Abstract
Quantitative craniometrical traits have been successfully incorporated into population genetic methods to provide insight into human population structure. However, little is known about the degree of genetic and non-genetic influences on the phenotypic expression of functionally based traits. Many studies have assessed the heritability of craniofacial traits, but complex patterns of correlation among traits have been disregarded. This is a pitfall as the human skull is strongly integrated. Here we reconsider the evolutionary potential of craniometric traits by assessing their heritability values as well as their patterns of genetic and phenotypic correlation using a large pedigree-structured skull series from Hallstatt (Austria). The sample includes 355 complete adult skulls that have been analysed using 3D geometric morphometric techniques. Heritability estimates for 58 cranial linear distances were computed using maximum likelihood methods. These distances were assigned to the main functional and developmental regions of the skull. Results showed that the human skull has substantial amounts of genetic variation, and a t-test showed that there are no statistically significant differences among the heritabilities of facial, neurocranial and basal dimensions. However, skull evolvability is limited by complex patterns of genetic correlation. Phenotypic and genetic patterns of correlation are consistent but do not support traditional hypotheses of integration of the human shape, showing that the classification between brachy- and dolicephalic skulls is not grounded on the genetic level. Here we support previous findings in the mouse cranium and provide empirical evidence that covariation between the maximum widths of the main developmental regions of the skull is the dominant factor of integration in the human skull.
Keywords: evolvability, heritability, human skull, quantitative genetics
Introduction
The human skull is an important source of information for phylogenetic and population-genetic studies (Strait, 2001; González-José et al. 2003; Ackermann & Cheverud, 2004a,b). The complex morphology of the skull is usually decomposed in a series of craniometric measurements and it has been demonstrated that moderate amounts of genetic heritable variation underlie these traits (Sjøvold, 1984; Sparks & Jantz, 2002; Carson, 2006a). To some extent this suggests that skull morphology has substantial potential to evolve and that craniometric characters have the potential to provide consistent phylogenetic signals. Nevertheless, most studies have disregarded the integrated nature of the skull (Lieberman et al. 2000a,b; McCarthy & Lieberman, 2001; Bookstein et al. 2003; González-José et al. 2004; Bastir et al. 2004; Bastir & Rosas, 2004, 2005, 2006). Morphological integration can constrain the evolvability of traits (Merilä & Björklund, 2004) and bias the results of phylogenetic analysis (Strait et al. 2007; Lockwood, 2007; Sherwood et al. 2008).
Although the most appropriate approach to address this issue is to account for genetic and phenotypic covariation patterns of multivariate skull shape (Klingenberg & Leamy, 2001; Klingenberg, 2004, 2005), an alternative approach is to assess both the patterns of genetic variation and the correlation of univariate craniometric measurements. Here we explore the genetic architecture underlying the skull following this latter approach, which is relevant for evolutionary biology because craniometric traits are still in full use. For instance, recent studies have extensively applied population-genetics-based models using classical measurements (Roseman, 2004; Neves & Hubbe, 2005; Schillaci & Stojanowski, 2005; Harvati & Weaver, 2006). Our goal is to reconsider the evolutionary potential of craniometric traits, accounting both for their heritabilities and for the patterns of genetic and phenotypic correlation among them. Furthermore, we will test hypotheses of cranial integration formulated on the basis of these kind of traits (Enlow & Hans, 1996; Hallgrímsson et al. 2007).
Genetic variation in the human skull
The estimation of the genetic and non-genetic components underlying the phenotypic variation of the human skull has long been a major focus of anthropological research (Boas, 1912; Kohn, 1991; Varela & Cocilovo, 1999; Konigsberg, 2000). The first studies addressing this issue date back to the first decades of the 20th century (Dahlberg, 1926) but the interest increased at the end of the century because evolutionary biologists reconsidered the use of skeletal remains to unravel human microevolutionary paths (Relethford & Lees, 1982; Relethford, 1994). This new paradigm was built upon the growing evidence that suggested that human patterns of craniofacial variation reflected the underlying genetic patterns of variation (Cheverud, 1988; Buikstra et al. 1990). Craniometric traits were thus regarded as useful tools to study the structure and history of human populations (Relethford & Lees, 1982) and population-genetic models were adapted to be used after craniometric traits (Relethford & Blangero, 1990; Relethford, 2002, 2004). The heritability of complex metric traits, considered in the narrow sense, expresses the proportion of total phenotypic variance due to additive genetic variance (Falconer & MacKay, 1996). Heritability provides a measure of the proportion of variance in a trait explained by genetic transmission and is therefore a key parameter in models of evolution of quantitative traits (Konigsberg, 2000).
A wide range of studies have estimated the heritability of craniofacial traits (Vandenberg, 1962; Hiernaux, 1963; Nakata et al. 1974; Susanne, 1975, 1977; Sjøvold, 1984; Devor et al. 1986; Sharma, 1987; Sharma & Susanne, 1991; Konigsberg & Ousley, 1995; Nikolova, 1996; Sharma, 1998; Sparks & Jantz, 2002; Arya et al. 2002; Johannsdottir et al. 2005; Carson, 2006a). The general conclusion of these studies is that human craniofacial traits have moderate to high degrees of genetic variation. However, the comparison of results from different studies is controversial as they have been computed on very different kinds of samples (living humans or skeletal remains) from different geographical regions, accounting for different familiar relationships (twins, nuclear or extended families) and using different statistical methods (regression, anova, path analysis or maximum likelihood analysis (ML). ML methods are considered the most efficient methods to estimate genetic parameters in natural populations (Konigsberg, 2000). However, they have not been used until recently because they are computationally highly demanding (Roff, 1997).
Moreover, one of the main problems concerning the heritability estimation of cranial measurements in humans is that suitable, large and pedigree-structured skull series are almost non-existent. Such a collection of skulls with genealogical-associated data exists in Hallstatt (Austria), and has been previously studied to measure the heritability of metric and non-metric cranial traits (Sjøvold, 1984; Carson, 2006a,b). The work by Sjøvold (1984) was one of the first surveys of heritability on a human skull pedigreed series and the heritabilities of cranial traits were estimated using regression analysis. Sjøvold (1984) concluded that most of Howell's measurements were significantly hereditable and suggested that the structures showing the highest heritabilities were those connected to the size of the brain, the orbits, the nose and the masticatory apparatus. In a recent study, Carson (2006a) used an ML method to provide alternative estimates of the heritability of Howell's measurements. The main conclusion of this study was in agreement with Sjøvold's study and reported that craniometric traits show low to moderate narrow sense heritabilities. However, Carson (2006a) pointed out some differences and concluded that facial dimensions and cranial breadth measures are the less heritable characters of the skull. According to Carson (2006a) these differences stem from the different statistical techniques used for the heritability estimation.
The patterns of genetic variation of craniometric traits have thus been analysed previously, but the patterns of genetic correlation among them are nearly unexplored. This issue is of crucial importance because morphological integration is pervasive in the human skull (Lieberman et al. 2000a,b; Bookstein et al. 2003; González-José et al. 2004; Bastir et al. 2004; Bastir & Rosas, 2004, 2005, 2006) and integration between characters can limit the evolvability of traits and determine their evolutionary response (McGuigan, 2006).
Morphological integration in the human skull
Integration is expressed through covariation between traits and it plays a key role in the evolution of complex morphological structures such as the human skull, as it can enhance or constrain the evolution of its morphology towards certain directions of shape change (Klingenberg, 2004, 2005). Morphological integration assumes that functionally and/or developmentally related traits will be coinherited and will produce coordinate responses to evolution (Olson & Miller, 1958; Cheverud, 1982, 1984, 1995, 1996a).
The human skull comprises three regions with different developmental origins and functional requirements (Carlson, 1999): the cranial base, the cranial vault and the face. The cranial base is formed from endochondral bone that arises from a cartilaginous precursor originated from mesoderm (Mooney et al. 2002). The base supports the inferior parts of the brain as well as the pons, the medulla oblongata and the brain stem (Richtsmeier, 2002). The cranial vault is formed from membranous bone of paraxial mesodermal and neural crest origin and it gives room and protects the cerebral hemispheres and the cerebellum (Sperber, 2001). The facial skeleton ossifies intramembranously from neural crest precursors (Sperber, 2002) and it surrounds the pharynx as well as the oral, respiratory and orbital cavities, supporting the functions of feeding, breathing and vision. The cranial base is the most ancient structure and has been highly preserved through phylogeny (Carlson, 1999). Therefore, it is considered that the cranial base is under stronger genetic control than the cranial vault and the face (Schilling & Thorogood, 2000; Sperber, 2001). Moreover, it is assumed that the face is the most sensitive skull region to non-genetic factors because it plays a key role in foraging and adaptation to environment and because facial growth is more extended into the postnatal period (Siebert & Swindler, 2002).
The level of integration between these skull regions is a matter of current research. Most studies of morphological integration in the skull of mammals (Hallgrímsson et al. 2004, 2006; Goswami, 2006, 2007), non-human primates (Cheverud, 1982, 1995; Marroig & Cheverud, 2001; Hallgrímsson et al. 2004; Ackermann & Cheverud, 2004b) and humans (Lieberman et al. 2000a,b; McCarthy & Lieberman, 2001; Bookstein et al. 2003; González-José et al. 2004; Bastir et al. 2004; Bastir & Rosas, 2004, 2005, 2006) have considered integration at the phenotypic level. However, researchers have not identified yet which phenotypic units reflect morphogenetic units (Lieberman et al. 2004) and little is known about genetic integration and constraint in the functional and developmental regions of the skull.
The first studies of cranial integration in primates were developed by Cheverud (1982, 1995) and evidenced that functionally and developmentally related traits were in fact integrated. These findings provided support to the functional matrix hypothesis (Moss & Young, 1960), which predicts that covariation within functional units is stronger than covariation within individual bones or osseous subdivisions with different developmental/tissue origins. Afterwards, Hallgrímsson et al. (2004) reported that this functional/developmental pattern of craniofacial integration was consistent in rhesus macaques but not in mice. More recent studies of modularity in mammals (Goswami, 2006, 2007) and primates (Ackermann & Cheverud, 2004b) have identified six phenotypic cranial modules, corresponding to four functional regions of the face (namely the oro-nasal, the molar, the orbital and the zygomatic-pterygoid regions), one neurocranial region (the vault) and one basicranial region (the basicranium). The patterns of covariation within and among regions indicated that the face (the oro-nasal and the molar regions) and the cranial base were the highest integrated structures of the skull, whereas the cranial vault showed differing levels of integration across taxa. According to Ackermann & Cheverud (2004b), the zygomatic region is one of the main sources of facial integration in African apes and humans. Furthermore, they report that the loose integration of the cranial vault provided the skull with more capability to evolve in response to encephalization.
Other studies (Lieberman et al. 2000a,b; Bastir & Rosas, 2004) support the existence of two modules in the human skull, namely the face and the braincase. Lieberman et al. (2000a,b) consider that the basicranium and the neurocranium form a highly integrated morphological unit, the neuro-basicranial complex, which is partially independent from the face. However, Bastir et al. (2006) highlighted that the cranial base can not be interpreted as an integrated unit, at least at the ontogenetic level, as midline and lateral basicranial structures show different growth patterns. Further differences in growth may also explain the lack of integration between the braincase and the face: whereas the basicranium and the neurocranium grow jointly following a rapid neural trajectory (Bastir et al. 2006), facial growth extends more into the postnatal period and is more influenced by environmental factors (especially mechanical loadings). According to this, the face would be more prone to plastic responses (Kohn, 1991; Strand Vidarsdóttir et al. 2002; Bastir & Rosas, 2004), and it has been suggested that from the phylogenetic point of view, facial traits would not be as informative as neuro- and basicranial traits, which are more conservative and would reflect more reliably the underlying genetic patterns (Collard & Wood, 2000; Collard & O’Higgins, 2001).
In the primate skull, the cranial base appears to have a key integrative role (Lieberman et al. 2000a,b; Bookstein et al. 2003; Zollikofer & Ponce de León, 2004). Anatomically, it is a hinge-structure between the face and the cranial vault and developmental and growth studies support this view. Enlow & Hans (1996) suggested that the craniofacial architecture is based on a system of hierarchical modules organized into several craniofacial levels, in which the basicranium responds to modifications of the brain and translates them epigenetically into changes of facial proportions along a cerebro-mandibular gradient. Therefore, the base is the structural foundation that sets out the spatial development of the face and to some extent regulates the overall cranial development via integration with the brain and the cranial vault. Regarding human craniofacial variation, Enlow & Hans (1996) considered that there are two extreme headform types along a continuous spectrum: the dolicocephalic type, which is characterized by a long and narrow skull associated with a flat base and a supero-inferiorly longer face; and the brachycephalic type, in which a short and broad skull is associated with a more flexed cranial base and the face reveals a decreased anterior height and increased breadths. However, this traditional hypothesis of integration is not supported by developmental models of craniofacial biology (Lieberman et al. 2000a; Bastir & Rosas, 2004).
Recent experimental research using mice as animal models (Hallgrímsson et al. 2007) suggests that integration in the mammalian skull is highly structured following a hierarchical scheme that is dominated by strong covariation between the widths of the neurocranium and the basicranium and also with that of the face, but to a lesser extent. This study has further emphasized the stronger integration of the neurocranium and the basicranium with respect to the face, which is more independent but still covaries with the braincase (Hallgrímsson et al. 2007). After analysing the influence of epigenetic factors in craniofacial variation, the authors conclude that phenotypic variation arises from a few key developmental processes (such as brain growth) that channel the underlying genetic variation towards certain phenotypic expressions that maintain an integrated functional skull.
In the present study we reanalyse the pedigreed skull collection from Hallstatt (Austria) to explore the genetic patterns of variation determining the phenotypic expression of the skull and to assess the levels of correlation in craniometric characters. This will allow us to account for both the heritable and the integration patterns of the human skull. Here we test several hypotheses regarding these issues.
Hypotheses
Hypothesis 1 (H1) examines the heritability patterns of facial, neurocranial and basicranial dimensions and tests whether there are differences in the amounts of genetic variation underlying these regions. The null hypothesis states that there are no significant differences among the heritability of each region, whereas rejection of the null hypothesis indicates differential genetic contribution to the phenotype of each region, suggesting that they are subject to different evolvabilities and levels of plasticity.
Hypothesis 2 (H2) explores genetic and phenotypic patterns of correlation of specific suites of craniofacial traits within and among major and minor developmental/ functional regions of the skull. The null hypothesis implies no correlation between the genetic (G) and phenotypic (P) matrices; that is, the patterns of phenotypic correlation do not reflect the genetic ones and show different strengths of morphological integration. The null hypothesis is rejected if the correlation of G and P is high and significant, which would suggest that genetic and environmental effects on development produce similar patterns of phenotypic variation. Thus, in those cases where G is not available, P could be used as a good proxy to G in population quantitative genetic models (Cheverud, 1988).
Hypothesis 3 (H3) tests the traditional hypothesis of integration of the human skull (Enlow & Hans, 1996). Under this hypothesis, maximum cranial breadth should be positively correlated with facial breadth and negatively correlated with facial height, neurocranial length and neurocranial height. The null hypothesis is rejected if the observed patterns of correlation between these pairs of distances do not fit the expected patterns of integration.
Hypothesis 4 (H4) tests whether the overall pattern of genetic integration in the human skull is dominated by the covariation between the maximum widths of the major developmental regions, namely, the face, the neurocranium and the basicranium. This hypothesis was put forward by Hallgrímsson et al. (2007), who investigated the influence of epigenetic factors in the patterns of morphological integration of mice skull. The null hypothesis predicts that the genetic correlations between facial, neurocranial and basicranial width are high and significant.
Materials and methods
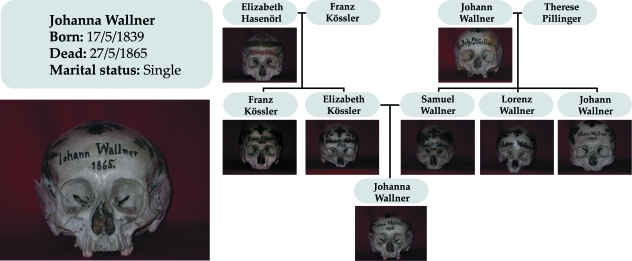
The sample examined here derives from the Hallstatt skull collection, which is a large sample of human skulls with identified familial relationships. It provides the unusual opportunity to perform quantitative genetic analysis in a human skeletal sample. This unique collection is made up of more than 700 decorated skulls that have been accumulating in the charnel house of Hallstatt from the beginning of the 18th century. It stems from a local tradition to honour predecessors (Burgstaller, 1961). On the request of the families, the gravedigger recovered the skeletal remains of their relatives, decorated their skulls with floral paintings and wrote the name of the individual on them (Fig. 1). This custom was widespread in Austrian and German regions surrounding the Alps (Sauser, 1952), but Hallstatt is the only place where it has provided such a large skull series and has endured for so long, the last skull being incorporated in 1996. The series covers a temporal span of more than 250 years, but most of the identified skulls date back to the 19th century.
Fig. 1.
Example of genealogy. The original name (Johanna Wallner) was misspelled (Johann Wallner) when the skull was repainted because of the fading decoration. Sjøvold's photographic records dating back from the seventies revealed the original name. In fact, discriminant analysis on sex confirmed that the skull belonged to a female.
Skull identification and genealogy reconstruction
The name and type of decoration of the skulls allowed us to identify at the parish demographical records almost 60% of the individuals. To reconstruct the genealogies of the Hallstatt population, we compiled the complete parish records of births, deaths and marriages from 1602 to 1900, a total of 18 134 individuals. The most complete families range back up to seven generations, including all kinds of familial relationships from first to fourth degree (Fig. 1). Most of the identified skulls are preserved at the charnel house in Hallstatt (n = 374), but a few of them are on loan to several Austrian Museums: the Musealverein in Hallstatt (n = 3), the Naturhistorisches Museum Wien (n = 17), the Österreichisches Museum für Volkskunde in Vienna (n = 1), and the Anatomisches Institut in Innsbruck (n = 11). Since the first surveys carried out by Sjøvold in 1974 and 1975, 25 identified skulls have disappeared from the charnel house and the names of several individuals have been changed because of recent renewed decoration (Fig. 1).
Morphometric analyses
In this study, we analysed a sample of 355 complete adult skulls of both sexes (144 females and 211 males), 317 of which fall into the extended, multigenerational genealogies. From the total sample of identified skulls (n = 406), subadult (n = 35) and fragmentary individuals (n = 16) were excluded to avoid sample bias. Adulthood was assessed by skeletal criteria, as determined by a fully closed spheno-occipital synchondrosis.
A set of 65 anatomical landmarks (Table 1) was recorded on each skull with a 3D digitizer (Microscribe, Inc.) in two consecutive recordings because it was impossible to access all the landmarks from a single orientation. The two recordings were matched automatically using a custom reference frame in the MUS software (Microscribe Utility Software) defined by three landmarks (nasion, bregma and hormion). Using this reference frame, landmark coordinates were also oriented along the sagittal plane. This means that sagittal landmarks had a z coordinate equal to 0, and symmetrical right and left landmarks only differed in the sign of the z coordinate, one being negative and the other one positive (for more details see Martínez-Abadías, 2007).
Table 1.
List of digitized landmarks. Codes and definitions used are provided (r, right; l, left)
| Code | Landmark | Definition |
|---|---|---|
| aam l | Anterior auditory meatus | Most anterior point at the external auditory meatus |
| al r/l | Alare | The most lateral point on the margin of the nasal aperture |
| alv l | Alveolar point | Posterior limit of the maxillary alveolar arch at the pterygo-alveolar suture |
| ast r/l | Asterion | The point where the lamboidal, parietomastoid, and occipitomastoid sutures meet |
| b | Bregma | The ectocranial point where the coronal and sagittal sutures intersect |
| ba | Basion | The midline point on the anterior margin of the foramen magnum |
| ek r/l | Ectoconchion | The most lateral and posterior point on the orbital margin |
| eu r/l | Euryon | The point of greatest breadth of the brain case perpendicular to the sagittal plane |
| fmo r/l | Frontomalare orbitale | The point where the frontozygomatic suture intersects the orbital margin |
| fmt l | Frontomalare temporale | The point where the frontozygomatic suture crosses the temporal line |
| ft r/l | Frontotemporale | The point where the temporal line reaches its most anteromedial position |
| g | Glabella | The most anterior midline point on the frontal bone, above the frontonasal suture |
| gle l | Glenoid fossa | The most posterior point on the margin of the glenoid fossa |
| ho | Hormion | The most posterior midline point on the vomer |
| i | Inion | Ectocranial midline point at the base of the external occipital protuberance |
| iam l | Inferior auditory meatus | Most inferior point at the external auditory meatus |
| izt l | Inferior zygo-temporal | Inferior point at the suture between temporal and zygomatic bones |
| ju r/l | Jugale | Depth point of the notch between the temporal and frontal processes of the malar |
| l | Lambda | Midline point of the intersection of the sagittal and lamboidal sutures |
| m | Metopion | Midline point where the elevation above the chord from n to b is greatest |
| mf r/l | Maxillofrontale | The point where the anterior lacrimal crest meets the frontomaxillary suture |
| ms l | Mastoidale | The most inferior point on the mastoid process |
| mw r/l | MW | Tip of the process at the infratemporal crest |
| n | Nasion | The midline point where the two nasal bones and the frontal intersect |
| nar r/l | Nariale | The most inferior point on the nasal aperture |
| o | Opisthion | The midline point at the posterior margin of the foramen magnum |
| oc l | Optic canal | Most superior, medial and anterior point of the optic canal |
| op | Opisthocranion | The posterior-most point of the skull in the medial sagittal plane |
| or r/l | Orbitale | The lowest point on the orbital margin |
| pam l | Posterior auditory meatus | Most posterior point at the external auditory meatus |
| pns | Posterior nasal spine | Vomer–palatin junction |
| po l | Porion | The uppermost point on the margin of the external auditory meatus |
| pt r/l | Pterion | The point where the frontal, parietal, temporal and sphenoides bones meet |
| ra r/l | Radicular | Lateral point on zygomatic process of the temporal bone at the postglenoid |
| ss | Subspinale | The deepest point seen in the profile below the anterior nasal spine |
| stf l | Stylomastoid foramen | Stylomastoid foramen |
| szt l | Superior zygo-temporal | Superior point at the suture between temporal and zygomatic bones |
| v | Vertex | Midsagittal superior point of the cranium when the skull is in Frankfurt |
| zy r/l | Zygion | The point of maximum lateral extent on the surface of the zygomatic arch |
| zym r/l | Zygomaxillare | The most inferior point of the zygomaticomaxillary suture |
| zyma r/l | Zygomaxillare anterior | The most anterior point on the zygomaticomaxillary suture |
| zyo r/l | Zygoorbitale | The point where the orbital rim intersects the zygomaticomaxillary suture |
From the total set of landmarks, five landmarks from the alveolar region (prosthion, inner prosthion, ectomolare right and left, and palate) were removed because they were missing in more than 50% of the cases due to tooth loss and high levels of alveolar bone resorption.
Measurement error was evaluated by a repeated recording of a subsample of 91 individuals each of which were digitized twice. Analysis of shape variation (anova on repeated measurements implemented with SAS) showed that the component of variation among individuals is 11.5 times the component of variation between repeated measurements. Thus, repeatability (the proportion of variance due to individual differences rather than measurement error) is 92%. Outlier points were detected by means of Box and Whisker plots assuming an outlier coefficient of 1.5. These points were deleted and considered missing data.
The overall percentage of missing values was 2.18%, and these were replaced by two different methods. If the missing landmark had a symmetric counterpart (as for example the asterions), it was directly replaced by coordinate reflection. As landmark coordinates were oriented along the sagittal plane, this was done by copying the x, y, z coordinates of the symmetric landmark and changing the sign of the z coordinate. If missing landmarks did not have a symmetric counterpart, they were replaced by multivariate regression.
To validate the individual identifications made by the gravediggers who decorated the skulls, we confirmed their sex assignment by performing a discriminant function analysis, considering sex as the discriminant variable. It is acknowledged that this is just a partial appraisal, as it only detects those cases in which a female and a male have been confused and neglects those cases where two individuals of the same sex may have been exchanged. Other kinds of errors are almost impossible to detect, but they are expected to be negligible because if any identification mistake was made, the most probable is that members of the same family buried within the same grave were confused. Thus, their influence is expected to produce slight underestimates of the additive genetic component of the morphological phenotype. Results showed that only eight skulls have an overall posterior probability higher than 0.85 of being the opposite sex. These individuals were considered misidentifications and were not taken into account for the estimation of the genetic parameters.
Finally, we estimated 58 linear inter-landmark distances from the three-dimensional landmark coordinates by applying the Pythagorean theorem. The linear distance between two landmarks is estimated using the following formula:
where (x1, y1, z1) are the landmark coordinates of landmark 1, and (x2, y2, z2) the landmark coordinates of landmark 2. Of these distances, 24 correspond to Howell's measurements (Howells, 1973) or are close approximations to them (i.e. the prosthion is substituted by the subspinale). The distances were assigned to the three major regions of skull, which have different developmental origins: the face, the neurocranium and the basicranium (Cheverud, 1995; Hallgrímsson et al. 2004, 2007). Distances within the face were also assigned to minor functional regions, such as the nasal, the orbital and the zygomatic regions (González-José et al. 2005; Sardi & Ramírez-Rozzi, 2007). Distances covering several regions were grouped into another category, the inter-regional dimensions.
All these calculations were performed with statistica 6.0 software package (Statsoft, Inc.).
Quantitative genetic analyses
Maximum likelihood methods were used to estimate the components of variance of the cranial measurements. With these estimates, the narrow sense heritability of each distance was computed as the proportion of phenotypic variance attributable to additive genetic effects (Lynch & Walsh, 1998). ML methods are usually applied under a mixed linear model that jointly accounts for fixed and random effects to describe the phenotype of each individual (Lynch & Walsh, 1998). The phenotypic variance is broken down into its components of additive genetic value and other random environmental and fixed effects. The components of variance are estimated by an iterative procedure that maximizes the likelihood of observing the actual data (Lynch & Walsh, 1998). ML analytical methods are advantageous compared with parent–offspring regression or sib analyses because they incorporate multigenerational information from unbalanced datasets (Konigsberg, 2000).
We computed the variance components of the craniometric traits using the solar 4.0.4 software package (Almasy & Blangero, 1998), which is available online at http://www.sfbr.org/solar/. solar provides estimates of the additive genetic variance and the variance of the residual errors, and computes the narrow sense heritability of the analysed traits. The significance of the heritability estimates is tested using likelihood ratio tests, in which the obtained likelihood of the model with the additive genetic variance component and covariates is compared with the obtained likelihood of the model with the additive genetic variance component constrained to be zero.
The mixed model used in this study included sex, year of birth and the interaction of sex and year of birth as covariates. Moreover, as 12.4% of the individuals showed slight dysmorphologies possibly related to craniosynostosis, deformation was also considered as a covariate. This kind of dysmorphology (occipital flattening and prominent forehead) was also reported in a very similar skull sample from Berg (Austria) (Howells, 1989). This author pointed to cradling practices as possible causes of these deformations, but did not rule out other non-artificial or genetic effects.
solar tested the significance of each covariate and computed the amount of variation explained by the significant ones at the P < 0.10 level. This is done by comparing the likelihood of a model that included each of the covariates separately with the likelihood of a model that excluded them. The final model used to compute the narrow sense heritabilities of the traits only retained the significant covariates.
Before analysis, the traits were inverse normalized using the ‘inormal’ procedure implemented in solar to avoid potential problems of kurtosis of continuous metric traits. Inverse normalization forces marginal (univariate) normality, but not bivariate normality. As all traits have a mean near 0.0 and a standard deviation near 1.0 after normalization, the variances and covariances are no longer available and only patterns of heritability and correlations can be explored. To compute the inverse normalization, the trait values are sorted from the minimal to the maximal value, and for any value a quantile is computed for it by the formula I/(N + 1), where I is the position in the sorted list and N is the total number of observations of the list. For example, the skull with the smallest value of a given measurement would receive a score of 0.0028 [the quantile 1/(355 + 1)], whereas the skull with the highest score, a score of 0.9971 [355/(355 + 1)]. The inverse normal cumulative density function is computed for each quantile and stored in an array keyed by the identification number of the skull. When different individuals show the same value of a measurement, the inverse normal is computed for each applicable quantile. Then the inverse normal is averaged and this average is the value that is stored for each individual.
To test whether there are differences in the amounts of genetic variation at each region (H1), we performed a two-tailed t-test that compared the average heritability estimations of the three sets of measurements (representing the facial, neurocranial and basicranial regions). To analyse the genetic and phenotypic correlation patterns of the skull (H2–H4), we computed the correlation between all possible pairs of distances of maximum breadth, height and length within and among major and minor developmental/functional regions of the skull. To estimate the genetic correlation between pairs of distances we used the solar bivariate models. The bivariate models work similarly to the univariate models, but also estimate the correlation between two traits: the phenotypic correlation is broken down into genetic and residual correlations (Lynch & Walsh, 1998). To estimate the phenotypic correlation we calculated the parametric Pearson's correlation using statistica 6.0. The correlations of each of the trait pairings were computed separately and then the correlation matrices were constructed manually in an excel spreadsheet.
To test the similarity between the genetic and the phenotypic correlation matrices (H2) we used a matrix correlation (Cheverud, 1988) and assessed its significance with a Mantel test (Mantel, 1967) after 100 000 permutations of the original matrices. According to Cheverud (1988), the level of heritability influences the similarity between genetic and phenotypic correlation patterns: if heritability is high it increases both the accuracy of the genetic correlation estimates and the similarity of G and P; if it is low or moderate, the accuracy is reduced and similarity of G and P suggests that genetic and epigenetic factors are channelled through the same developmental process. In this latter case, the levels of genetic correlation usually exceed that of phenotypic correlations. To assess the reliability of the genetic correlation estimates, we measured the effective sample size (Nes) used in our analyses, as suggested by Cheverud (1988). This is a rough measure of the actual sample size and is derived as the product of the number of nuclear families on which the estimation of the genetic parameters and the mean heritability of the traits are based. Previous evidence suggests that an effective sample size of at least 40 should be used to guarantee the reliability of the data (Cheverud, 1988).
Finally, to test the integration hypotheses (H3 and H4) we compared the expected patterns of genetic correlations between the involved measurements with the observed ones. The null hypothesis is rejected if the observed patterns of correlation between these pairs of distances do not fit the expected patterns of integration.
Results
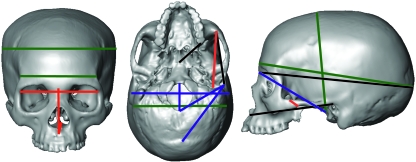
The univariate maximum likelihood estimates of heritability of facial, neurocranial and basicranial dimensions are presented in Tables 2–5. As a summary of these results, we present in Fig. 2 the five most heritable traits of each region. The obtained heritabilities are comparable between regions because the estimation of the phenotypic and genetic variance components was always based on the same number of individuals. Results show that craniofacial traits are low to moderate heritable characteristics. Heritability values ranged from 0.00 to 0.43, and 72.2% of them were significant at the 0.05 level. Regarding the regional patterning of heritabilities, the face is the skull region with a highest number of significantly heritable traits (81%) and the highest mean heritability (0.26), followed by the basicranium (73% and 0.23) and the neurocranium (61.5% and 0.19). The percentage of significant heritability estimates within the inter-regional dimensions was 50%. Despite these slight differences, there is no clearcut difference among regions and the t-test showed that the comparisons of the genetic amounts of variation among regions were not statistically different. The statistical significance of the differences between the average heritability of the three regions was as follows: facial vs. neurocranial (P = 0.053); facial vs. basicranial (P = 0.433); and neurocranial vs. basicranial (P = 0.336). Therefore the H1 null hypothesis cannot be rejected.
Table 2.
Facial dimensions: Narrow-sense heritability estimations (h2) and associated standard errors (SE). Statistical significant estimations (α = 0.05) are in bold. The proportion of variation explained by the significant covariates (α = 0.1) is also provided. Each measure corresponds to the distance between two landmarks (see Table 1 for definitions) and has been assigned to a minor function region within the face (although some distances may cover several regions)
| Distance | Covariates | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Landmarks | Howells | h2 | SE | P | Variance explained | Significant covariates | |
| Total | height | ss>n | NPH* | 0.34 | 0.13 | 0.002 | 0.15 | sex |
| length | ss>ba | BPL* | 0.32 | 0.12 | 0.001 | 0.14 | sex | |
| breadth | zy r>zy l | ZYB | 0.28 | 0.13 | 0.008 | 0.43 | sex, year birth | |
| Orbital | breadth | fmor r>fmo l | FMB | 0.40 | 0.13 | 0.001 | 0.19 | sex |
| length | or l>oc l | 0.35 | 0.14 | 0.004 | 0.06 | sex | ||
| breadth | ek r>ek l | EKB | 0.34 | 0.14 | 0.005 | 0.17 | sex, year birth | |
| breadth | mf r>mf l | DKB* | 0.33 | 0.13 | 0.003 | 0.05 | sex | |
| height | or l>fmo l | 0.29 | 0.14 | 0.015 | − | − | ||
| breadth | mf l>ek l | OBB* | 0.28 | 0.14 | 0.013 | 0.07 | sex | |
| Nasal | height | n>nar r/l | NLH | 0.43 | 0.13 | 0.000 | 0.13 | sex |
| length | ss>pns | 0.38 | 0.14 | 0.001 | 0.19 | sex, year birth | ||
| breadth | al r>al l | NLB | 0.00 | 0.00 | 0.500 | 0.05 | sex, year birth | |
| Zygomatic | height | szt l>izt l | 0.38 | 0.13 | 0.001 | 0.11 | sex, deformation, year birth | |
| length | zym l>gle l | 0.37 | 0.12 | 0.000 | 0.15 | sex, year birth | ||
| height | zyma l>fmo l | 0.34 | 0.13 | 0.004 | 0.10 | sex | ||
| length | izt l>mw l | 0.28 | 0.11 | 0.002 | 0.15 | sex | ||
| height | zym l> or l | WMH | 0.24 | 0.12 | 0.014 | 0.13 | sex | |
| height | zyo l>fmo l | 0.23 | 0.14 | 0.029 | 0.32 | sex | ||
| length | or l>izt l | 0.22 | 0.12 | 0.024 | 0.17 | sex, year birth | ||
| length | fmo l>fmt l | 0.22 | 0.12 | 0.020 | 0.04 | sex | ||
| length | zyma l>izt l | IML | 0.22 | 0.13 | 0.037 | 0.13 | sex, deformation, year birth | |
| length | zyo l>izt l | XML | 0.20 | 0.11 | 0.018 | 0.23 | sex, deformation, year birth | |
| breadth | ju r>ju l | JUB | 0.19 | 0.13 | 0.071 | 0.38 | sex, year birth | |
| height | zyo l>zyma l | 0.09 | 0.10 | 0.143 | 0.13 | sex | ||
| breadth | zymar>zyma l | ZMB | 0.07 | 0.10 | 0.232 | 0.23 | sex, year birth | |
| height | or l>zyma l | 0.03 | 0.10 | 0.364 | 0.13 | sex | ||
A close approximation to the exact Howell's measurements (1973).
Table 5.
Inter-regional dimensions: Narrow-sense heritability estimations (h2) and associated standard errors (SE). For coding details see Table 2
| Distance |
Covariates |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Landmarks | Howells | h2 | SE | P | Variance explained | Significant covariates | |
| Other | length | zym l>ra l | 0.34 | 0.12 | 0.001 | 0.19 | sex | |
| length | n>op | NOL | 0.34 | 0.13 | 0.001 | 0.15 | sex | |
| length | po l>ss | 0.32 | 0.12 | 0.003 | 0.24 | sex, year birth | ||
| breadth | ho>alv l | 0.29 | 0.16 | 0.034 | 0.30 | sex | ||
| height | op>i | 0.13 | 0.12 | 0.116 | 0.04 | sex, deformation | ||
| length | n>b | FRC | 0.11 | 0.12 | 0.161 | 0.14 | sex, deformation | |
| length | po l>n | 0.07 | 0.11 | 0.267 | 0.21 | sex, year birth | ||
| height | po l>b | 0.03 | 0.12 | 0.383 | 0.22 | sex, deformation | ||
Fig. 2.
Frontal, lateral and inferior views of a human skull showing the cranial dimensions with higher heritabilities. Colours indicate dimensions from the facial (red), neurocranial (green), and basicranial (blue) regions. Inter-regional dimensions are depicted in black.
Regional heritability estimations
Total facial dimensions (maximum facial breadth, length and height) have moderate heritabilities, showing that additive genetic variation accounts for approximately 30% of the phenotypic variation of these traits (Table 2). Minor functional regions within the face show diverse patterns of genetic variation: the orbital and the nasal regions show some of the highest amounts of genetic variance and thus the highest heritabilities of the skull (Fig. 2), whereas the zygomatic or masticatory apparatus tend to show lower estimates (Table 2). The mean heritabilities of the nasal, orbital and zygomatic regions are 0.27, 0.33 and 0.22, respectively. Total breadth, length and height orbital measurements show moderate to high significant heritability estimates. Other breadth measures such as the bi-orbital breadth and the interorbital breadth also show moderate heritabilities. Nasal height and length show high heritability estimates, but nasal breadth shows no additive genetic variance at all (Table 2). The t-test comparison for functional facial regions showed that the orbital region is significantly more heritable than the zygomatic (P = 0.044). Further comparisons did not provide any significant differences.
Total neurocranial dimensions (Table 3) also have moderate significant heritabilities. The anterior breadth measure and the maximum cranial breadth measure have indeed high estimates (Fig. 2). The other neurocranial measurements tend to show low heritability estimates, whereas Howell's chord distances show no genetic variation at all. All neurocranial breadth measures are significantly heritable, except the distance between pterions.
Table 3.
Neurocranial dimensions: Narrow-sense heritability estimations (h2) and associated standard errors (SE). For coding details see Table 2
| Distance | Covariates | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Landmarks | Howells | h2 | SE | P | Variance explained | Significant covariates | |
| Total | breadth | eu r>eu l | XCB | 0.36 | 0.14 | 0.002 | 0.17 | sex, year birth |
| length | g>op | GOL | 0.31 | 0.12 | 0.002 | 0.18 | sex | |
| height | b>ba | BBH | 0.24 | 0.12 | 0.016 | 0.18 | sex, deformation | |
| Other | breadth | ast r>ast l | ASB | 0.23 | 0.14 | 0.034 | 0.05 | sex |
| breadth | ft r>ft l | 0.23 | 0.12 | 0.024 | 0.07 | sex | ||
| length | m>b | 0.22 | 0.12 | 0.020 | ||||
| breadth | pt r>pt l | 0.21 | 0.15 | 0.072 | 0.13 | sex, year birth | ||
| height | g>m | 0.20 | 0.12 | 0.031 | 0.16 | sex, deformation | ||
| length | v>l | 0.19 | 0.12 | 0.043 | 0.03 | sex | ||
| breadth | mw r>mw l | WCB* | 0.16 | 0.11 | 0.050 | 0.05 | sex | |
| length | b>l | PAC | 0.06 | 0.10 | 0.262 | 0.07 | sex | |
| height | l>op | OCC | 0.04 | 0.12 | 0.379 | 0.02 | sex, year birth | |
| length | b>v | 0.00 | 0.00 | 0.500 | ||||
The heritability estimates of the basicranial region (Table 4) were moderate and significant, except for the distances between the inion and the opisthion, the mastoid height and the otic height. The length of the foramen magnum as well as the auricular breadth showed some of the highest heritability estimates, whereas total cranial base length and height show more moderate estimates (Fig. 2).
Table 4.
Basicranial dimensions: Narrow-sense heritability estimations (h2) and associated standard errors (SE). For coding details see Table 2
| Distance |
Covariates |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Landmarks | Howells | h2 | SE | P | Variance explained | Significant covariates | |
| Total | breadth | ba>po l | 0.29 | 0.12 | 0.005 | 0.20 | sex | |
| length | n>ba | BNL | 0.24 | 0.10 | 0.003 | 0.17 | sex | |
| Other | breadth | ra r>ra l | AUB* | 0.40 | 0.12 | 0.000 | 0.19 | sex |
| length | o>ba | FOL | 0.38 | 0.13 | 0.001 | 0.13 | sex | |
| breadth | i>po l | 0.27 | 0.14 | 0.011 | 0.17 | sex, year birth, sex*year birth | ||
| breadth | adml>pam l | 0.23 | 0.14 | 0.030 | − | − | ||
| breadth | po l>ho | 0.21 | 0.14 | 0.049 | 0.22 | sex, year birth | ||
| breadth | ba>ho | 0.20 | 0.12 | 0.034 | 0.04 | sex | ||
| length | i>o | 0.16 | 0.12 | 0.080 | 0.02 | year birth | ||
| height | ms l>stf l | 0.15 | 0.12 | 0.081 | 0.15 | sex | ||
| height | po l>iam l | 0.00 | 0.12 | 0.486 | 0.07 | sex, deformation, year birth | ||
Finally, inter-regional dimensions show two different patterns (Table 5). Those distances that mostly cover the face show moderate and significant heritabilities, whereas those mostly covering the cranial vault tend to show low and non-significant estimates, although one exception is the distance from the nasion to the opisthocranion (Fig. 2).
Regarding the covariates included in the analyses (Tables 2–5), sexual dimorphism was the most important effect as it affected more than 90% of the measurements, especially the facial ones. The second most important effect was the temporal span of the sample, which could be reflecting morphological secular trends: year of birth significantly affected facial (41.7%), neurocranial (25%) and basicranial dimensions (40%). Finally, deformation had a smaller effect but significantly affected three facial dimensions, two neurocranial, one basicranial and three inter-regional dimensions. The joint effect of sex and year of birth only influenced one measurement from the cranial base.
Genetic and phenotypic integration
Our results show that the observed genetic and phenotypic correlation patterns of skull integration are consistent in our sample. The matrix correlation between G and P was high (r = 0.74) and the Mantel test revealed that it was highly significant (P < 0.000). Thus, we reject the H2 null hypothesis, which expected independence between these matrices. This suggests that P can be used as a good proxy of G. However, a closer look at the correlation matrices (see Appendix 1) reveals that genetic integration is more constrained to specific dimensions, whereas phenotypic integration is more widespread throughout the skull. Almost all phenotypic correlations were highly statistically significant, even when the correlation was low. Genetic correlations were usually higher than the phenotypic ones, but few of them were statistically significant due to large standard errors. This was an expected result as heritabilities were all low to moderate. To confirm that the genetic correlations were well estimated, we computed the effective sample size (Nes) and we found that it exceeds the minimal threshold value suggested by Cheverud (1988). In fact, there were skull data for 209 families, the mean heritability was 0.23 and thus the effective sample size was 47.4. This result confirms that the genetic correlations are reliable and that G and P are similar because both genetic and environmental components of phenotypic variation are channelled through the same developmental pathways.
Regarding integration hypotheses, the patterns of genetic and phenotypic correlations between facial and neurocranial dimensions do not follow Enlow's expected pattern of craniofacial variation and headform in humans (Enlow & Hans, 1996). As predicted by the hypothesis, maximum cranial breadth is positively correlated with facial breadth (r = 0.89, P = 0.007), but it does not correlate negatively with facial height (r = 0.47, P = 0.11), neurocranial length (r = 0.49, P = 0.06) or neurocranial height (r = 0.16, P = 0.72). Thus, we reject the H3 null hypothesis because neither the genetic nor the phenotypic observed patterns of correlation fit the pattern expected by the traditional hypothesis of integration (Enlow & Hans, 1996).
Finally, our results confirm the hypothesis of strong covariation between the breadth measures of major developmental regions of the skull (Hallgrímsson et al. 2007). The genetic correlations of facial, neurocranial and basicranial breadth measures were high and statistically significant and dominate the patterns of integration of the human skull (rf-b = 0.90, P = 0.014; rn-b = 0.93, P = 0.007; rf-n = 0.89, P = 0.007). Therefore we do not reject H4 null hypothesis and support the hypothesis that this correlation pattern prevails in integration of the skull.
Discussion
This study explored the levels of genetic variation and correlation of craniometric traits through a developmental/functional approach to assess the evolutionary potential of the human skull. The above results confirm that the human skull has substantial amounts of genetic variation, which confers to the skull a high ability to evolve (Tables 2–5). However, evolvability is compromised by complex patterns of genetic integration that may constrain the potential evolution of the skull towards certain directions of change (Appendix 1). That is, free evolution of the skull is unlikely because of morphological integration, and this suggests that the developmental system plays an important role, channelling the paths through which genetic and environmental components of phenotypic variation can be expressed (Cheverud, 1988, Lieberman et al. 2004).
It has been suggested that the different cranial regions could be subject to different levels of evolvability and/or plasticity (Kohn, 1991; Strand Vidarsdóttir et al. 2002; Bastir & Rosas, 2004). We tested this assumption in hypothesis H1 and we did not find significant differences between the amounts of genetic variation underlying the three major developmental regions of the skull. Craniometric traits from the face, the cranial vault and the base show similar percentages of significant heritability estimations and low to moderate levels of genetic components of variation. This result confirms previous evidence indicating that within the primate skull basicranial, neurocranial and facial dimensions show similar levels of heritability (Cheverud & Buikstra, 1982; Sjøvold, 1984; Cheverud, 1996b). Moreover, there is no evidence suggesting that the face is the most plastic region of the skull. For instance, our results showed that some facial dimensions associated with functional regions (such as the nasal, the orbital and the zygomatic regions) have some of the highest heritabilities of the skull (Fig. 2). Characters with no heritability, with all their variation due to environmental effects, are not limited to the face but are widespread through the whole skull and can also be found at the neurocranium and the basicranium (Tables 2–5).
Our results support the hypothesis that the cranial base is more conservative and may be under slightly stronger genetic control, as most distances within the basicranium show moderate and significant heritabilities, and phenotypic and genetic correlations between the width and length of the cranial base are strong (Appendix 1). Also, we corroborate the hypothesis that the cranial base acts as the ‘skull's central integrator’ (Lieberman et al. 2000a,b, 2002). In fact, the cranial base strongly influences the overall cranial shape, constraining facial breadth, height and length, as well as neurocranial breadth and length. This mechanism would contribute to preventing the different regions from evolving independently and would preserve the functional and architectural requirements of the skull.
Craniofacial traits have substantial amounts of genetic variation, but are significantly affected by other non-genetic factors such as sex and year of birth, as revealed by the genetic analyses. This may be reflecting the influences of sexual dimorphism and secular trends in the Hallstatt population. Sexual dimorphism is one of the main sources of intraspecific variation in skull morphology, which is probably the result of allometric factors and differences in body composition between males and females (O’Higgins & Dryden, 1993; Rosas & Bastir, 2002). Therefore, it is not an unexpected result that sex was a significant covariate affecting most of the measurements; especially because linear distances were not corrected for size.
Secular changes are also a well-known source of morphological variation involving both genetic and environmental components (Jantz & Meadows Jantz, 2000). Secular trends may have been driven by random genetic changes by gene flow (Lahr, 1996) and admixture, as well as by specific adaptations due to a release in selective pressures due to masticatory, dietary, and technological changes (Larsen, 1997). Secular trends have been reported in American (Jantz & Meadows Jantz, 2000) and European (Rösing & Schwidetzky 1979; 1984) populations, including Hallstatt (Sjøvold 1990, 1995; Carson, 2006a). These studies showed that the secular trends detected in Hallstatt follow the general trend of gracilization of European modern populations (Henneberg et al. 1978; Rösing & Schwidetzky, 1979, 1984). In Hallstatt, at least from the transition between the 18th and the 19th centuries, there was a reduction of maximum cranial breadth, accompanied by an increase of neurocranial height (Sjøvold 1990, 1995; Carson, 2006a). Here, we have not assessed the temporal gradient of cranial measurements, but we have found evidence that the minor functional regions (i.e. the zygomatic and nasal regions) of the skull are the most affected (Table 2), which could be reflecting dietary and climatic changes (Jantz & Meadows Jantz, 2000). Neurocranial and basicranial dimensions are also affected, especially the maximum cranial breath (Table 3), and these changes might be caused both by genetic and non-genetic factors.
Hypothesis 2 (H2) tested the similarity between the genetic and the phenotypic correlation matrices, and the Mantel test revealed that they are significantly similar. This is important because many studies are using phenotypic data in population genetic models without any knowledge of the genetic architecture of the skull (Steadman, 2001; González-José et al. 2003, 2005, 2007; Ackermann & Cheverud, 2004a; Roseman, 2004; Schillaci & Stojanowski, 2005; Stojanowski, 2005; Martínez-Abadías et al. 2006; Stojanowski & Schillaci, 2006). This is done assuming that the G and P matrices are similar and proportional, a conclusion drawn from Cheverud's (1988) work. This study compared genetic and phenotypic correlation matrices obtained from 23 published studies, which included a wide range of animals (from human to amphipods) and of kinds of traits (from morphological to cognitive). Here we provide empirical data exclusively for human craniometric traits and support the view that G and P display consistent patterns of morphological variation (Cheverud, 1988).
The proportionality of G and P, however, is not a straightforward consequence of the similarity between these correlation matrices. We could not directly assess the proportionality of G and P variance-covariance matrices because variances and covariances are not available after the inverse normalization. However, in another study (Martínez-Abadías, 2007) we tested this assumption using a set of multivariate landmark data representing the shape of the cranium and applying geometric morphometric methods. Our data strongly contradicted this expectation (Martínez-Abadías, 2007). This result, along with earlier findings from mice (Klingenberg & Leamy, 2001) and humans (Sherwood et al. 2008), supports theoretical arguments (Willis et al. 1991) and suggests that phenotypic data may introduce a potential bias in population and quantitative genetic studies unless the sample size is sufficiently large or familial information is available (Sherwood et al. 2008). In conclusion, our analyses suggest that the genetic and the phenotypic covariation matrices are similar but not identical or proportional (Martínez-Abadías, 2007). Genetic covariation matrices show more complex and structured patterns of morphological integration than the phenotypic covariation matrices. This should be taken into account in studies using P as a proxy of G variance-covariance matrix.
Regarding the pattern of genetic correlations between facial and neurocranial dimensions considered in H3, our results show that these patterns do not follow Enlow's expected pattern of craniofacial variation and headform in humans (Enlow & Hans, 1996). Under this hypothesis, maximum cranial breadth should be positively correlated with facial breadth and negatively correlated with facial height, neurocranial length and neurocranial height. However, we only found a significant correlation between neurocranial and facial breadth, as has been previously hypothesized (Weidenreich, 1941) and supported by studies of artificial cranial deformation (Antón, 1989). Therefore, we conclude that the traditional classification between dolico- and brachycephalic skulls does not reflect the genetic architecture of the human skull or provide any valuable hypothesis of morphological-genetic integration. This is relevant because many bio-anthropological issues are still being synthesized in terms of dolico- vs. brachycephalic forms. For instance, the classical study of Boas on European immigrants to USA (Boas, 1912; Gravlee et al. 2003; Relethford, 2004), studies of morphological variation among ancient and modern Native Americans (Gonzalez et al. 2003; Fiedel, 2004) and studies analysing the relationship among head shape and climate (Beals, 1972; Goodman, 1995, 1997) still use this terminology to describe human craniofacial variation.
The clearest integrated module is formed by breadth dimensions covering the neurocranium, the basicranium and the face: the overall pattern of integration in the human skull is dominated by the covariation between the maximum widths of the major developmental regions. This pattern was first reported in the mice cranium (Hallgrímsson et al. 2007) and here we extend it to humans. Evolutionary developmental studies use model organisms such as mice to identify candidate genes that are involved in the phenotypic expression of skull morphology (Lieberman et al. 2004; Hallgrímsson et al. 2004, 2006, 2007). To extrapolate the results obtained from such organisms to humans it is important to compare them with other primate species. Hallgrímsson et al. (2004) compared phenotypic and genetic correlations in macaques and two strains of mice and did not find a consistent pattern of modularity in these groups. Therefore, it is relevant to find the same predicted pattern of integration in humans and mice. This suggests that covariation between cranial widths is an integrated feature that has been conserved across the evolution of the mammalian craniofacial form.
The present study presents similarities but also some differences to previous analyses carried out with the Hallstatt skull collection (Sjøvold, 1984, Carson, 2006a). Although they are all grounded on the same population, results are not totally coincident. However, this is not an unexpected output as each study took its point of departure from different familiar data, accounted for different sources of covariation and did not use exactly the same crania. As sample size is limited, standard errors are substantially large (Falconer & MacKay, 1996) and slight differences in sample composition, model definition and data treatment can alter the results. Therefore, general trends are more reliable quantitative parameters than the exact value of the heritability estimations. In common, all studies have shown that craniometric traits are low to moderate hereditary characteristics. However, we do not confirm previous evidence suggesting that breadth and facial dimensions are the less heritable characters of the human skull (Carson, 2006a). This study reports low to moderate heritability estimates for breadth measures (Tables 2–5) and has tested statistically that there are no significant differences in the amount of genetic variation underlying the main developmental regions of the skull. Although we used the same statistical method to estimate heritability (ML), inconsistencies between studies might also arise due to other methodological issues regarding the number of skulls included in the analyses and the complexity of the pedigree structure. In this study, we extended and revised the pedigrees constructed by Sjøvold (1984), checked the identifications made by the gravedigger by sex confirmation, and thanks to Sjøvold's photographic records from the mid seventies we could identify the original names of the individuals (Fig. 1). In comparison with previous studies, our analysis included a larger skull sample, did not contain missing values and used larger and more complex genealogies since the whole population was reconstructed.
Understanding the patterns of morphological integration among skull regions will improve our ability to make evolutionary and phylogenetic inferences about human evolution. The use of craniodental characters in phylogenetic analyses of primate and hominid evolution is widespread (Strait et al. 1997; Strait & Grine, 1999; Strait et al. 2007; Lockwood, 2007) and they are essential because cranial remains are one of the main sources of information on extant and fossil species (Ackermann & Cheverud, 2004b; Lockwood, 2007). Although skull morphology is affected to some extent by environmental factors and is under lower genetic control than molecular traits, it is accepted that craniometric traits are phylogenetically informative (Collard & Wood, 2007; Lockwood, 2007). However, as there is strong evidence that morphological integration plays an important role in evolutionary biology and can bias the results of such cladistic analyses (Strait et al. 2007; Lockwood, 2007), further understanding about how and why morphological complexes arise in the skull is needed.
Our analysis reports that the human skull has substantial amounts of genetic variation that are constrained by integration. Furthermore, it demonstrates that craniometric traits from the face, the neurocranium and the basicranium do not differ in their heritability patterns. We also provide empirical evidence that genetic and phenotypic correlation patterns in the human skull are consistent and show similar morphological variation patterns. Regarding integration, results suggest that traditional integration hypotheses (Enlow & Hans, 1996) do not have a genetic basis, but confirm recent modularity patterns found in mice, emphasizing strong covariation between relative widths of the neurocranium, the basicranium and the face as the most dominant integration pattern in the mammal skull (Hallgrímsson et al. 2007).
Our results concerning the heritability and correlation patterns of craniometric traits shed light into the genetic architecture of the human skull. Also, they are especially useful to provide an evolutionary context based on quantitative genetics for classic morphometric studies and databases using univariate measurements. For a greater comprehension of modularity and integration patterns in the skull, future analyses should account for the multivariate nature of shape (Klingenberg, 2004). This could be achieved by combining quantitative genetic methods with geometric morphometric tools, as suggested by Klingenberg & Leamy (2001). It would then be possible to discuss in greater detail the genetic and modular basis of complex phenotypes.
Acknowledgments
We thank the authorities of the Hallstatt Parish, Institut für Anatomie (Innsbruck), Naturhistorisches Museum Wien, Musealverein Hallstatt, and Österreichisches Museum für Volkskunde (Wien) for permission to access their collections. We thank Karl Mager, Maria Teschler-Nicola and Karin Wiltschke-Schrotta for assistance with digitizing. We are grateful to Christian Peter Klingenberg, Marta Muñoz-Tudurí, Yann Heuzé and two anonymous referees for helpful suggestions on earlier versions of the manuscript. This work was supported by the Wenner Gren Foundation for Anthropological Research (Gr. 7149), the Universitat de Barcelona (Research and Teaching Postgraduate grant to NMA) and by the Spanish Ministerio de Educación y Ciencia MEC-FEDER (CGL2004-00903/BTE).
Appendix 1
Genetic correlations (lower left) and phenotypic correlations (upper right) among cranial distances. For genetic correlations, the associated standard errors (SE) are also provided. Significant correlations at the 0.05 level are in bold. Note that the genetic correlations involving nasal breadth were non-computable because its heritability was 0.00. B, breadth; H, height; L, length
| Facial | Neural | Basal | Nasal | Orbital | Zygomatic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | H | L | B | H | L | B | H | B | H | L | B | H | L | B | H | L | ||
| Facial | B | 0.47 | 0.43 | 0.68 | 0.41 | 0.47 | 0.7 | 0.49 | 0.25 | 0.46 | 0.49 | 0.42 | 0.17 | 0.4 | 0.61 | 0.45 | 0.56 | |
| H | 0.57 ± 0.24 | 0.24 | 0.31 | 0.31 | 0.41 | 0.41 | 0.34 | 0.05 | 0.88 | 0.42 | 0.37 | 0.28 | 0.14 | 0.42 | 0.55 | 0.37 | ||
| L | 0.42 ± 0.24 | 0.42 ± 0.26 | 0.21 | 0.3 | 0.48 | 0.37 | 0.8 | 0.2 | 0.18 | 0.72 | 0.27 | −0.11 | 0.41 | 0.43 | 0.06 | 0.37 | ||
| Neural | B | 0.89 ± 0.11 | 0.47 ± 0.25 | 0.46 ± 0.24 | 0.28 | 0.33 | 0.52 | 0.26 | 0.18 | 0.28 | 0.28 | 0.31 | 0.15 | 0.24 | 0.36 | 0.3 | 0.29 | |
| H | −0.01 ± 0.45 | 0.17 ± 0.39 | 0.43 ± 0.30 | 0.16 ± 0.40 | 0.36 | 0.49 | 0.52 | 0 | 0.29 | 0.34 | 0.25 | 0.04 | 0.33 | 0.3 | 0.23 | 0.33 | ||
| L | 0.56 ± 0.20 | 0.69 ± 0.21 | 0.50 ± 0.21 | 0.49 ± 0.20 | 0.61 ± 0.25 | 0.41 | 0.61 | 0.11 | 0.41 | 0.44 | 0.31 | 0.07 | 0.33 | 0.33 | 0.29 | 0.34 | ||
| Basal | B | 0.90 ± 0.13 | 0.78 ± 0.26 | 0.62 ± 0.26 | 0.93 ± 0.14 | 0.40 ± 0.35 | 0.50 ± 0.24 | 0.5 | 0.14 | 0.38 | 0.42 | 0.25 | 0.1 | 0.34 | 0.45 | 0.3 | 0.41 | |
| L | 0.43 ± 0.23 | 0.45 ± 0.24 | 0.96 ± 0.07 | 0.63 ± 0.22 | 0.56 ± 0.25 | 0.75 ± 0.15 | 0.79 ± 0.23 | 0.13 | 0.33 | 0.59 | 0.36 | −0.01 | 0.4 | 0.39 | 0.19 | 0.46 | ||
| Nasal | B | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | 0.17 | 0.07 | 0.21 | 0.05 | 0.23 | 0.36 | 0.14 | 0.06 | |
| H | 0.57 ± 0.23 | 0.94 ± 0.05 | 0.23 ± 0.24 | 0.48 ± 0.23 | 0.27 ± 0.34 | 0.52 ± 0.21 | 0.61 ± 0.26 | 0.25 ± 0.23 | −1 | 0.36 | 0.35 | 0.31 | 0.16 | 0.42 | 0.58 | 0.28 | ||
| L | 0.71 ± 0.18 | 0.77 ± 0.16 | 0.86 ± 0.08 | 0.61 ± 0.19 | 0.75 ± 0.23 | 0.60 ± 0.17 | 1 | 0.91 ± 0.10 | 1 | 0.57 ± 0.19 | 0.28 | −0.02 | 0.36 | 0.4 | 0.21 | 0.45 | ||
| Orbital | B | 0.19 ± 0.37 | 0.98 ± 0.36 | 0.60 ± 0.34 | 0.35 ± 0.32 | 0.26 ± 0.47 | 0.00 ± 0.33 | 0.27 ± 0.40 | 0.45 ± 0.30 | −1 | 0.64 ± 0.32 | 0.75 ± 0.29 | 0.26 | 0.18 | 0.31 | 0.32 | 0.25 | |
| H | −0.08 ± 0.30 | 0.59 ± 0.30 | −0.63 ± 0.22 | 0.1 ± 0.32 | −0.44 ± 0.38 | −0.16 ± 0.30 | −0.07 ± 0.35 | −0.43 ± 0.26 | 1 | 0.65 ± 0.26 | −0.62 ± 0.24 | 0.31 ± 0.41 | −0.05 | −0.01 | 0.52 | 0.08 | ||
| L | 0.00 ± 0.33 | 0.08 ± 0.31 | 0.36 ± 0.24 | 0.45 ± 0.27 | 1 | 0.30 ± 0.26 | 0.66 ± 0.29 | 0.71 ± 0.22 | 1 | 0.03 ± 0.27 | 0.23 ± 0.26 | −0.03 ± 0.38 | −0.41 ± 0.28 | 0.41 | 0.15 | 0.25 | ||
| Zygomatic | B | 0.72 ± 0.37 | 0.17 ± 0.55 | 0.79 ± 0.41 | 0.68 ± 0.43 | 0.53 ± 0.72 | 0.16 ± 0.51 | 1 | 0.73 ± 0.56 | −1 | 0.23 ± 0.48 | 0.82 ± 0.28 | 0.87 ± 0.63 | −0.22 ± 0.60 | 0.41 ± 0.54 | 0.34 | 0.34 | |
| H | 0.19 ± 0.33 | 0.82 ± 0.19 | 0.03 ± 0.31 | 0.49 ± 0.29 | −0.42 ± 0.52 | 0.12 ± 0.30 | 0.27 ± 0.32 | −0.04 ± 0.31 | 1 | 0.90 ± 0.17 | 0.34 ± 0.28 | 0.12 ± 0.41 | 1 | −0.28 ± 0.34 | 0.02 ± 0.66 | 0.34 | ||
| L | 0.61 ± 0.24 | 0.30 ± 0.32 | 0.49 ± 0.25 | 0.51 ± 0.31 | 0.03 ± 0.52 | 0.70 ± 0.27 | 0.78 ± 0.22 | 0.47 ± 0.26 | 1 | 0.27 ± 0.30 | 0.77 ± 0.15 | −0.11 ± 0.50 | −0.60 ± 0.40 | 0.30 ± 0.34 | 0.14 ± 0.66 | −0.40 ± 0.48 | ||
References
- Ackermann RR, Cheverud JM. Detecting genetic drift versus selection in human evolution. Proc Natl Acad Sci U S A. 2004a;101:17946–17951. doi: 10.1073/pnas.0405919102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann RR, Cheverud JM. Morphological integration in primate evolution. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford: Oxford University Press; 2004b. pp. 302–319. [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative trait linkage analysis in general pedigrees. Am J Hum Gen. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón SC. Intentional cranial vault deformation and induced changes of the cranial base and face. Am J Phys Anthropol. 1989;79:253–267. doi: 10.1002/ajpa.1330790213. [DOI] [PubMed] [Google Scholar]
- Arya R, Duggirala R, Comuzzie AG, et al. Heritability of anthropometric phenotypes in caste populations of Visakhapatnam, India. Hum Biol. 2002;74:325–344. doi: 10.1353/hub.2002.0026. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Facial heights: evolutionary relevance of postnatal ontogeny for facial orientation and skull morphology in humans and chimpanzees. J Hum Evol. 2004;47:359–381. doi: 10.1016/j.jhevol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Hierarchical nature of morphological integration and modularity in the human posterior face. Am J Phys Anthropol. 2005;128:26–34. doi: 10.1002/ajpa.20191. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Arch Oral Biol. 2006;51:814–824. doi: 10.1016/j.archoralbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Kuroe K. Petrosal orientation and mandibular ramus breadth: evidence for an integrated petroso-mandibular developmental unit. Am J Phys Anthropol. 2004;123:340–350. doi: 10.1002/ajpa.10313. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, O’Higgins P. Craniofacial levels and the morphological maturation of the human skull. J Anat. 2006;209:637–654. doi: 10.1111/j.1469-7580.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals KM. Head form and climatic stress. Am J Phys Anthropol. 1972;37:85–92. doi: 10.1002/ajpa.1330370111. [DOI] [PubMed] [Google Scholar]
- Boas F. Changes in the bodily form of descendants of immigrants. Am Anthropol. 1912;14:530–562. [Google Scholar]
- Bookstein FL, Gunz P, Mitteroecker P, Prossinger H, Schaefer K, Seidler H. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–187. doi: 10.1016/s0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Buikstra JE, Frankenberg SR, Konigsberg LW. Skeletal biological distance studies in American Physical Anthropology: recent trends. Am J Phys Anthropol. 1990;82:1–7. doi: 10.1002/ajpa.1330820102. [DOI] [PubMed] [Google Scholar]
- Burgstaller E. Schädelbeschriftung und Bemalung in den Österreichischen Alpenländer. In: Koren H, Kretzenbacher L, editors. Volkskunde im Ostalpenraum (Alpes Orientales II) Steir: Steirisches Volksmuseum; 1961. pp. 71–84. [Google Scholar]
- Carlson BM. Human Embryology and Developmental Biology. Philadelphia: Mosby Elsevier; 1999. [Google Scholar]
- Carson EA. Maximum likelihood estimation of human craniometric heritabilities. Am J Phys Anthropol. 2006a;131:169–180. doi: 10.1002/ajpa.20424. [DOI] [PubMed] [Google Scholar]
- Carson EA. Maximum-likelihood variance components analysis of heritabilities of cranial nonmetric traits. Hum Biol. 2006b;78:383–402. doi: 10.1353/hub.2006.0054. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution. 1982;36:499–516. doi: 10.1111/j.1558-5646.1982.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Quantitative genetics and developmental constraints on evolution by selection. J Theor Biol. 1984;110:155–171. doi: 10.1016/s0022-5193(84)80050-8. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. A comparison of genetic and phenotypic correlations. Evolution. 1988;42:958–968. doi: 10.1111/j.1558-5646.1988.tb02514.x. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Morphological integration in the saddle-back tamarin (Saguinus fuscicollis) cranium. Am Nat. 1995;145:63–89. [Google Scholar]
- Cheverud JM. Developmental integration and the evolution of pleiotropy. Am Zool. 1996a;36:44–50. [Google Scholar]
- Cheverud JM. Quantitative genetic analysis of cranial morphology in the cotton-top (Saguinus oedipus) and saddle-back (S. fuscicollis) tamarins. J Evol Biol. 1996b;9:5–42. [Google Scholar]
- Cheverud JM, Buikstra JE. Quantitative genetics of skeletal nonmetric traits in the rhesus macaques of Cayo Santiago. III. Relative heritability of skeletal nonmetric and metric traits. Am J Phys Anthropol. 1982;59:151–155. doi: 10.1002/ajpa.1330590205. [DOI] [PubMed] [Google Scholar]
- Collard M, O’Higgins P. Ontogeny and homoplasy in the papionin monkey face. Evol Dev. 2001;3:322–331. doi: 10.1046/j.1525-142x.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- Collard M, Wood B. How reliable are human phylogenetic hypotheses? Proc Natl Acad Sci USA. 2000;97:5003–5006. doi: 10.1073/pnas.97.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard M, Wood B. Hominin homoiology: an assessment of the impact of phenotypic plasticity on phylogenetic analyses of humans and their fossil relatives. J Hum Evol. 2007;52:573–584. doi: 10.1016/j.jhevol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Dahlberg G. Twin Births and Twins from a Hereditary Point of View. Stockholm: Tidens Tryckeri; 1926. [Google Scholar]
- Devor EJ, McGue M, Crawford MH, Lin PM. Transmissible and non transmissible components of anthropometric variation in the Alexanderwohl Mennonites: II. Resolution by path analysis. Am J Phys Anthropol. 1986;69:83–92. doi: 10.1002/ajpa.1330690110. [DOI] [PubMed] [Google Scholar]
- Enlow D, Hans MG. Essentials of Facial Growth. Philadelphia: Saunders Co; 1996. [Google Scholar]
- Falconer DS, MacKay TFC. Introduction to Quantitative Genetics. Essex: Longman Group, Ltd; 1996. [Google Scholar]
- Fiedel SJ. The Kennewick Follies: ‘New’ theories about the peopling of the Americas. J Anthropol Res. 2004;60:75–110. [Google Scholar]
- Gonzalez S, Jiménez-López JC, Hedges R, et al. Earliest humans in the Americas: new evidence from México. J Hum Evol. 2003;44:379–387. doi: 10.1016/s0047-2484(03)00004-6. [DOI] [PubMed] [Google Scholar]
- González-José R, Gonzalez-Martin A, Hernandez M, et al. Craniometric evidence for Palaeoamerican survival in Baja California. Nature. 2003;425:62–65. doi: 10.1038/nature01816. [DOI] [PubMed] [Google Scholar]
- González-José R, Martínez-Abadías N, González-Martín A, et al. Detection of a population replacement at the Classic-Postclassic transition in Mexico. Proc R Soc London Ser B. 2007;274:681–688. doi: 10.1098/rspb.2006.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-José R, Ramírez-Rozzi F, Sardi M, Martínez-Abadías N, Hernández M, Pucciarelli H. Functional-craniology approach to the influence of economic strategy on skull morphology. Am J Phys Anthropol. 2005;128:757–771. doi: 10.1002/ajpa.20161. [DOI] [PubMed] [Google Scholar]
- González-José R, Van der Molen S, González-Pérez E, Hernández M. Patterns of phenotypic covariation and correlation in modern humans as viewed from morphological integration. Am J Phys Anthropol. 2004;123:69–77. doi: 10.1002/ajpa.10302. [DOI] [PubMed] [Google Scholar]
- Goodman AH. The problematics of ‘race’ in contemporary biological anthropology. In: Boaz NT, Wolfe LD, editors. Biological Anthropology: the State of the Science. Bend, OR: International Inst. of Human Evolutionary Research; 1995. pp. 215–239. [Google Scholar]
- Goodman AH. Bred in the bone? The Sciences. 1997;37:20–25. [Google Scholar]
- Goswami A. Cranial modularity shifts during Mammalian evolution. Am Nat. 2006;168:270–280. doi: 10.1086/505758. [DOI] [PubMed] [Google Scholar]
- Goswami A. Cranial modularity and sequence heterochrony in mammals. Evol Dev. 2007;9:290–298. doi: 10.1111/j.1525-142X.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- Gravlee CC, Bernard HR, Leonard WR. Heredity, environment, and cranial form: a reanalysis of Boas's immigrant data. Am Anthropol. 2003;105:125–138. [Google Scholar]
- Hallgrímsson B, Brown JA, Ford-Hutchinson AF, Sheets DH, Zelditch ML, Jirik FR. The brachymorph mouse and the developmental-genetic basis for canalization and morphological integration. Evol Dev. 2006;8:61–73. doi: 10.1111/j.1525-142X.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Lieberman DE, Liu W, Ford-Hutchinson AF, Jirik FR. Epigenetic interactions and the structure of phenotypic variation in the cranium. Evol Dev. 2007;9:76–91. doi: 10.1111/j.1525-142X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Willmore K, Dorval C, Cooper DML. Craniofacial variability and modularity in macaques and mice. J Exp Zool (Mol Dev Evol) 2004;302B:207–225. doi: 10.1002/jez.b.21002. [DOI] [PubMed] [Google Scholar]
- Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat Rec. 2006;288A:1225–1233. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- Henneberg M, Piontek J, Strzalko J. Natural selection and morphological variability: the case of Europe from Neolithic to modern times. Current Anthropology. 1978;19:67–82. [Google Scholar]
- Hiernaux J. Heredity and environment: their influence on human morphology. A comparison of two independent lines of study. Am J Phys Anthropol. 1963;21:575–589. doi: 10.1002/ajpa.1330210414. [DOI] [PubMed] [Google Scholar]
- Howells WW. Cranial Variation in Man. Cambridge, MA: Harvard University; 1973. [Google Scholar]
- Howells WW. Skull Shapes and the Map. Papers of the Peabody Museum. Cambridge, MA: Harvard University Press; 1989. [Google Scholar]
- Jantz RL, Meadows Jantz L. Secular change in craniofacial morphology. Am J Hum Biol. 2000;12:327–338. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<327::AID-AJHB3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Johannsdottir B, Thorarinsson F, Thordarson A, Magnusson TE. Heritability of craniofacial characteristics between parents and offspring estimated from lateral cephalograms. Am J Orthod Dent Orthoped. 2005;127:200–207. doi: 10.1016/j.ajodo.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Integration, modules and development: molecules to morphology to evolution. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. New York: Oxford University Press; 2004. pp. 213–230. [Google Scholar]
- Klingenberg CP. Developmental constraints, modules and evolvability. In: Hallgrímsson B, Hall BK, editors. Variation: a Central Concept in Biology. San Diego: Academic Press; 2005. pp. 219–247. [Google Scholar]
- Klingenberg CP, Leamy LJ. Quantitative genetics of geometric shape in the mouse mandible. Evolution. 2001;55:2342–2352. doi: 10.1111/j.0014-3820.2001.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Kohn LPA. The role of genetics in craniofacial morphology and growth. Ann Rev Anthropol. 1991;20:261–278. [Google Scholar]
- Konigsberg LW. Quantitative variation and genetics. In. In: Stinson S, Bogin B, Huss-Ashmore R, O’Rourke D, editors. Human Biology: an Evolutionary and Biocultural Perspective. New York: Wiley Liss; 2000. pp. 135–162. [Google Scholar]
- Konigsberg LW, Ousley SD. Multivariate quantitative genetics of anthropometric traits from the Boas data. Hum Biol. 1995;67:481–498. [PubMed] [Google Scholar]
- Lahr MM. The evolution of Modern Human Diversity: a Study of Cranial Variation. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Larsen CS. Bioarcheology: Interpreting Behavior from the Human Skeleton. New York: Cambridge University Press; 1997. [Google Scholar]
- Lieberman DE, Mowbray KM, Pearson OM. Basicranial influences on overall cranial shape. J Hum Evol. 2000a;38:291–315. doi: 10.1006/jhev.1999.0335. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Ross CR, Ravosa M. The primate cranial base: ontogeny, function and integration. Ybk Phys Anthropol. 2000b;43:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McBratney BM, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proc Natl Acad Sci U S A. 2002;99:1134–1139. doi: 10.1073/pnas.022440799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz G, McBratney B. Testing hypotheses about tinkering in the fossil record: the case of the human skull. J Exp Zool (Mol Dev Evol) 2004;302B:284–301. doi: 10.1002/jez.b.21004. [DOI] [PubMed] [Google Scholar]
- Lockwood CA. Adaptation and functional integration in primate phylogenetics. J Hum Evol. 2007;52:490–503. doi: 10.1016/j.jhevol.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics analysis of quantitative traits. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- Marroig G, Cheverud JM. A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of New World monkeys. Evolution. 2001;55:2576–2600. doi: 10.1111/j.0014-3820.2001.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Abadías N. Evolutionary patterns of the human skull. A quantitative genetic analysis of craniofacial phenotypic variation. Barcelona: Universitat de Barcelona; 2007. PhD. Dissertation. [Google Scholar]
- Martínez-Abadías N, González-José R, González-Martín A, et al. Phenotypic evolution of human craniofacial morphology after admixture: a geometric morphometrics approach. Am J Phys Anthropol. 2006;129:387–398. doi: 10.1002/ajpa.20291. [DOI] [PubMed] [Google Scholar]
- McCarthy RC, Lieberman DE. Posterior maxillary (PM) plane and anterior cranial architecture in primates. Anat Rec. 2001;264:247–260. doi: 10.1002/ar.1167. [DOI] [PubMed] [Google Scholar]
- McGuigan K. Studying phenotypic evolution using multivariate quantitative genetics. Mol Ecol. 2006;15:883–896. doi: 10.1111/j.1365-294X.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- Merilä J, Björklund M. Phenotypic integration as a constraint and adaptation. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford: Oxford University Press; 2004. pp. 107–129. [Google Scholar]
- Mooney MP, Siegel MI, Smith TD, Burrows AM. Evolutionary changes in the cranial vault and base: establishing the primate form. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Ethiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 275–294. [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–291. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Nakata M, Yu PL, Davis B, Nance WE. Genetic determinants of cranio-facial morphology: a twin study. Ann Hum Genet. 1974;37:431–443. doi: 10.1111/j.1469-1809.1974.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Neves WA, Hubbe M. Cranial morphology of early Americans from Lagoa Santa, Brazil: implications for the settlement of the New World. Proc Natl Acad Sci USA. 2005;102:18309–18314. doi: 10.1073/pnas.0507185102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M. Similarities in anthropometrical traits of children and their parents in a Bulgarian population. Ann Hum Genet. 1996;60:517–525. doi: 10.1111/j.1469-1809.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- O’Higgins P, Dryden IL. Sexual dimorphism in hominoids: further studies of craniofacial shape differences in Pan, Gorilla and Pongo. J Hum Evol. 1993;24:183–205. [Google Scholar]
- Olson EC, Miller RL. Morphological Integration. Chicago: University of Chicago Press; 1958. [Google Scholar]
- Relethford JH. Craniometric variation among modern human populations. Am J Phys Anthropol. 1994;95:53–62. doi: 10.1002/ajpa.1330950105. [DOI] [PubMed] [Google Scholar]
- Relethford JH. Apportionment of global human genetic diversity based on craniometrics and skin color. Am J Phys Anthropol. 2002;118:393–398. doi: 10.1002/ajpa.10079. [DOI] [PubMed] [Google Scholar]
- Relethford JH. Boas and beyond: migration and craniometric variation. Am J Hum Biol. 2004;16:379–386. doi: 10.1002/ajhb.20045. [DOI] [PubMed] [Google Scholar]
- Relethford JH, Blangero J. Detection of differential gene flow from patterns of quantitative variation. Hum Biol. 1990;62:5–25. [PubMed] [Google Scholar]
- Relethford JH, Lees FC. The use of quantitative traits in the study of human population structure. Yearb Phys Anthropol. 1982;25:113–132. [Google Scholar]
- Richtsmeier JT. Cranial vault dysmorphology and growth in craniosynostosis. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Ethiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 321–341. [Google Scholar]
- Roff DA. Evolutionary Quantitative Genetics. New York: Chapman & Hall; 1997. Heritability; pp. 5–72. [Google Scholar]
- Rosas A, Bastir M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am J Phys Anthropol. 2002;117:236–245. doi: 10.1002/ajpa.10023. [DOI] [PubMed] [Google Scholar]
- Roseman CC. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc Natl Acad Sci USA. 2004;101:12824–12829. doi: 10.1073/pnas.0402637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösing FW, Schwidetzky I. Comparative statistical studies on the physical anthropology of the Early Medieval period (500–1000 A.D.) J Hum Evol. 1979;8:755–758. [Google Scholar]
- Rösing FW, Schwidetzky I. Comparative statistical studies on the physical anthropology of the Late Medieval period (A.D. 1000–1500) J Hum Evol. 1984;13:325–329. [Google Scholar]
- Sardi M, Ramírez-Rozzi F. Developmental connections between cranial components and the emergence of the first permanent molar in humans. J Anat. 2007;210:406–417. doi: 10.1111/j.1469-7580.2007.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauser G. Actes du IVe Congrés International des Sciences Anthropologiques et Ethnologiques. Vienna: 1952. Bemalte Ossuarienschädel aus Hallstatt; pp. 112–116. [Google Scholar]
- Schillaci MA, Stojanowski CM. Craniometric variation and population history of the Prehistoric Tewa. Am J Phys Anthropol. 2005;126:404–412. doi: 10.1002/ajpa.20150. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Thorogood P. Development and evolution of the vertebrate skull. In: O’Higgins P, Cohn M, editors. Development, Growth and Evolution. Implications for the Study of the Hominid Skeleton. London: Academic Press; 2000. pp. 57–83. [Google Scholar]
- Sharma K. Maximum-likelihood approaches applied to quantitative genetics of natural populations. Evolution. 1987;48:812–826. doi: 10.1111/j.1558-5646.1987.tb05855.x. [DOI] [PubMed] [Google Scholar]
- Sharma K. Sex differences in genetic determinants of craniofacial variations – a study based on twin kinships. Acta Genet Med Gemellol. 1998;47:31–41. doi: 10.1017/s0001566000000350. [DOI] [PubMed] [Google Scholar]
- Sharma K, Susanne C. Comparative genetic variance and heritability of head and facial traits in northwest Indian and Belgian twins. Am J Hum Biol. 1991;3:315–324. doi: 10.1002/ajhb.1310030402. [DOI] [PubMed] [Google Scholar]
- Sherwood RJ, Duren DL, Demerath EW, Czerwinski SA, Siervogel RM, Towne B. Quantitative genetics of modern human cranial variation. J Hum Evol. 2008;54:909–914. doi: 10.1016/j.jhevol.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Swindler DR. Evolutionary changes in the midface and mandible: establishing the primate form. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Ethiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 345–378. [Google Scholar]
- Sjøvold T. A report on the heritability of some cranial measurements and non-metric traits. In: Van Vark GN, Howells WW, editors. Multivariate Statistical Methods in Physical Anthropology. Dordrecht: Reidel Publishing Company; 1984. pp. 223–246. [Google Scholar]
- Sjøvold T. Brachicephalization in microevolutionary terms: The evidence from the Hallstatt cranial collection. In: Sloutkal M, editor. Diachronic Trends in Historical Anthropology. Prague: Acta Musei Nationalis; 1990. pp. 196–201. [Google Scholar]
- Sjøvold T. Testing assumptions for skeletal studies by means of identified skulls from Hallstat, Austria. In: Saunders SR, Herring A, editors. Grave reflections: Portraying the past through cemetery studies. Toronto: Canadian Scholars Press Inc; 1995. pp. 241–581. [Google Scholar]
- Sparks CS, Jantz RL. A reassessment of human cranial plasticity: Boas revisited. Proc Natl Acad Sci USA. 2002;99:14636–14639. doi: 10.1073/pnas.222389599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber GH. Craniofacial Development. London: BC Decker Inc; 2001. [Google Scholar]
- Sperber GH. Craniofacial embryogenesis: normal developmental mechanisms. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies. The Ethiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 31–60. [Google Scholar]
- Steadman DW. Mississippians in motion? A population genetic analysis of interregional gene flow in west-central Illinois. Am J Phys Anthropol. 2001;114:61–73. doi: 10.1002/1096-8644(200101)114:1<61::AID-AJPA1006>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stojanowski CM. Spanish colonial effects on Native American mating structure and genetic variability in Northern and Central Florida: evidence from Apalachee and Western Timucua. Am J Phys Anthropol. 2005;128:273–286. doi: 10.1002/ajpa.20053. [DOI] [PubMed] [Google Scholar]
- Stojanowski CM, Schillaci MA. Phenotypic approaches for understanding patterns of intracemetery biological variation. Am J Phys Anthropol. 2006;131:49–88. doi: 10.1002/ajpa.20517. [DOI] [PubMed] [Google Scholar]
- Strait DS. Integration, phylogeny, and the hominid cranial base. Am J Phys Anthropol. 2001;114:273–297. doi: 10.1002/ajpa.1041. [DOI] [PubMed] [Google Scholar]
- Strait DS, Grine FE. Cladistics and early hominid phylogeny. Science. 1999;285:1209. doi: 10.1126/science.285.5431.1209c. [DOI] [PubMed] [Google Scholar]
- Strait DS, Grine FE, Moniz MA. A reappraisal of early hominid phylogeny. J Hum Evol. 1997;32:17–82. doi: 10.1006/jhev.1996.0097. [DOI] [PubMed] [Google Scholar]
- Strait DS, Richmond BG, Spencer MA, Ross CF, Dechow PC, Wood B. Masticatory biomechanics and its relevance to early hominid phylogeny: an examination of palatal thickness using finite-element analysis. J Hum Evol. 2007;52:585–599. doi: 10.1016/j.jhevol.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Strand Vidarsdóttir US, O’Higgins P, Stringer CB. A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. J Anat. 2002;201:211–229. doi: 10.1046/j.1469-7580.2002.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susanne C. Genetic and environmental influences on morphological characteristics. Ann Hum Biol. 1975;2:279–287. doi: 10.1080/03014467500000851. [DOI] [PubMed] [Google Scholar]
- Susanne C. Heritability of anthropological characters. Hum Biol. 1977;49:573–580. [PubMed] [Google Scholar]
- Vandenberg SG. How ‘stable’ are heritability estimates? A comparison of heritability estimates from six anthropometric studies. Am J Phys Anthropol. 1962;20:331–338. doi: 10.1002/ajpa.1330200316. [DOI] [PubMed] [Google Scholar]
- Varela HH, Cocilovo JA. Evaluation of the environmental component of the phenotypic variance in prehistoric populations. Homo. 1999;5:46–53. [Google Scholar]
- Weidenreich F. The brain and its role in the phylogenetic transformation of the human skull. Trans Am Philos Soc. 1941;31:321–442. [Google Scholar]
- Willis JH, Coyne JA, Kirkpatrick M. Can one predict the evolution of quantitative characters without genetics? Evolution. 1991;45:441–444. doi: 10.1111/j.1558-5646.1991.tb04418.x. [DOI] [PubMed] [Google Scholar]
- Zollikofer CP, Ponce de León MS. Kinematics of cranial ontogeny: heterotopy, heterochrony, and geometric morphometric analysis of growth models. J Exp Zool (Mol Dev Evol) 2004;302B:322–340. doi: 10.1002/jez.b.21006. [DOI] [PubMed] [Google Scholar]