Abstract
Dental tissues contain regular microscopic structures believed to result from periodic variations in the secretion of matrix by enamel- and dentine-forming cells. Counts of these structures are an important tool for reconstructing the chronology of dental development in both modern and fossil hominids. Most studies rely on the periodicity of the regular cross-banding that occurs along the long axis of enamel prisms. These prism cross-striations are widely thought to reflect a circadian rhythm of enamel matrix secretion and are generally regarded as representing daily increments of tissue. Previously, some researchers have argued against the circadian periodicity of these structures and questioned their use in reconstructing dental development. Here we tested the periodicity of enamel cross-striations – and the accuracy to which they can be used – in the developing permanent dentition of five children, excavated from a 19th century crypt in London, whose age-at-death was independently known. The interruption of crown formation by death was used to calibrate cross-striation counts. All five individuals produced counts that were strongly consistent with those expected from the independently known ages, taking into account the position of the neonatal line and factors of preservation. These results confirm that cross-striations do indeed reflect a circadian rhythm in enamel matrix secretion. They further validate their use in reconstructing dental development and in determining the age-at-death of the remains of children whose dentitions are still forming at the time of death. Significantly they identify the most likely source of error and the common difficulties encountered in histological studies of this kind.
Keywords: age-at-death, archaeology, circadian, cross-striations, dental development, enamel, neonatal line, periodicity
Introduction
The fundamental units of enamel structure are bundles of crystallites known as prisms (or rods). In sections parallel to the long axis of human teeth, regular markings can be observed along enamel prisms approximately every 3–4 µm in various types of light microscopy, and in backscattered mode scanning electron microscopy (Berkovitz et al. 2002; Hillson, 2005). It has been widely argued that these so-called prism cross-striations are the result of a circadian rhythm in the metabolic activity of the enamel-forming cells, the ameloblasts (see Boyde, 1976, 1989, 1990a; Risnes, 1986; Dean, 1987, 1989). Circadian rhythms, reflecting periodicities that are very close to a 24-h cycle, are near-universal in living organisms (Hastings, 1997). Counts of cross-striations within one tooth, from first formed enamel to completion of the crown, correspond well with independent estimates of crown formation timing (Asper, 1916; Gysi, 1931; Boyde, 1963, 1990a). Experiments with injected markers in laboratory animals (Mimura, 1939; Bromage, 1991; Dean, 1998; Smith, 2006) and known age primates (Smith et al. 2006) also indicate a circadian rhythm. Despite the increasing and widely accepted use of dental incremental structures to reconstruct the timing and sequence of hominid dental development (e.g. Bromage & Dean, 1985; Dean, 1987; Beynon & Dean, 1988; Ramirez-Rozzi, 1998; FitzGerald & Rose, 2000; Dean et al. 2001; Smith et al. 2007a), some researchers have in the past questioned their periodicity and pointed to this as a likely major source of error and, therefore, their reliability when used as a tool to study development (e.g. Warshawsky et al. 1984; Mann et al. 1990, 1991; Lampl et al. 2000; Macho et al. 2003). Indeed, little work has been done to evaluate the most likely sources of error and their periodicity in human teeth: a direct test on human dental enamel appears to be a logical step forward. A pilot study (Antoine et al. 1999) on four teeth from a single archaeologically preserved individual with documentary evidence of age-at-death concluded that cross-striations reflect the circadian rhythm of enamel matrix secretion and suggested that a very detailed reconstruction of crown formation in several teeth from one individual was possible. The work presented in this paper expands that study to include 14 teeth from five known-age individuals using a total of 15 thin sections. The present study differs from previous studies in that we have attempted to identify sources of error when counts of daily cross-striations are extended from birth in one tooth to subsequent teeth developing in the same individual up until the time of death. This particular material was of exceptional quality and, unlike most histological studies of this type, it was possible to observe and count cross-striations throughout each of the crowns. This careful approach was designed not only to add to our understanding of the periodicity of such structures but also to allow us to evaluate potential sources of error that may affect histological studies of this type. In particular, we address issues such as the absence of a recognizable neonatal line, indistinct accentuated striae and the potential affects of prism decussation on crown formation time estimates (e.g. Risnes, 1986; Macho et al. 2003).
Materials
Fundamental to this study was that teeth were available from children whose age-at-death was independently known and whose tooth crowns were still forming at the time of death. This rare opportunity was provided by the archaeological excavation of the 18th/19th century crypt of Christ Church in Spitalfields, London (Molleson & Cox, 1993; Cox, 1996), in which human remains were recovered from 968 coffins. Of these, 387 were of named individuals whose age-at-death could be determined from coffin plates and parish records. These included 63 young children aged between birth and 6 years, with tooth crowns still being formed at the time of death. Dentitions from five children showing good enamel preservation and an advanced state of crown development were selected for the test described here (Table 1). In all five individuals, the teeth showing the best crown preservation were selected for thin sectioning (28 teeth; 37 sections) and, out of these, well-centred intact sections with sufficient crown development and clear enamel growth structures were used to record the prism cross-stations (14 teeth; 15 sections). The teeth were embedded in methylmethacrylate prior to sectioning in order to protect the highly friable enamel formed immediately prior to death (Boyde, 1984; Antoine et al. 1999; Antoine 2001).
Table 1.
The five individual children and their independently known ages-at-death
| Individual | 2365 | 2431 | 2456 | 2520 | 2815 |
| Sex | Male | Male | Female | Female | Female |
| Date of birth in parish records | June 1824 | May 1820 | Feb. 1835 | Jan. 1837 | 14 Sept. 1779 |
| Date of death in parish records | 7 Nov. 1827 | 14 Dec. 1822 | 25 Sept. 1838 | 28 Oct. 1839 | 30 Nov. 1782 |
| Age on coffin plate | 3 years 4 months | 2 years 6 months 3 weeks | 3 years 7 months 17 days | 2 years 9 months 23 days | 3 years 2 months |
| Age from Parish records and coffin plate evidence (days) | 1225–1247* | 934 | 1325† | 1026 | 1173† |
A precise age-at-death is not available due to the lack of detailed information. A previous estimate (Antoine et al. 1999) of 1216 days (3 years 4 months) had not taken into account the month of birth and the revised age-at-death is between 1225 and 1247 days.
Leap years affecting calculations: 1780, 1836.
Methods
The teeth were sectioned in a longitudinal plane from cusp tip to root apex. Section planes were oriented buccal-lingual/palatal (or labial-lingual/palatal) and centred through the tips of the unworn cups – or central mamelon in incisors – and the underlying dentine horns (Fig. 1). The exact section plane varies between tooth types and is strictly a radial plane in incisors and canines (see Schmidt & Keil, 1971; Hillson, 1996). In molars, it does not include the axis of rotation of the tooth, nor is it perpendicular to it, so it is strictly speaking a tangential section centred through the tips of both the buccal/labial and lingual/palatal cusps, and the underlying dentine horns (Fig. 1). This provides the most complete record of the enamel incremental structures, from the first layer formed above the dentine horn to the last layers formed immediately prior to death. A rotating diamond-bladed saw (Buehler Isomet) was used to cut 50-µm-thin sections which were lapped down to a thickness of approximately 100 µm using a Logitech PM2 lapping machine with 3-µm aluminium oxide abrasive slurry. The thin sections were examined and photographed at ×300 between crossed polars with a polarizing transmitted light microscope (Olympus BH-2), using overlapping series of digital colour photographs to create photomontages that provided sufficient detail to show the cross-striations clearly throughout the enamel. To cover the entire crown formation, several photomontages were required for each section, each covering the whole enamel thickness at different parts of the crown (Antoine et al. 1999; Antoine, 2001). One photomontage was employed to record the early enamel formation, found immediately above the dentine horn, whilst additional montages were used to record the rest of the enamel formation, along the side of each crown (Fig. 2). Cross-striations were marked on the photomontages as they were counted. Warshawsky in particular – but also Macho – have emphasized the difficulty of distinguishing prisms sectioned ‘end on’ with those sectioned along their full length (e.g. Warshawsky & Bai, 1983; Macho et al. 2003). Some histologists (Weber & Glick, 1975) have also suggested that cross-striations might be confused with section artifacts more common in electron microscopy (such as ‘knife shatter’). These ideas have now been largely dispelled with the use of laser confocal scanning microscopy (e.g. Dean, 2000) and phase contrast X-ray synchrotron microtomography (e.g. Tafforeau & Smith, 2008). Indeed, the cross-striations and their spacing were confirmed in the Spitalfields specimens used in this study through the use of laser confocal scanning microscopy, which focuses on such a thin plane that artifacts and error resulting from prism cross-section plane can be ruled out (Fig. 3).
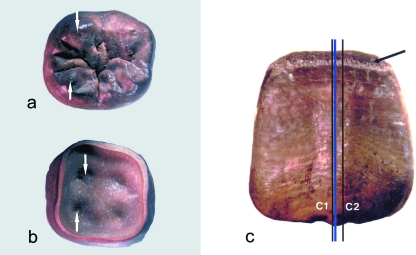
Fig. 1(a–c).
Location of the section planes in (a,b) a developing lower 1st molar (occlusal and apical view) and (c) an upper central incisor (labial view). (a,b) To provide the most complete record of enamel incremental structures, the sections were centred through (a) the tips of the unworn cups (arrows) – or central mamelon in an incisor – and (b) the underlying dentine horns (arrows). Each thin section is achieved by cutting the tooth twice. (c) The first cut (C1) is taken 50 µm away from the ‘ideal plane’ (blue line), with the second cut (C2), taken 500 µm away from the first cut. The sections were then lapped down to a thickness of approximately 100 µm. The friable appearance of the poorly mineralised enamel formed immediately prior to death (arrow) can be seen in (c). (a–c) During the decomposition of the soft tissues, the body fluids appear to have reacted with the wood and lead of the coffins. This unusual archaeological burial environment stained most of the bones and teeth brown (see also Fig. 2).
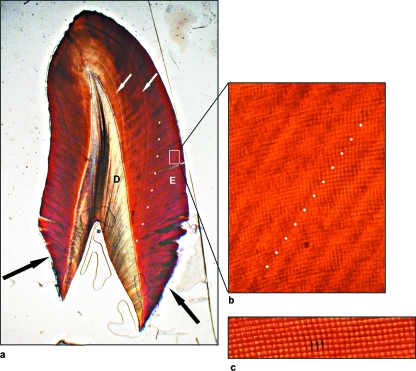
Fig. 2(a–c).
Transmitted light microscope images of long- and short-period incremental lines in the enamel from a section of a developing upper lateral incisor. Section thickness approximately 100 µm. (a) Low magnification overview of the section, with the enamel (E) on the outside, the underlying dentine (D) in the centre and the occlusal edge to the top. The long-period incremental lines, known as striae of Retzius, can be seen radiating away (following the direction of the dotted line) from the enamel-dentine junction. Examples of the more pronounced or marked accentuated striae’ are also present (white arrows). The black arrows point to the last formed enamel prior to death. (b) High-magnification image of the outer enamel showing several striae of Retzius (an example of which is highlighted by the dotted line) running from the bottom left to the top right. The enamel prisms run almost horizontally, with a slight upwards angle, from left to right, with cross-striations marking the prisms at approximately 4-µm intervals. (c) High-magnification image of several enamel prisms running almost horizontally from left to right. Cross-striations (arrows) can clearly be seen along their lengths.
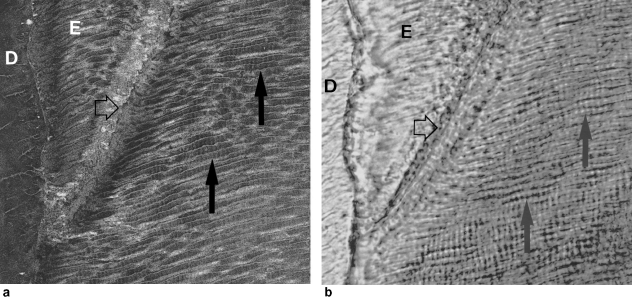
Fig. 3(a,b).
The appearance of enamel cross-striations in a human incisor with the same field of view seen in laser confocal scanning microscopy (a) and transmitted light microscopy (b). Field width approximately 170 µm. Section thickness approximately 100 µm. (a) The confocal microscope (Leica TCS SP; reflection mode; 488 nm laser) used an oil immersion objective, which improves the light-gathering ability, with the oil applied on top of the cover-slip to prevent the enamel being contaminated by oil, as this may cause imaging problems (see Watson, 1997). Confocal microscopy can, by combining laser illumination with a series of apertures, eliminate scattered, reflected, or fluorescent light from out-of-focus planes, and focus into the thickness of the section (in this case 50 µm below the surface) to produce an image of the very thin layer (less than 1 µm) on which the laser beam is focused (see Boyde, 1990b). Boyde (1987, 1989) used confocal microscopy to show that cross-striations were ‘less likely’ to be artifactual, as this type of imaging greatly reduces the likelihood of any optical interference problems that may occur in light microscopy, when light has to pass through several layers of prisms (or in this case, 100 µm of enamel). (a,b) The dentine (D) is to the left of both images, with the enamel-dentine junction running vertically. The rest of both images is all enamel (E) and includes arrows marking features that can be matched between the two types of microscopy. The enamel prisms run almost horizontally, with a slight upwards angle, across the images. Significantly, regular cross-striations (vertical solid arrows), marking the prisms at approximately 4-µm intervals, can be seen on both images. The line of a strongly defined accentuated stria (horizontal open arrow) can also be seen running from halfway across the top of each image to the enamel-dentine junction. In (a), this can be recognised as a sharp line of disruption to the enamel prisms. The confocal image also reveals that the path of the enamel prisms does not always run straight and, whereas the boundaries of the prism appear to run unbroken in the transmitted light image, it is clear in (a) that some are longitudinally sectioned (see lower solid arrow) and some are more transversely sectioned (below the upper solid arrow). There is, however, a clear difference between the cross-striations pointed out by both solid arrows, and the more transversely sectioned prisms. Where the prisms were longitudinally sectioned, the cross-striations are clearly visible and their spacing is almost identical in both images (a, b). This strongly suggests that prism cross-striations are not artifactual, and that it is possible to correctly record such structures using transmitted light microscopy in areas where the prisms are parallel, or close to being parallel, to the plane of section.
To continue cross-striation counts within a tooth from one photomontage to another, and thus record its entire developmental history, the photomontages from each tooth were matched by wider-spaced incremental lines described originally in transmitted light by Anders Retzius as ‘brownish parallel lines’ and now more usually referred to as brown striae of Retzius or Retzius lines (e.g. Retzius, 1837; Boyde, 1989; FitzGerald & Rose, 2000). These striae represent successive outlines of the developing enamel front and, similarly to tree-rings, reveal the layered growth of each tooth (Fig. 2). In transmitted light microscopy, they are visible as a series of light to dark brown lines, which are believed to be the result of near-weekly (or circaseptan) variations in the structure of enamel (Dean, 1987; Boyde, 1989, 1990a; Dean & Scandrett, 1996; FitzGerald & Rose, 2000; Smith et al. 2007b). For any one individual, the count of prism cross-striations between regular striae of Retzius is constant throughout all teeth. In a review of many studies, FitzGerald & Rose (2000) found some individuals with counts as low as 5 or as high as 12 (see also Smith et al. 2007b). The striae of Retzius are not always regular and may appear darker, broader or more accentuated (Fig. 2). These variations in pattern affect all of the enamel matrix being formed at any one age in a given child, so the more pronounced or marked ‘accentuated striae’ can be used to match different teeth from the one dentition and this demonstrates that they represent some systemic physiological change (Fujita, 1939; Gustafson, 1955; Gustafson & Gustafson, 1967; Boyde, 1990a; Antoine et al. 1999). Accentuated striae follow the direction of the other striae of Retzius and some are associated with surface hypoplastic defects (Rose, 1979; Goodman & Rose, 1990; Hillson et al. 1999; FitzGerald & Rose, 2000). They may either enhance a single stria of Retzius or occur between regular striae of Retzius, and are likely to relate to the same kinds of systemic growth disruptions as enamel hypoplasia (Hillson, 1996; Hillson et al. 1999). These kinds of markings are also referred to in the literature as ‘accentuated lines’ (e.g. Smith et al. 2006) or, when associated with hypoplastic defects, as ‘Wilson bands’ (e.g. Goodman & Rose, 1990). Variation in the prominence of various kinds of brown striae does not appear to affect the regularity of cross-striation counts between them. Groups of accentuated striae can therefore be used as reference points by which cross-striation counts can be transferred between different parts of one crown section, and between sections from different teeth in one individual (Antoine et al. 1999; Antoine, 2001).
Expectations
It is unusual for histological material to preserve clearly visible prism cross-striations across large areas of a crown and some estimation is generally required to establish crown formation times (e.g. Smith et al. 2006), such as multiplying Retzius line counts by their cross-striation periodicity (the number of striae of Retzius multiplied by the number of cross-striations between striae of Retzius). In this material, the exceptional quality of the sections made it possible to observe cross-striations throughout the crowns, and no estimates were used. This approach should allow us to identify the most likely difficulties in this type of histological analysis. If prism cross-striations are circadian, as generally accepted, is it to be expected that that their total count from first- to last-formed enamel matrix would match exactly the known age in days? The answer is probably ‘no’. A number of factors affect the count, and need to be taken into account when judging the results and estimating crown formation times.
Accentuated striae
Accentuated striae are important in matching sequences of cross-striations between photomontages. They are produced in the transmitted light microscope image by a light-scattering effect which is different from the optical effects which produce the cross-striations (Osborn & Ten Cate, 1983; Boyde, 1989). The apparent position of an accentuated stria marker is likely to be affected by such factors as the plane of section and orientation of the brown stria plane within the enamel, so the count of cross-striations between markers may vary slightly. The overall cross-striation count for a tooth will, however, be unaffected by this so the maximum count through the sequence is the appropriate one to compare with the known age, rather than a count composed, for example, of mean counts between markers. Boyde (1963, 1990a) also employed accentuated striae to match cross-striation counts up and down the crown. Importantly, he recommended that, as the optical effect of an accentuated stria may include more than one cross-striation, care should be taken when restarting counts from the same stria further down the crown, or in a different tooth. This is unlikely to produce counting errors of more than a few cross-striations but, as noted by Boyde (1963, 1990a), it is possible to test the consistency of the cross-striation counts by comparing the results obtained between corresponding pairs of accentuated striae, both within one tooth and in other teeth developing at the same time (see results below).
Neonatal line
One particular accentuated stria is a special case, because it marks the event of birth (Rushton, 1933; Schour, 1936; Schour & Kronfeld, 1938; Weber & Eisenmann, 1971; Whittaker & Richards, 1978; FitzGerald et al. 1999). This so-called neonatal line is recognized because it divides the apparently smooth course of prenatal enamel matrix formation, which is marked only by cross-striations (and by an absence of clearly marked brown striae), from the ‘normal’ structure of postnatal enamel matrix. The neonatal line is found in all deciduous teeth, as they start to form enamel matrix in utero. It is also usually, but not always, found in the earliest enamel formation of the permanent first molars (Fig. 4). It is sometimes difficult to recognize the neonatal line in permanent first molars as it is often close to the enamel–dentine junction but, where it can be identified, it is an important reference point for the start of cross-striation counts. It has been suggested that this stria may be caused by the decrease in plasma calcium (hypocalcaemia) which occurs in the first 48 h after birth (Nóren, 1984), although others have proposed that the trauma associated with birth causes this type of accentuated stria (Gustafson & Gustafson, 1967; Eli et al. 1989).
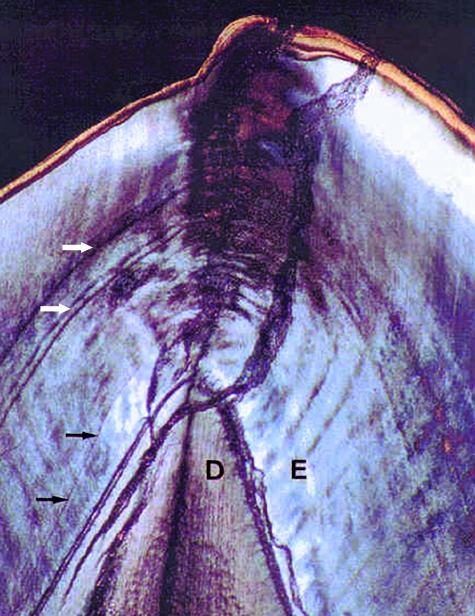
Fig. 4.
Transmitted light microscope image of the neonatal line in the mesio-buccal cusp of the first molar of specimen 2365 seen in crossed polars. Section thickness approximately 100 µm. The cusp tip is to the top of the image. The neonatal line (black arrows) can clearly be seen in the enamel (E) formed immediately above the dentine (D) horn. Examples of accentuated striae (white arrows) can also be seen halfway up the cusp.
In their study of the permanent first molar in tooth germs dissected from aborted foetuses, Christensen & Kraus (1965) showed that in all their specimens one or more of the first molar cusps were mineralized at birth. Lower first molars start to form in utero slightly earlier than upper first molars (Christensen & Kraus, 1965; Kraus & Jordan, 1965), and the mesiobuccal cusp starts to form earlier than the others, so it is to be expected that there will be differences in cross-striation counts between cusps. In any case, the prenatal cross-striation count must vary with the length of gestation at birth and with variation in the timing of the initiation of tooth development. Christensen & Kraus's (1965) research indicates that the upper and lower first molar mesiobuccal cusps initiate enamel formation between 28 and 32 weeks after fertilization, or 12 to 8 weeks (84 to 56 days) before a full-term birth at 40 weeks of gestation. In all but preterm births, at least some prenatal enamel matrix should be present under the mesiobuccal cusp, as the variation of ‘full term’ is commonly taken to be 37–42 weeks (Beischer et al. 1997).
Previous histological work by Schour (1936) recorded the presence of a neonatal line in only approximately 10% of first molar mesio-buccal cusps, whereas Weber & Eisenmann (1971) noted that it was only ‘occasionally present’ in the permanent first molars. It has been suggested that failure to identify a neonatal line in sections may be due its poor visibility in certain specimens, as opposed to a real absence (Eli et al, 1989; Skinner, 1992). As well as variations in the circumstances of birth (Schour & Kronfeld, 1938) – such as differences in trauma, post-natal health or period of hypocalcaemia – the appearance of the neonatal line is likely to be affected by the same factors determining the light-scattering effect of other accentuated striae (see above), such as the plane of section and orientation of the brown stria plane. To include the earliest enamel formation, it is therefore crucial to centre the section on the sharply pointed dentine horn under the mesiobuccal cusp. Even a few micrometers either way could result in either the first cross-striations or the neonatal line being missed. This could be an important source of error. In this project, the crowns were still developing and it was possible to position the plane of section carefully in the earliest-formed enamel as the dentine horns were clearly visible. From the discussion above there is a strong expectation that prenatal enamel matrix would be present, even if a neonatal line is not visible, when the section has been carefully centred in this way.
Immature enamel
In developing teeth, the last formed enamel matrix would not have been fully mineralized at the time of death. The matrix as initially secreted is about 30% mineral by weight (Ten Cate, 1994). This increases to about 96% through the process of maturation (Rosser et al. 1967; Ten Cate, 1994). In the enamel of a developing tooth, the growing edge has a matrix secretion zone, a transition zone and a maturation zone (Boyde, 1989; Suga, 1989; Robinson et al. 1997). Mineral content rises abruptly through the maturation period/zone (see Rosser et al. 1967). The surviving latest formed matrix in most of the specimens from Spitalfields was friable and more porous in appearance than the previously formed enamel (Figs 1 and 5), but it is difficult to estimate which developmental zone it represents or how much of the very last secreted matrix is preserved. This is likely to vary between specimens and must result in the loss of a variable and an unpredictable number of final cross-striation counts. Indeed, previous research on developing teeth from archaeological sites (Stringer et al. 1990) and from the crypt of Christ Church in Spitalfields itself (Dean & Beynon, 1991) has noted the loss of the last formed immature enamel. This has also been observed by Boyde (1990a), although his studies of the maturation of cervical enamel in human permanent teeth have shown that enamel attains a high percentage of its mature mineral content within a few days of its secretion. He thus argued that ‘the amount of tissue likely to be lost from the rotting of immature enamel probably will not amount to more than a week or two's worth of growth’ (Boyde, 1990a, p. 240). Nevertheless, the loss of immature enamel is expected to result in a total count of cross-striations which is less than the known age in days.
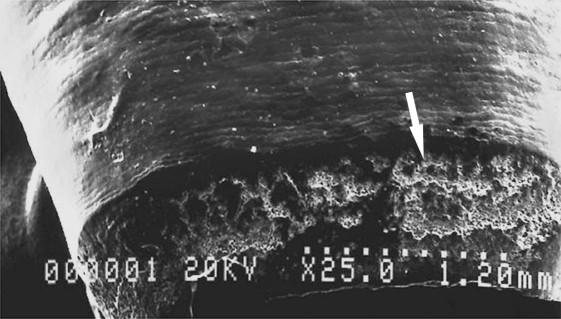
Fig. 5.
Scanning electron microscope image of the developing edge of the labial surface of a lower central incisor. Epoxy replica examined in a Hitachi S570 scanning electron microscope, operated a 20 kV, using an Everhart-Thronley detector. The poorly mineralised enamel matrix of the last-formed increments can be seen at the bottom (white arrow). Running across the surface of the enamel (above the arrow) are the wave-like incremental structures known as perikymata, the surface expression of the brown striae of Retzius.
Interruption of crown formation
In humans, the main period of tooth crown formation takes place during the first 6 years of a child's life. If a child experiences periods of poor health during this time, such as episodes of fever or nutritional deficiencies (Pindborg, 1982), this can disrupt the secretion of enamel matrix and create defects that are visible on the surfaces of teeth. Such defects can take the form of furrows, pits or prominent steps and are usually referred to as enamel hypoplasia (Rose, 1979; Goodman & Rose, 1990; Hillson et al. 1999; FitzGerald & Rose, 2000; Hillson, 2005) or, to distinguish them from defects of genetic origin, developmental defects of enamel (Commission on Oral Health, 1982; Hillson, 1996). The precise mechanisms by which the defects are created during enamel matrix secretion, and their potential impact on the continuity of crown formation, are little understood (Boyde, 1989; Hillson et al. 1999). As discussed above, they are likely to relate to the same systemic growth disruptions as the accentuated striae (Hillson, 1996; Hillson et al. 1999). It is possible that disruptions to crown formation, including an accentuated stria such as the neonatal line (Boyde, 1990a) or a hypoplastic defect, may affect cross-striation counts, but previous research on the Spitalfields teeth (Hillson et al. 1999) suggests that it does not, as the cross-striation counts between regular striae of Retzius do not vary as they pass through the region of a hypoplastic defect.
Prism decussation
The paths of enamel prisms do not run straight throughout the thickness of enamel and, in parts, groups of prisms undulate from side to side in a sinusoidal or helicoidal fashion. This phenomenon is described as prism decussation (Osborn & Ten Cate, 1983; Boyde, 1989). Decussation is most marked directly under the tips of cusps (gnarled enamel) where it swivels in a spiral. Outside this small area of enamel, decussation is much less marked and takes the form of parallel sinusoidal undulations in neighbouring prisms. As shown in Fig. 3, prism paths may appear to be continuous in a two-dimensional thin section but must be a composite of several prisms weaving in and out of the plane of focus (FitzGerald & Rose, 2000). This may affect the total cross-striation counts (Risnes, 1986; Macho et al. 2003) but, if the cross-striations maintain a regular spacing and the majority of the prisms are parallel to section plane (see Fig. 3), the error is unlikely to be large.
Results
The growth structures in the enamel of the Spitalfields teeth proved to be particularly clear and, using the methods outline above for all five individuals, counts of prism cross-striations were made from the first formed enamel matrix under the cusps of permanent first molars to the matrix which was being formed at the time of death. It was possible to follow several paths through each section and, using the accentuated striae, several matches between different teeth from each individual. The similarity between the counts from contemporaneous pairs of accentuated striae indicates that, in sections where the growth structures are clearly visible, cross-striations can be recorded with a good degree of consistency.
From these counts, the maximum count of cross-striations for dental development in each individual was compared with the independently known age-at-death in days. A neonatal line could only be clearly identified in the permanent first molars of Individual 2365 (Table 2). In both its upper and lower molars, the mesiobuccal cusps had the highest counts of prenatal cross-striations at 73 and 38, respectively. The maximum cross-striation count from the neonatal line in the first molar to the last remnants of enamel actively being formed in the second incisor was 1197; short of the known age by 14–38 days (depending on the independent age used, see Table 1). For the first molars of Individuals 2431, 2456, 2520 and 2815 it was not possible to identify clear neonatal lines (Table 3). In each them, their maximum cross-striation counts are between 11 and 45 higher than the known age-in-days.
Table 2.
Neonatal enamel in Individual 2365
| Cusp sectioned | No. of prenatal cross-striations |
|---|---|
| Lower 1st molar – mesiobuccal cusp | 73 |
| Lower 1st molar – mesiolingual cusp | 13 |
| Lower 1st molar – distobuccal cusp | 28 |
| Upper 1st molar – mesiobuccal cusp | 38 |
Table 3.
Cross-striation counts for Individual 2365 (in which neonatal line was identified)
| Tooth | Neonatal line to accentuated striae | Accentuated striae to last formed enamel | Overall count | Known age (days) | Count minus age |
|---|---|---|---|---|---|
| Lower 1st molar | 654 | 544 | 1198* | ||
| Upper 1st incisor | 557 | 1211 | 1225–1247 | –14 to –36 | |
| Upper 2nd incisor | 555 | 1209 | 1225–1247 | –16 to –38 |
1st molar crown was complete before death and a small amount of root formed. The crowns of the other teeth were still forming at death.
Discussion
It was predicted above that the cross-striation counts would be affected by several factors.
Neonatal line
In one individual (2365), the neonatal line was positively identified, whereas in the four others it was not. For Individual 2365, 73 prenatal cross-striations were counted under the mesiobuccal cusp of the lower first molar and 38 for the upper first molar. Dean & Beynon (1991) counted 20–28 prenatal cross-striations for an upper first molar in another individual from the Christ Church crypt whose age was not independently known. Christensen & Kraus's (1965) research on dissected tooth buds (see discussion above) suggests that between 56 and 84 days of prenatal enamel can be expected in the mesiobuccal cusps of first molars (not specifying upper or lower). This fits well with the lower first molar mesiobuccal cusp count from Individual 2365 of 73 prenatal cross-striations. Overall, the cross-striation count for this individual was less than that expected from the independently known age, but this was anticipated for reasons discussed below. In the four specimens where a neonatal line was not identified, the lower first molar mesiobuccal cusp was also the first to initiate but the overall cross-striation count was higher than expected by 11 to 45 striations. At least part of this can be attributed to the difficulty of identifying a neonatal line. If such a line is missed and the section still includes prenatal enamel matrix then, according to the discussion above, up to 84 prenatal cross-striations could be expected to be added to the postnatal striations in the total count. The higher counts are therefore consistent with the lack of an identifiable neonatal line in these specimens. As pointed out above, there is a strong expectation that neonatal enamel matrix would be present in spite of the lack of a recognizable neonatal line.
Immature enamel
For Individual 2365 (Table 3) the maximum cross-striation count, from the neonatal line in the first molar to the last enamel increments surviving, is between 14 and 38 counts lower than the independently known age in days (depending on how it is reckoned from the historical data). This number of cross-striations might represent perhaps 60–150 µm of immature enamel matrix, which is a reasonable amount to have been lost through poor preservation of the most friable part formed immediately prior to death (above). With this in mind, the count makes a good approximation to the known age and, indeed, if the count had been higher than the known age, it would have suggested an error in counting. For Individuals 2431, 2456, 2520 and 2815 the maximum cross-striation counts were all higher than the known age (Table 4). As discussed above this was expected because the neonatal line could not be identified in the lower first molars for these dentitions, so that 56–84 additional prenatal cross-striations might be included in the total count. As in addition some 14–38 cross-striations would be expected to be missing from the last matrix formed, the counts approximate very well to those expected from known age at death. At a maximum the total counts could be expected to exceed age by 84 – 14 = 70 counts. Using similar estimates, the minimum might be 53 – 38 = 15 counts. Overall, therefore, the total cross-striation counts matched well with those expected from the independently known age.
Table 4.
Cross-striation counts for Individuals 2431, 2456, 2520 and 2815
| Individual | Tooth | First formed enamel to accentuated stria | Accentuated stria to last enamel formed | Overall count | Known age (days) | Count minus age |
|---|---|---|---|---|---|---|
| 2431 | Lower 1st molar | 287 | 665 | 952 | 934 | 18 |
| Lower 1st incisor | 658 | 945 | 934 | 11 | ||
| Upper 1st incisor | 661 | 948 | 934 | 14 | ||
| 2456 | Lower 1st molar | 1146 | 218 | 1364 | 1325 | 39 |
| Lower 1st incisor | 206 | 1352 | 1325 | 27 | ||
| Upper canine | 213 | 1359 | 1325 | 34 | ||
| 2520 | Lower 1st molar | 269 | 799 | 1068 | 1026 | 42 |
| Lower 1st incisor | 784 | 1053 | 1026 | 27 | ||
| Lower 2nd incisor | 802 | 1071 | 1026 | 45 | ||
| 2815 | Lower 1st molar | 481 | ||||
| Lower 2nd incisor | 711 | 1192 | 1173 | 19 |
Interruption of crown formation
Cross-striation counts in the crown of Individual 2812 (Table 4) were complicated by the presence of large developmental defects of the type described as enamel hypoplasia. The effect of these defects depends upon their position on the crown in different teeth, but the maximum cross-striation count for the dentition as a whole nevertheless matched well with the known age. This confirms a previous observation that the regular circadian rhythm of enamel matrix secretion continues even during the physiological disturbance that initiates a hypoplastic defect and creates an accentuated stria (above; Hillson et al. 1999). There is then no evidence that enamel formation stops at any kind of regular or accentuated stria, something that has previously been suggested, for example, with respect to the neonatal line at birth (Schour & Massler, 1937; Boyde, 1990a).
Prism decussation
The counts of all five specimens were consistent with those expected from the independently known ages, indicating that prism decussation did not have a noticeable impact on the cross-striation counts. It is likely that the careful centring of sections minimized its effect and areas of obvious decussation or poorly defined growth structures were avoided (Antoine, 2001; Dean, 2004; Smith et al. 2006).
Conclusion
The purpose of this research was twofold: firstly, to test, in human enamel, the generally accepted view that prism cross-striations represent a regular circadian rhythm and, second, to use these unusually clear enamel thin sections to investigate the potential sources of error in histological studies of this kind. Archaeological tooth specimens were used, taken from five children excavated in the crypt of Christ Church, Spitalfields in London. Their ages-at-death were independently known from coffin plates and parish records. Counts of cross-striations were made from the enamel matrix formed in utero, at the start of tooth development, to the last matrix secreted before death. Once the position of the neonatal line in the enamel was taken into account, together with the potential loss of the poorly mineralized matrix which was being deposited at the end, the total cross-striation counts were highly consistent with those expected from the known ages. If we ignore all this, the age estimates were still within 1.2% (Individual 2431) to 4.4% (Individual 2520) of the known ages. We therefore confirm that the cross-striations represent a circadian rhythm in humans, and that it is possible to make reliable counts of them using routine sections and light microscopy. The rhythm appears to have been very close to 24-hourly. The effects of departure from a circadian rhythm would result in considerably different age estimates and, for example, assuming a 12-hourly cross-striation rhythm instead of a 24-hourly one would double the count. If the rhythm was 22-hourly, the total count of Individual 2815, for example, would represent 1093 days rather than 1192, 80 days from the known age of 1173 days. Even a small change in the rhythm would thus make a large difference to the match with known age.
The recording of enamel incremental structures therefore offers a powerful tool with which to investigate in detail the dental development and crown formation of both modern and archaeological remains. This approach provides an independent chronology of crown formation enabling ancient and modern hominids to be compared without relying on the small collection of modern dental developmental standards. It has in any case been argued that such standards are unlikely to be applicable to archaeological populations and fossil hominids (FitzGerald & Rose, 2000; Dean et al. 2001). Enamel incremental structures also allow us to determine an independent age-at-death in individuals whose dentitions are still forming at the time of death and calibrate their skeletal development. This is the only way in which a true growth rate can be calculated for archaeological or fossil specimens. Finally, prism cross-striations provide detailed sequences from which the timing and duration of the growth disruptions seen in hypoplastic defects and accentuated striae can be established (Hillson et al. 1999). The results also confirm that the presence of hypoplastic defects and – in carefully centred sections – the effect of prism decussation do not noticeably affect crown formation time estimates. Significantly, the absence of a recognizable neonatal line can add up to 12 weeks to any estimates and, in developing crowns, the loss of the last-formed poorly mineralized enamel may result in a shortfall of 2–5 weeks.
Acknowledgments
The authors are very grateful for the help of the Natural History Museum, and in particular Theya Molleson and Louise Humphrey, for allowing us access to the Spitalfields skeletal assemblage. We are also very grateful to Sandra Bond, Don Reid and Pam Walton for their help in sectioning the material, and generally providing help and advice. The authors would also like to thank Stuart Laidlaw for his help with the illustrations. Finally, our thanks go to Charles FitzGerald, Helen Liversidge and Gary Schwartz for their help and useful comments during the research. Two anonymous reviewers provided helpful comments on an earlier version of the manuscript. The project was funded by the Wellcome Trust (067257/Z/02/Z).
References
- Antoine D. Evaluating the periodicity of incremental structures in dental enamel as a means of studying growth in children from past human populations. London: University College London, University of London; 2001. Ph.D. Thesis. [Google Scholar]
- Antoine D, Dean C, Hillson S. The periodicity of incremental structures in dental enamel based on the developing dentition of Post-Medieval known-age children. In: Mayhall JT, Heikkinen T, editors. Dental Morphology 1998 – Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 1999. pp. 48–55. [Google Scholar]
- Asper H. Ueber die ‘Braune Retzius'sche Parallelstreifung’ im Schmelz der Menschlichen Zähne. Schweiz Vierteljahrschr Zahnheilkd. 1916;26:275. [Google Scholar]
- Beischer NA, Mackay EV, Cole PE. Obstetrics and the Newborn. 3rd edn. London: WB Saunders; 1997. An Illustrated Textbook. [Google Scholar]
- Berkovitz BKB, Holland GR, Moxham BJ. Oral Anatomy, Histology and Embryology. London: Mosby International Ltd; 2002. [Google Scholar]
- Beynon AD, Dean MC. Distinct dental development patterns in early fossil hominids. Nature. 1988;335:509–514. doi: 10.1038/335509a0. [DOI] [PubMed] [Google Scholar]
- Boyde A. Estimation of age at death of young human skeletal remain from incremental lines in dental enamel. Third International Meeting in Forensic Immunology, Medicine, Pathology and Toxicology. 1963. pp. 36–46.
- Boyde A. Amelogenesis and the structure of enamel. In: Cohen B, Kramer IRH, editors. Scientific Foundations of Dentistry. London: William Heinemann Medical Books Ltd; 1976. pp. 335–352. [Google Scholar]
- Boyde A. Methodology of calcified tissue specimen preparation for scanning electron microscopy. In: Dickson GR, editor. Methods of Calcified Tissue Preparation. Amsterdam: Elsevier; 1984. pp. 251–307. [Google Scholar]
- Boyde A. A 3-D model of enamel development at the scale of one inch to the micron. Adv Dent Res. 1987;1:135–140. doi: 10.1177/08959374870010020101. [DOI] [PubMed] [Google Scholar]
- Boyde A. Enamel. In: Berkovitz BKB, Boyde A, Frank RM, Höhling HJ, Moxham BJ, Nalbandian J, Tonge CH, editors. Teeth. Handbook of Microscopic Anatomy. New York: Springer Verlag; 1989. pp. 309–473. [Google Scholar]
- Boyde A. Developmental interpretations of dental microstructure. In: DeRousseau CJ, editor. Primate Life History and Evolution, Monographs in Primatology Vol 14. New York: Wiley-Liss; 1990a. pp. 229–267. [Google Scholar]
- Boyde A. Confocal optical microscopy. In: Duke PJ, Michette AG, editors. Modern Microscopies: Techniques and Applications. New York: Plenum Press; 1990b. pp. 185–204. [Google Scholar]
- Bromage TG. Enamel incremental periodicity in the pig-tailed macaque: a polychrome fluorescent labeling study of dental hard tissues. Am J Phys Anthropol. 1991;86:205–214. [Google Scholar]
- Bromage TG, Dean MC. Re-evaluation of the age at death of immature fossil hominids. Nature. 1985;317:525–527. doi: 10.1038/317525a0. [DOI] [PubMed] [Google Scholar]
- Christensen GJ, Kraus BS. Initial calcification of the human permanent first molar. J Dent Res. 1965;44:1338–1342. [Google Scholar]
- Commission on Oral Health RE. An epidemiological index of developmental defects of dental enamel (DDE Index) Int Dent J. 1982;52:159–167. [PubMed] [Google Scholar]
- Cox M. Life and Death in Spitalfields: 1700–2850. York: Council for British Archaeology; 1996. [Google Scholar]
- Dean MC. Growth layers and incremental markings in hard tissues: a review of the literature and some preliminary observations about enamel structure in Paranthropus boisei. J Hum Evol. 1987;16:157–172. [Google Scholar]
- Dean MC. The developing dentition and tooth structure in hominoids. Folia Primatol (Basel) 1989;53:160–170. doi: 10.1159/000156414. [DOI] [PubMed] [Google Scholar]
- Dean MC. A comparative study of cross striation spacings in cuspal enamel and of four methods of estimating the time taken to grow molar cuspal enamel in Pan, Pongo and Homo. J Hum Evol. 1998;35:449–462. doi: 10.1006/jhev.1998.0208. [DOI] [PubMed] [Google Scholar]
- Dean MC. Incremental markings in enamel and dentine: what they can tell us about the way teeth grow. In: Teaford MF, Smith MM, Ferguson MWJ, editors. Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. pp. 119–132. [Google Scholar]
- Dean MC. 2D or not 2D, and other interesting questions about enamel: reply to Macho et al. (2003) J Hum Evol. 2004;46:633–640. doi: 10.1016/j.jhevol.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Dean MC, Beynon AD. Histological reconstruction of crown formation times and initial root formation times in a modern human child. Am J Phys Anthropol. 1991;86:215–228. [Google Scholar]
- Dean MC, Scandrett AE. The relation between long-period incremental markings in dentine and daily cross-striations in enamel in human teeth. Arch Oral Biol. 1996;41:233–241. doi: 10.1016/0003-9969(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Dean MC, Leakey MG, Reid D, et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature. 2001;414:628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- Eli I, Sarnat H, Talmi E. Effects of the birth process on the neonatal line in primary tooth enamel. Pediatr Dent. 1989;11:220–223. [PubMed] [Google Scholar]
- FitzGerald C, Rose J. Reading between the lines: dental development and subadult age assessment using the microstructural growth markers of teeth. In: Katzenberg MA, Saunders SR, editors. Biological Anthropology of the Human Skeleton. London: Wiley-Liss; 2000. pp. 163–186. [Google Scholar]
- FitzGerald CM, Saunders SR, Machhiarelli R, Bondioli L. Large scale histological assessment of deciduous crown formation. In: Mayhall JT, Heikkinen T, editors. Dental Morphology 1998 – Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 1999. pp. 92–101. [Google Scholar]
- Fujita T. Neue Feststellungen uber Retzius'schen Parallelstreifung des Zahnschmelzes. Anat Anz. 1939;87:350–355. [Google Scholar]
- Goodman AH, Rose JC. Assessment of systematic physiological pertubations from dental enamel hypoplasias and associated histological structures. Yearb Phys Anthropol. 1990;31:59–110. [Google Scholar]
- Gustafson AG. The similarity between contralateral pairs of teeth. Odontol Tidskr. 1955;63:245–248. [PubMed] [Google Scholar]
- Gustafson G, Gustafson AG. Microanatomy and histochemistry of enamel. In: Miles AEW, editor. Structural and Chemical Organization of Teeth. London: Academic Press; 1967. pp. 135–162. [Google Scholar]
- Gysi A. Metabolism in adult enamel. Dent Dig. 1931;37:661–668. [Google Scholar]
- Hastings MH. The vertebrate clock: localisation, connection and entrainment. In: Redfern PH, Lemmer B, editors. Physiology and Pharmacology of Biological Rhythms. Berlin: Springer-Verlag; 1997. pp. 1–24. [Google Scholar]
- Hillson S. Dental Anthropology. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Hillson S. Teeth. 2nd edn. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Hillson S, Antoine D, Dean C. A detailed developmental study of the defects of dental enamel in a group of post-medieval children from London. In: Mayhall JT, Heikkinen T, editors. Dental Morphology 1998 – Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 1999. pp. 102–111. [Google Scholar]
- Kraus BS, Jordan RE. The Human Dentition before Birth. Philadelphia: Lea & Febiger; 1965. [Google Scholar]
- Lampl M, Mann A, Monge J. A comparison of calcification staging and histological methods for ageing immature modern human specimens. Anthropologie. 2000;XXXVIII/1:51–62. [Google Scholar]
- Macho GA, Jiang Y, Spears IR. Enamel microstructure – a truly three-dimensional structure. J Hum Evol. 2003;45:81–90. doi: 10.1016/s0047-2484(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Mann A, Monge J, Lampl M. Dental caution (correspondence) Nature. 1990;348:202. doi: 10.1038/348202a0. [DOI] [PubMed] [Google Scholar]
- Mann AE, Monge JM, Lampl M. Investigation into the relationship between perikymata counts and crown formation times. Am J Phys Anthropol. 1991;86:175–188. [Google Scholar]
- Mimura T. Horoshitsu ni mirareru Seicho-sen no shuki (The periodicity of growth lines seen in the enamel) Kobyo-shi. 1939;13:454–455. [Google Scholar]
- Molleson TI, Cox M. The People of Spitalfields: the Middling Sort. York: Council for British Archaeology; 1993. [Google Scholar]
- Nóren JG. Microscopic study of enamel defects in deciduous teeth of infants of diabetic mothers. Acta Odontol Scand. 1984;42:153–156. doi: 10.3109/00016358408993866. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Ten Cate AR. Advanced Dental Histology. 4th edn. Bristol: John Wright; 1983. [Google Scholar]
- Pindborg JJ. Aetiology of developmental enamel defects not related to fluorosis. Int Dent J. 1982;32:123–134. [PubMed] [Google Scholar]
- Ramirez-Rozzi F. Enamel structures and development and its application in hominid evolution and taxonomy. J Hum Evol. 1998;35:327–330. doi: 10.1006/jhev.1998.0257. [DOI] [PubMed] [Google Scholar]
- Retzius A. Bemerkungen über den inneren Bau der Zähne, mit besonderer Rücksicht auf dem in Zahnknochen vorkommenden Röhrenbau. (Müllers) Arch Anat Physiol. 1837;1837:486–566. [Google Scholar]
- Risnes S. Enamel apposition rate and the prism periodicity in human teeth. Scand J Dent Res. 1986;94:394–404. doi: 10.1111/j.1600-0722.1986.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Robinson C, Brookes WA, Bonass WA, Shore RC, Kirkham J. Enamel maturation. In: Chadwick DJ, Cardew G, editors. Dental Enamel, Ciba Foundation Symposium 205. Chichester: Wiley; 1997. pp. 156–174. [DOI] [PubMed] [Google Scholar]
- Rose JC. Morphological variations of human enamel prisms within abnormal striae of Retzius. Hum Biol. 1979;51:139–151. [PubMed] [Google Scholar]
- Rosser H, Boyde A, Stewart ADG. Preliminary observations of the calcium concentration in developing enamel assessed by scanning electron-probe x-ray emission microanalysis. Arch Oral Biol. 1967;12:431–440. doi: 10.1016/0003-9969(67)90018-0. [DOI] [PubMed] [Google Scholar]
- Rushton MA. On the fine contour lines of the enamel of milk teeth. Dent Rec. 1933;53:170–171. [Google Scholar]
- Schmidt WJ, Keil A. Polarizing Microscopy of Dental Tissues. Theory, Methods and Results from the Structural Analysis of Normal and Diseased Hard Dental Tissues and Tissues Associated with Them in Man and Other Vertebrates. Oxford: Pergamon Press; 1971. [Google Scholar]
- Schour I. Neonatal line in enamel and dentin of human deciduous teeth and first permanent molar. J Am Dent Assoc. 1936;23:1946–1955. doi: 10.1177/00220345460250030601. [DOI] [PubMed] [Google Scholar]
- Schour I, Kronfeld R. Tooth ring analysis: IV. Neonatal dental hypoplasia analysis of the teeth of an infant with injury of the brain at birth. Arch Pathol. 1938;26:471–490. [Google Scholar]
- Schour I, Massler M. The rate and gradient of growth in human deciduous teeth with special reference to neonatal ring. J Dent Res. 1937;16:349–350. [Google Scholar]
- Skinner M. Gestation length and the location of the neonatal line in human enamel. In: Goodman AH, Capasso LL, editors. Recent Contribution to the Study of Enamel Developmental Defects. Cheiti, Italy: Associazione Antropologica Abruzzese; 1992. pp. 41–50. [Google Scholar]
- Smith TM. Experimental determination of the periodicity of incremental features in enamel. J Anat. 2006;208:99–113. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Reid JD, Sirianni JE. The accuracy of histological assessments of dental development and age at death. J Anat. 2006;208:125–138. doi: 10.1111/j.1469-7580.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Tafforeau P, Reid DJ, et al. Earliest evidence of modern human life history in North African early Homo sapiens. Proc Natl Acad Sci U S A. 2007a;104:6128–6133. doi: 10.1073/pnas.0700747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Reid DJ, Dean MC. New perspectives on chimpanzee and human molar crown development. In: Bailey SE, Hublin JJ, et al., editors. Dental Perspectives on Human Evolution: State of the Art Research in Dental Paleoanthropology. Dordrecht: Springer; 2007b. pp. 177–192. [Google Scholar]
- Stringer CB, Dean MC, Martin RD. A comparative study of cranial and dental development within a recent British sample and among Neanderthals. In: DeRousseau CJ, editor. Primate Life History and Evolution, Monographs in Primatology Vol 14. New York: Wiley-Liss; 1990. pp. 115–152. [Google Scholar]
- Suga S. Enamel hypomineralization viewed from the pattern of progressive mineralization of human and monkey developing enamel. Adv Dent Res. 1989;3:188–189. doi: 10.1177/08959374890030021901. [DOI] [PubMed] [Google Scholar]
- Tafforeau P, Smith TM. Nondestructive imaging of hominoid dental microstructure using phase contrast X-ray synchrotron microtomography. J Hum Evol. 2008;54:272–278. doi: 10.1016/j.jhevol.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Ten Cate AR. Oral Histology: Development, Structure and Function. St. Louis: Mosby; 1994. [Google Scholar]
- Warshawsky H, Bai P. Knife chatter during thin sectioning of rat incisor enamel can cause periodicities resembling cross-striations. Anat Rec. 1983;206:533–538. doi: 10.1002/ar.1092070315. [DOI] [PubMed] [Google Scholar]
- Warshawsky H, Bai P, Nanci A. Lack of evidence for the rhythmicity in enamel development. In: Belcourt AB, Ruch JV, editors. Tooth Morphogenesis and Differentiation. Paris: INSERN; 1984. pp. 241–255. [Google Scholar]
- Watson TF. Fact or artefact in confocal microscopy. Adv Dent Res. 1997;11:433–441. doi: 10.1177/08959374970110040901. [DOI] [PubMed] [Google Scholar]
- Weber DF, Eisenmann D. Microscopy of the neonatal line in developing human enamel. Am J Anat. 1971;132:375–392. doi: 10.1002/aja.1001320307. [DOI] [PubMed] [Google Scholar]
- Weber DF, Glick PL. Correlative microscopy of enamel prism orientation. Am J Anat. 1975;144:407–420. doi: 10.1002/aja.1001440402. [DOI] [PubMed] [Google Scholar]
- Whittaker DK, Richards D. Scanning electron microscopy of the neonatal line in human enamel. Arch Oral Biol. 1978;23:45–50. doi: 10.1016/0003-9969(78)90052-3. [DOI] [PubMed] [Google Scholar]