient with bi-temporal discharges. Here are shown EMG correlates of spread of excitation to M. biceps and M. deltoideus, but no correlates of “after-discharges” are distinguishable during residual muscle activity following clonic contractions.
No other adverse effects were observed and no possible TMS-related symptoms such as headache, lightheadedness, hearing change, tinnitus, vertigo, paraesthesia or weakness were reported. Some patients reported fatigue after the testing which lasted for several hours, but denied mood or other mental changes.
EEG
In addition to the patient with bi-temporal spikes, pre-testing EEG findings included diffuse delta activity during drowsiness in an older patient and slowing of the background activity (8 Hz) in another. Diffuse delta activity during drowsiness has been described in mild encephalopathy of various origins which remained undetermined in this otherwise healthy patient (Schaul et al., 1981). Slowing of background activity can be seen in PD (Soikkeli et al., 1991) and also may be attributed to intake of a selective serotonin re-uptake inhibitor (SSRI) (Bauer and Bauer, 2004). No changes were found in the immediate post-testing EEG.
Cognitive and motor testing
Cognitive and motor tests after 50 Hz rTMS testing yielded no statistical significant differences to baseline (see Table 1). None of the patients experienced a significant worsening (as defined by a >30 % lower performance) in any of the cognitive and motor tests after 50 Hz rTMS except for a single patient in the Combined Movements Test with the left (non-stimulated) hand (−30.9%). Mean results of cognitive tests pre- and post-50 Hz rTMS with standard deviations are given with the median and range of changes. Procedural learning was not observed in the SRTT. There was no learning across blocks 2–6 before or after 50 Hz rTMS (F(4,36)=0.39, p=0.81, and F(4,36)=0.69, p=0.6, respectively) and no sequence-specific learning (difference block 6 and 7; p=0.31, and p=0.38, respectively). Accuracy as determined by the ER remained also unchanged (across blocks 2–6 (F(4,36)=2.31, p=0.08, and F(4,36)=0.35, p=0.84, and p=0.51, and p=0.41 respectively). These results reflect consistency in overall performance in the SRTT and also with regard to speed and accuracy without evidence of trading-off increased speed with reduced accuracy or vice versa.
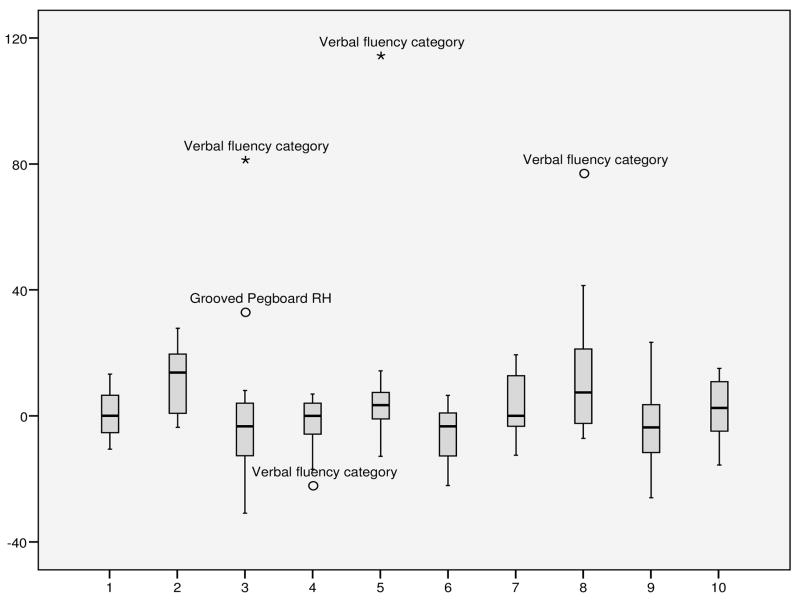
Pre- and post-50 Hz rTMS test performance across patients and across motor and cognitive tasks varied widely with both worsening and improvement in each patient although some showed large improvements in single tests (see Figure 2). The median change in all cognitive and motor test performances for each patient ranged between −3.7 and 13.7% and the median of all those medians amounts to 0% which would indicate no change in the global performance of the entire group.
Figure 2.
Median of changes in motor and cognitive tests of post-50 Hz rTMS testing to baseline (in percentage, outliers > 1.5 inter-quartile range [IQR]) in individual patients (1–10),. MMSE Folstein: Mini-mental status examination; UDPRS III: Unified Parkinson’s disease rating scale, motor part; SRTT: serial reaction time test
Discussion
The principal finding of this study is that 50 Hz sub-threshold rTMS at 90% RMT of the motor cortex for up to 2 sec in PD patients appears to be safe. Clinical and neurophysiological monitoring did not record signs of increased cortical excitability and seizure activity during or after consecutive 50 Hz rTMS trains of up to 100 stimuli and a cumulative total of 1000 stimuli applied over 2 h. Motor and cognitive tests showed neither significant worsening from pre-test baseline performance nor in any patient. Fifty Hz rTMS also appears to be safe in patients on SSRIs or anti-cholinergics, considered a relative contraindication for rTMS, which stringently applied would restrict the number of potential candidates.
To our knowledge, this is the first study to test the safety of 50 Hz rTMS in humans; this is double the stimulation frequency previously considered safe. Safety of low-intensity 50 Hz rTMS by means of short (5 and 15 stimuli) and repeated bursts in TBS in healthy subjects has been postulated, but these studies lacked EEG and EMG monitoring (Huang and Rothwell, 2004; Huang et al., 2005). For the opposite purpose of provoking a seizure in a presurgical evaluation of medically intractable temporal lobe epilepsy, suprathreshold (120% RMT) 50 Hz rTMS was applied for 1 sec over either frontal or temporal lobe of either side, but failed to induce a seizure and even reduced number of spikes and sharp waves (Jennum et al., 1994).
The purpose of this study to establish safety limits of rTMS in PD patients was to provide safe stimulation pattern for future therapeutic trials. Since experiences have been promising, we considered the potential benefits to outweigh the risk of seizure by rTMS. We tried to minimize this risk by approaching safety limits by stepwise increase of stimulation intensity and duration. We limited testing to PD patients since healthy subjects could not expect a benefit. Thus, these findings preclude drawing conclusions regarding current safety guidelines established in healthy subjects. Twenty and 25 Hz rTMS at suprathreshold (110 – 130 % RMT) intensity are within safety limits in healthy subjects, but not in patients with chronic stroke (Lomarev et al., 2007). Thus, extrapolating these findings to healthy subjects or other disease conditions could only be guided by knowledge of cortical excitability and mechanisms of epileptogenesis assuming similar responses to high-frequency rTMS in different conditions with similar physiology. Whether our findings suggest a higher seizure threshold in PD which could indicate decreased cortical excitability remains unknown. Safety of high-frequency rTMS at intensities below motor threshold has not yet been explored and review of neurophysiological studies indicate increased motor cortex excitability at rest and decreased facilitation with voluntary activity in PD (Cantello et al., 2002; Berardelli and Hallet, 2008). Several drugs used in PD can also decrease cortical excitability, foremost levodopa as well as some of the dopaminergic agonists (Ziemann et al., 1997; Ziemann, 2004). A few patients took GABA-ergic drugs such as diazepam (GABA A) and baclofen (GABA B) which enhance intra-cortical inhibition and reduce intra-cortical facilitation (Ziemann, 2004), and oxycodon, an opioid with sedative effects, whose effect on cortical physiology is unknown.
Although 50 Hz rTMS at the tested parameters appears safe in PD patients, caveats apply for any co-morbidities predisposing to seizures. The contralateral jerks with concurrent EMG correlates of spread of excitation indicated increased cortical excitability during 50 Hz rTMS which may lead to a seizure at higher intensities. The precise timing with the rTMS train and absence of those phenomena in the continuous recording for > 120 min at lower stimulation intensities strongly suggest 50 Hz rTMS to be the cause. This is supported by the temporary increase of spikes in the temporal lobe exclusively on the stimulated side which can result from rTMS (Hufnagel et al., 1990).
This event raises concerns for the safety of these stimulation parameters in patients with a similar condition. Those spikes were located distant from the stimulated motor cortex in the temporal lobes, but neurophysiological studies suggest the possibility that a distant epileptogenic focus can increase motor cortex excitability (Werhahn et al., 2000; Hamer et al., 2005). Apart from the spread of excitation, RMT and MEP amplitudes in this patient did not indicate increased cortical excitability, but other physiological measures had not been investigated. Repetitive and even single-pulse TMS of the primary motor cortex in focal epilepsy patients with an epileptic focus outside the motor cortex have been shown to provoke typical seizures (Schrader et al., 2004; Bae et al., 2007) and simple motor seizures (Dhuna et al., 1991; Michelucci et al., 1994). Subdural electrodes in patients evaluated for temporal lobe epilepsy surgery have shown that rTMS can activate an epileptic focus and even drive a non-active theta focus to a state of sustained epileptic activity (Hufnagel et al., 1990). Thus, 50Hz rTMS of the motor cortex most likely caused the jerking which was possibly facilitated by increased cortical excitability either resulting from a distant epileptic focus which was activated by the stimulation or a generalized brain disorder.
This study was based on the concept of a “safety threshold” (Pascual-Leone et al., 1993b; Pascual-Leone et al., 1994; Wassermann, 1998) which supposes that rTMS frequency, intensity and/or duration exceeding a certain threshold increases cortical excitability and ultimately induces a seizure. Spread of excitation with increasing stimulation frequency and intensity presumably indicates increased cortical excitability and has been observed preceding TMS-induced seizures (unpublished observation). SE is postulated to reflect lateral, i.e., intra-cortical propagation (Pascual-Leone et al., 1993b; Pascual-Leone et al., 1994). The pattern of muscle activation which follows their somatotopic representation and the delay in MEP latencies of proximal muscles compared to when stimulated over their optimal position has been argued to point to intra-cortical propagation. The precise mechanism is unknown. Lateral inhibitory circuits have been postulated to prevent focally increased cortical excitability and epileptic activity from propagating to surrounding cortex (Pascual-Leone et al., 1993b; Pascual-Leone et al., 1994). Another explanation postulates that the proximal arm muscles with a higher threshold are recruited only at a certain “amount” of stimulation. Brief EMG bursts (BEBs) after the rTMS train has ceased may be more specific for epileptic activity, but were not observed. BEBs are considered EMG correlates of after-discharges in the electro-corticogram (EcoG) and EEG after cortical stimulation which are a recognized sign of local epileptic activity (Ajmone Marsan, 1972; Wassermann, 1998). We did not observe increased MEP amplitudes after consecutive 50 Hz rTMS trains presumed to reflect increased excitability of the motor cortex. This is also in line with findings of a high-frequency rTMS study (5 Hz at 120 % RMT) in Parkinson’s disease which have been explained to reflect reduced facilitation of motor cortex (Gilio et al., 2002). However, we cannot exclude a short-lasting increase of MEP as reported in healthy subjects after 5-pulse sub-threshold 50 Hz burst (Huang and Rothwell, 2004), TBS for 2 sec (Huang et al., 2005) or 20 stimuli of supra-threshold 20Hz rTMS (Modugno et al., 2001) since test settings did not allow the first single TMS pulse to be applied within 10 sec after the 50- Hz rTMS train. On the other hand, a longer-lasting increase reported after 10 stimuli at 20 Hz was not observed (Pascual-Leone et al., 1994). Since we did not observe signs of increased excitability during rTMS, an interaction of successive stimulation trains is unlikely also considering a minimum inter-train interval of 2 min which is longer than inter-train intervals found to be safe (Chen et al., 1997).
Safety considerations constrained the order of testing instead of randomly applying rTMS trains of various stimulation intensity and duration. Random testing would have allowed to control for potential confounding effects such as the effect of cumulative test stimuli and the patient’s mental state anticipating increasing stimulation intensity/duration and anxiety which may influence cortical excitability (Palmieri et al., 1999; Wassermann et al., 2001).
We did not find significant mean declines in performance of motor and cognitive tests and none of the patients deteriorated in his/her performance in any of those tests. Performance varied widely in these tests across patients and also across tests within an individual patient. There was no distinguishable trend in either improvement or deterioration which suggests that these results reflect variability within a normal range. Fatigue after the lengthy testing may have contributed to a lower performance in some of the patients. In therapeutic rTMS studies in PD, motor performance has never been reported to decline after rTMS of the primary motor cortex but worsening in complex movements was seen in the suprathreshold high-frequency rTMS of the supplementary motor area (SMA) (Boylan et al., 2001). The same variability in general performance was also seen in the rate of improvement suggesting no immediate beneficial effects of rTMS, although some showed large improvements. This study was designed to detect adverse motor and cognitive effects of 50 Hz rTMS, but not to assess clinical benefit. Methodological differences in the study design, stimulation parameters, number of trains, stimuli, intervention sessions and assessment preclude comparison with therapeutic rTMS studies.
Cognitive abilities appeared preserved in all patients, but procedural learning in the SRTT before or after testing was impaired procedural as has been described in PD. The length of the repeating sequence could have contributed since shorter sequences of 8 and 10 are better learnt than 12 items used here (Pascual-Leone et al., 1993a). Prefrontal cortex is involved in procedural learning as its disruption by high-frequency suprathreshold rTMS of the dorsolateral prefrontal cortex (DLPFC) but not of the SMA suggests (Pascual-Leone et al., 1996). This finding, thus, indicates a decline of prefrontal functions which characterizes cognitive impairment in PD. Motor function assessed by visuomotor speed and accuracy in the SRTT remained unchanged after 50 Hz rTMS testing.
No other adverse events were observed. Mental or mood changes were explicitly denied, but we had not performed a more detailed psychiatric assessment, since they were not reported in motor cortex stimulation in PD patients.
We conclude that 50 Hz sub-threshold rTMS is safe and future therapeutic trials in PD patients are feasible, but these findings cannot be extrapolated to other disease conditions or to healthy subjects. Safety of longer train duration, suprathreshold intensity and multiple stimulation sessions remains to be established; stimulation of other (non-motor) targets such as the dorsolateral prefrontal cortex would require appropriate tests (Machii et al., 2006). Comprehensive screening of candidates for rTMS is indispensable and requires an EEG; moreover, patients need to be monitored during high-frequency rTMS by an experienced physician. Some drugs with relative contraindication for rTMS which are frequently taken in PD such as SSRIs and anti-cholinergics may not exclude study participation.
Acknowledgments
Acknowledgements to Elaine Considine, David Bates and David Prosper for help in the research, Sungyoung Auh for statistical analysis and Devee Schoenberg for skillful editing.
This research was supported by the Intramural Research Program of the NINDS, NIH, and in part by a grant from the USAMRMC (W81XWH-06-1-0534). E. H. was funded by the Fyssen Foundation.
Footnotes
Disclosure: The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajmone Marsan C. Focal electrical stimulation. In: Purpura DP, Penry JK, Tower DB, Woodbury DM, Walter RD, editors. Experimental models of epilepsy. Raven; New York: 1972. pp. 147–172. [Google Scholar]
- Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy & Behavior. 2007;10:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bauer G, Bauer R. EEG, drug effects, and central nervous system poisoning. In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography: basic principles, clinical applications, and related fields. Williams & Wilkins; Baltimore: 2004. [Google Scholar]
- Berardelli A, Hallet M. TMS in movement disorders. In: Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, editors. The Oxford Handbook of Transcranial Stimulation. Oxford University Press; Oxford: 2008. pp. 329–336. [Google Scholar]
- Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clinical Neurophysiology. 2001;112:259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Research Reviews. 2002;38:309–327. doi: 10.1016/s0165-0173(01)00158-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Classen J, Wassermann EM, Hallett M, Cohen LG. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electromyography and Motor Control-Electroencephalography and Clinical Neurophysiology. 1997;105:415–421. doi: 10.1016/s0924-980x(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Dhuna A, Gates J, Pascual-Leone A. Transcranial Magnetic Stimulation in Patients with Epilepsy. Neurology. 1991;41:1067–1071. doi: 10.1212/wnl.41.7.1067. [DOI] [PubMed] [Google Scholar]
- Gilio F, Curra A, Inghilleri M, Lorenzano C, Manfredi M, Berardelli A. Repetitive magnetic stimulation of cortical motor areas in Parkinson’s disease: Implications for the pathophysiology of cortical function. Movement Disorders. 2002;17:467–473. doi: 10.1002/mds.1255. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Reis J, Mueller HH, Knake S, Overhof M, Oertel WH, Rosenow F. Motor cortex excitability in focal epilepsies not including the primary motor area - a TMS study. Brain. 2005;128:811–818. doi: 10.1093/brain/awh398. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clinical Neurophysiology. 2004;115:1069–1075. doi: 10.1016/j.clinph.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Elger CE, Durwen HF, Boker DK, Entzian W. Activation of the Epileptic Focus by Transcranial Magnetic Stimulation of the Human-Brain. Annals of Neurology. 1990;27:49–60. doi: 10.1002/ana.410270109. [DOI] [PubMed] [Google Scholar]
- Jennum P, Winkel H, Fuglsangfrederiksen A, Dam M. EEG Changes Following Repetitive Transcranial Magnetic Stimulation in Patients with Temporal-Lobe Epilepsy. Epilepsy Research. 1994;18:167–173. doi: 10.1016/0920-1211(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Movement Disorders. 2006;21:2201–2205. doi: 10.1002/mds.21089. [DOI] [PubMed] [Google Scholar]
- Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Movement Disorders. 2006;21:325–331. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- Lomarev MP, Kim DY, Richardson SP, Voller B, Hallett M. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clinical Neurophysiology. 2007;118:2072–2075. doi: 10.1016/j.clinph.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clinical Neurophysiology. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Michelucci R, Valzania F, Passarelli D, Santangelo M, Rizzi R, Buzzi AM, Tempestini A, Tassinari CA. Rapid-Rate Transcranial Magnetic Stimulation and Hemispheric Language Dominance - Usefulness and Safety in Epilepsy. Neurology. 1994;44:1697–1700. doi: 10.1212/wnl.44.9.1697. [DOI] [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, Mackinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Experimental Brain Research. 2001;140:453–459. doi: 10.1007/s002210100843. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130:2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- Palmieri MG, Iani C, Scalise A, Desiato MT, Loberti M, Telera S, Caramia MD. The effect of benzodiazepines and flumazenil on motor cortical excitability in the human brain. Brain Research. 1999;815:192–199. doi: 10.1016/s0006-8993(98)01164-0. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural Learning in Parkinsons-Disease and Cerebellar Degeneration. Annals of Neurology. 1993a;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, Vallssole J, Brasilneto JP, Wassermann EM, Cohen LG, Hallett M. Safety of Rapid-Rate Transcranial Magnetic Stimulation in Normal Volunteers. Electroencephalography and Clinical Neurophysiology. 1993b;89:120–130. doi: 10.1016/0168-5597(93)90094-6. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Wassermann EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Experimental Brain Research. 1996;107:479–485. doi: 10.1007/BF00230427. [DOI] [PubMed] [Google Scholar]
- Schaul N, Lueders H, Sachdev K. Generalized, Bilaterally Synchronous Bursts of Slow Waves in the Eeg. Archives of Neurology. 1981;38:690–692. doi: 10.1001/archneur.1981.00510110050006. [DOI] [PubMed] [Google Scholar]
- Schrader LM, Stern JM, Koski L, Nuwer MR, Engel J. Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (TMS) in individuals with epilepsy. Clinical Neurophysiology. 2004;115:2728–2737. doi: 10.1016/j.clinph.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Soikkeli R, Partanen J, Soininen H, Paakkonen A, Riekkinen P. Slowing of EEG in Parkinsons-Disease. Electroencephalography and Clinical Neurophysiology. 1991;79:159–165. doi: 10.1016/0013-4694(91)90134-p. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Evoked Potentials-Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biological Psychiatry. 2001;50:377–382. doi: 10.1016/s0006-3223(01)01210-0. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Lieber J, Classen J, Noachtar S. Motor cortex excitability in patients with focal epilepsy. Epilepsy Research. 2000;41:179–189. doi: 10.1016/s0920-1211(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clinical Neurophysiology. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electromyography and Motor Control-Electroencephalography and Clinical Neurophysiology. 1997;105:430–437. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]