Abstract
This study analyses some morphological and histological aspects that could have a role in the development of the condylar cartilage (CC). The specimens used were serial sections from 49 human fetuses aged 10–15 weeks. In addition, 3D reconstructions of the mandibular ramus and the CC were made from four specimens. During weeks 10–11 of development, the vascular canals (VC) appear in the CC and the intramembranous ossification process begins. At the same time, in the medial region of the CC, chondroclasts appear adjacent to the vascular invasion and to the cartilage destruction. During weeks 12–13 of development, the deepest portion of the posterolateral vascular canal is completely surrounded by the hypertrophic chondrocytes. The latter emerge with an irregular layout. During week 15 of development, the endochondral ossification of the CC begins. Our results suggest that the situation of the chondroclasts, the posterolateral vascular canal and the irregular arrangement of the hypertrophic chondrocytes may play a notable role in the development of the CC.
Keywords: development, mandibular condylar cartilage, vascular canal
Introduction
The primary and secondary cartilages have different embryonic origins. Primary cartilages are considered to be part of the primary skeletal cartilage. Secondary cartilages appear later in embryonic development and are independent of the primary skeletal cartilage. The two types of cartilages show differences in histological organization and in the pattern of cell proliferation (Delatte et al. 2004). In primary cartilages, interstitial cell proliferation occurs in chondrocytes, whereas secondary cartilage shows appositional proliferation (Durkin, 1972; Copray et al. 1986). During development, primary cartilage reacts primarily to systemic growth stimuli such as hormones. By contrast, the secondary cartilage only secondarily follows these overall stimuli after additional modulation by local growth factors (Enlow, 1992; Sperber, 2001).
Mandibular condylar cartilage (CC) is often classified as secondary cartilage because it differs to some extent from primary skeletal cartilage (Proff et al. 2007). Most studies on the CC during development have dealt with the temporomandibular joint (TMJ) (see Mérida Velasco et al. 1999). The terminology used for the histological description of CC layers during development differs among authors (Table 1); in the present study, we will follow the terminology proposed by Gómez de Ferraris & Campos (1999) because it best matches our histological observations.
Table 1.
Overview of terminology of the layers identified in the mandibular condylar cartilage during development
| Study | Layers identified | ||||
|---|---|---|---|---|---|
| Copray et al. (1986) | Articular | prechondroblastic | transitional | hypertrophic chondrocytes | |
| Takenoshita (1987) | Articular | proliferative | hypertrophic | non mineralized | mineralized |
| Livne et al. (1990) | Perichoarthral | proliferative (chondroprogenitor cells) | chondroblasts | chondrocytes | hypertrophic chondrocytes |
| Ben-Ami et al. (1992) | Articular | chondroprogenitor | chondroblast | non mineralized hypertrophic | mineralized hypertrophic |
| Ben-Ami et al. (1993) | Articular | progenitor | chondroblast | hypertrophic chondrocytes | |
| Gómez de Ferraris & Campos Muñoz (1999) | Superficial | proliferative (mesenchymal) | chondroblasts | chondrocytes | hypertrophic chondrocytes |
| Habib et al. (2005) | Mesenchymal | prehypertrophia | hypertrophic chondrocytes | ||
| Shen & Darendeliler (2005) | Articular | resting | proliferative | hypertrophic chondrocytes | erosive |
| Shibukawa et al. (2007) | Fibroblastic | polymorphic | flattened chondrocytes | hypertrophic chondrocytes | |
Vascular canals (VC), the structures of vascularized mesenchyme present in the epiphyseal and diaphyseal cartilage of bones prior to ossification (Blumer et al. 2004), are found in mammals, birds, reptiles, and amphibians (Haines, 1933; Kuettner & Pauli, 1983). Some authors have pointed out that the function of the VC is the nutrition of cartilages (Haines, 1933; Lufti, 1970; Wilsman & Van Sickle, 1970). In this regard, some studies have shown that VC may play a certain role in the process of endochondral bone formation (Burkus et al. 1993; Ganey et al. 1995; Roach et al. 1998; Blumer et al. 2004, 2006). However, little attention has been paid to VC in the CC during its development. Blackwood (1965) reported that VC were constantly present in the CC from human specimens of 130-mm crown-rump length (CRL) and in specimens of 2 years 9 months.
According to Blackwood (1965), the true function of VC is to increase the nutrition of the cartilage during a period of rapid growth. Thilander et al. (1976) reported a reduction of the VC in the CC of 5- and 6-year-old specimens, whereas Takenoshita (1987) reported a continual reduction up to the age of 10.
In a previous study, we identified three phases in TMJ development: (1) the blastematic stage (weeks 7–8 of development); (2) the cavitation stage (weeks 9–11 of development); and (3) the maturation stage (after week 12 of development). The chondrification of the mandibular condyle was observed to occur in week 9 of development, at the same time as the inferior articular cavity appears (Mérida Velasco et al. 1999). In the present study, we analyse the histological characteristics of the CC as well as the structure and layout of the VC during weeks 10–15 of human fetal development.
In this work we examine the development of the CC in 10–15-week-old human fetuses using light microscopy, with the aim of clarifying some morphological and histological aspects which may have a bearing on the growth of the CC. There have been studies on the development of the mandible and the TMJ using 3D reconstructions (Radlanski et al. 2003). However, there is no available 3D description of the normal development of the CC in 10–15-week-old human fetuses. This study furnishes such 3D images, making it possible to observe CC morphology during that developmental period.
Material and methods
In this study, 49 human specimens were used, ranging from 10 to 15 weeks of development (postfertilization weeks). All the specimens studied belong to the collection of the Institute of Embryology of the Complutense University of Madrid. The specimens were obtained from the Department of Obstetrics after miscarriages and ectopic pregnancies. The absence of external and congenital malformations was verified. Specimens were fixed in 10% formalin and transferred to the laboratory. The parameters used to determine the age of gestation were CRL, weight, and cranial perimetry (O’Rahilly & Müller, 1996). All the specimens, which had previously been decalcified in trichloroacetic acid, were then dehydrated in a graded series of ethanol and finally embedded in paraffin wax. The usual laboratory procedures were used to prepare 10–20-µm-thick transverse, frontal and sagittal serial sections, which were stained with haematoxylin-eosin and azocarmine (McManus & Mowry, 1968). All sections were examined under light microscopy. The study was carried out using a Nikon Eclipse E400 microscope and a Nikon DXM 1200 digital camera coupled to a Pentium IV PC.
The study was approved by the Ethics Committee of the Medical Faculty of the Complutense University of Madrid.
In specimens Be-503, Be-101, JR6 and B29, the mandibular ramus and the condylar cartilage were reconstructed in 3D. Three-dimensional images were constructed using the StudioMax 7 3D Reconstruction programme, a software specifically designed for 3D modelling (Autodesk Inc.). Each of the cross-sections generated by the microscope can be converted into a set of complex polygonal lines, automatically sectorizing contours of each section as if they were the kind of level curves or splines used in topography. The curves then have to be corrected manually to avoid identification errors. These two-dimensional curves are converted to three dimensions by assigning them a width equivalent to the distance between the cross-sections.
The vertices of the splines are superimposed and joined to produce a continuous three-dimensional surface. Smoothing techniques are used to remove any irregularities in the three-dimensional mesh. Finally, the programme assigns different textures to the surfaces of the reconstruction to make it more realistic and to distinguish the different structures. The result is a set of objects which can be visualized from any point of view and which allows the use of real light effects, shadows and transparencies.
Results
Weeks 10 and 11
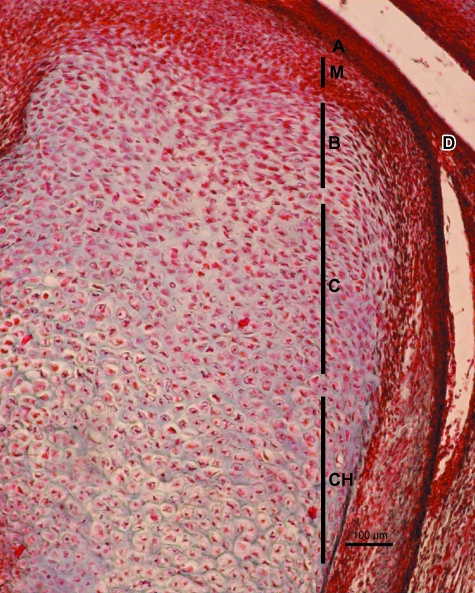
At week 10 of development the CC presented an inverted-cone shape pointing in the direction of the future mandibular foramen (Fig. 1). The CC is linked cranially with the lower articular cavity of the TMJ. The CC presents a compact surface layer of flattened cells corresponding to the articular layer. This layer is continuous with the cell layer surrounding the mandible. More deeply, there is a mesenchymal layer composed of small, round cells. The third layer is composed of polymorphous cells surrounded by a scanty cartilaginous intercellular matrix (chondroblastic layer). The fourth layer is composed of chondrocytes surrounded by slightly basophilic cartilaginous intercellular matrix (Fig. 2).
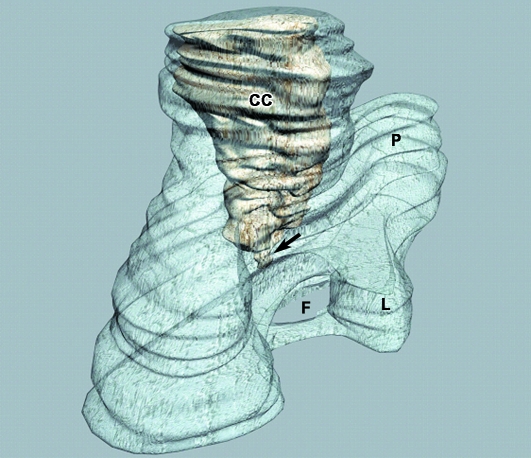
Fig. 1.
3D reconstruction of the mandibular ramus and condylar cartilage (CC) of human fetus Be-101 (65 mm CRL; 11 weeks of development). Posteromedial view. P, coronoid process; L, lingula; F, mandibular foramen; vertex of condylar cartilage (arrow).
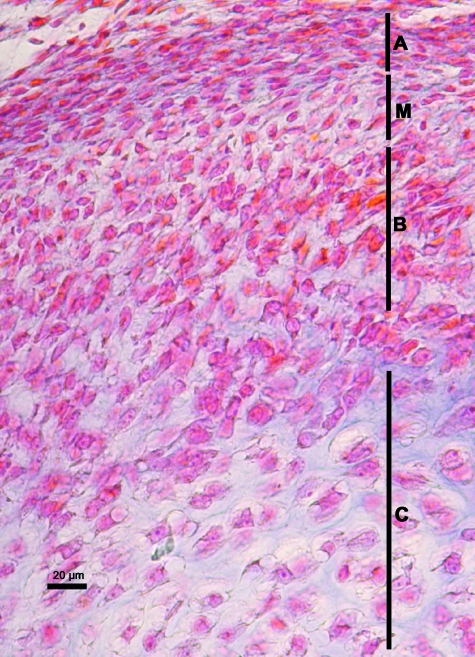
Fig. 2.
Human fetus Ca-6 (52-mm CRL; 11 weeks of development). Frontal section. Haematoxylin-eosin staining. A, articular layer; M, mesenchymal layer; B, chondroblastic layer; C, chondrocyte layer. The mesenchymal layer is diminished on the left hand side of the figure due to the obliquity of the section. Bar: 20 µm.
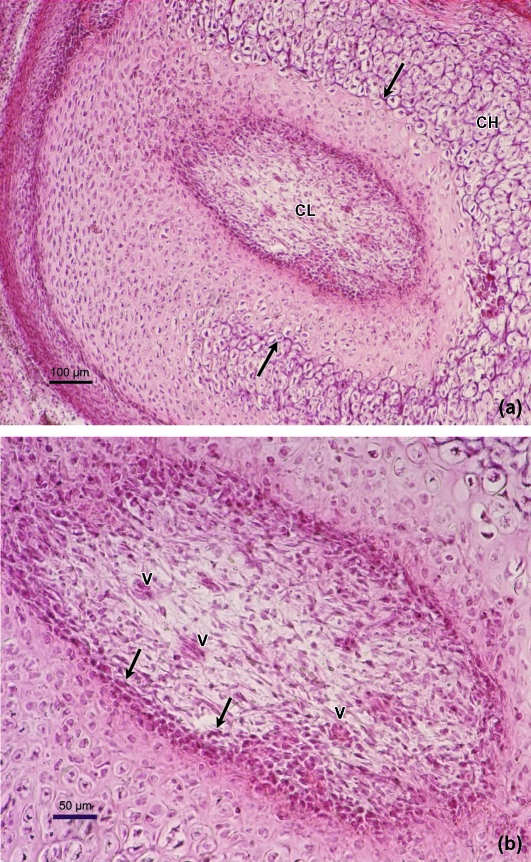
Mesenchymal invaginations forming the VC were observed in week 10 of development; these were located in two regions of the CC, posteromedial and posterolateral, and VC differed considerably. The first difference was in the location. The canals in the posteromedial region are located in the area where the mandibular condyle links with Meckel's cartilage and the auriculotemporal nerve and the maxillary vessels run between the two. Moreover, the posteromedial VC oscillated between one and three in number (Fig. 3a,b). In contrast, there is only one posterolateral canal, larger than posteromedial canals and located dorsal to the future outer pole of the condyle. The 3D reconstruction of the specimens from this developmental period shows the depth and length of the posterolateral canal, which extends nearly as far as the CC vertex (Fig. 4a,b). The VC are composed of a central mesenchymatous axis containing vessels, with a superficial layer of cuboid cells as a pocket-like formation, and associated with the adjacent cartilaginous tissue (Fig. 5a,b).
Fig. 3.
(a) Human fetus Be-503 (48 mm CRL; 10 weeks of development). Frontal section. Haematoxylin-eosin staining. There are two posteromedial VC (arrows) associated with the auriculotemporal nerve (AT). LP, lateral pterygoid muscle; CM, Meckel's cartilage; T, temporalis muscle; D, articular disc; asterisk, lower articular cavity. Bar 200 µm. (b) Reconstruction of condylar cartilage of human fetus Be-101 (65 mm CRL; 11 weeks of development). Arrows indicate posteromedial VC.
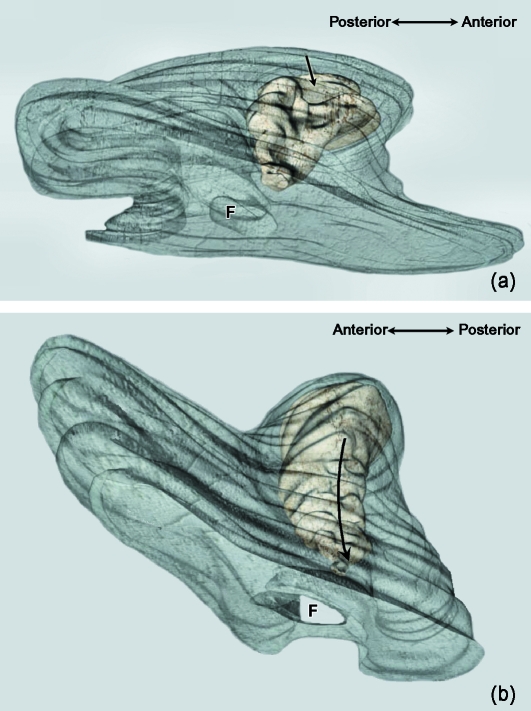
Fig. 4.
(a) 3D reconstruction of the mandibular ramus and condylar cartilage of human fetus Be-503 (48 mm CRL; 10 weeks of development). Inferolateral view. Arrow indicates posterolateral canal; F, mandibular foramen. (b) 3D reconstruction of the mandibular ramus and condylar cartilage of human fetus Be-101 (65-mm CRL; 11 weeks of development). Inferolateral view of the posterolateral canal; F, mandibular foramen.
Fig. 5.
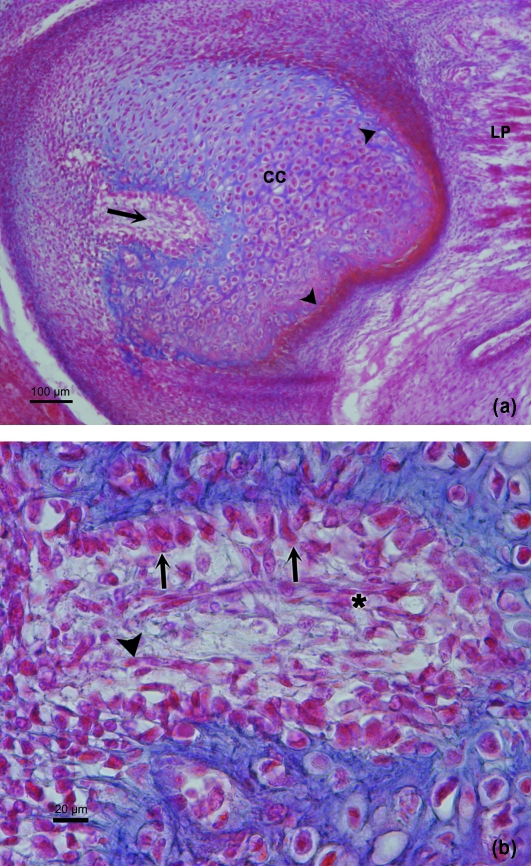
(a) Human fetus Be-503 (48-mm CRL; 10 weeks of development). Azocarmine staining. Frontal section. The posterolateral canal (arrow) deepens into the condylar cartilage (CC). Arrowhead, intramembranous ossification; LP, lateral pterygoid muscle. Bar 100 µm. (b) Human fetus Be-503 (48-mm CRL; 10 weeks of development) Frontal section. Azocarmine staining. The periphery of the posterolateral canal shows cuboid cells in band formation (arrows), associated with the cartilaginous intercellular matrix. In the lower part of the canal the band arrangement is not clearly discernible because of the obliqueness of the section. Inside the canal, mesenchymal cells (arrowhead) and vessels (asterisk) appear. Bar: 20 µm.
During week 11 of development a process of intramembranous ossification began. This ossification extended from the area of insertion of the lateral pterygoid muscle to the posteromedial region of the CC (Fig. 5a) and was continued by the bony tissue of the posterior edge of the mandibular ramus.
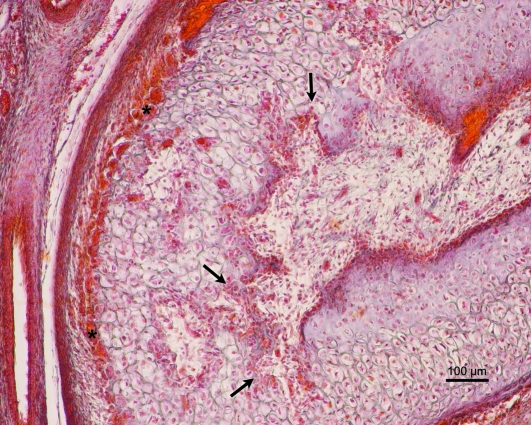
During this period, hypertrophic chondrocytes were observed in the deepest part of the CC. This zone is limited by bony tissue of intramembranous origin. In addition, large multinucleated cells were observed in the medial region of the CC, along with instances of vascular invasion and cartilage destruction (Figs 6 and 7a,b).
Fig. 6.
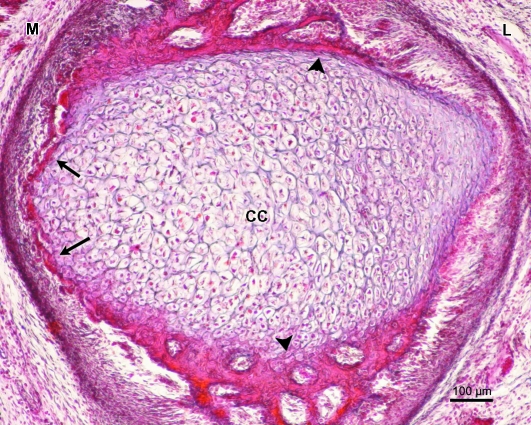
Human fetus Be-101 (65-mm CRL; 11 weeks of development). Frontal section. Azocarmine staining. The condylar cartilage (CC) is surrounded by intramembranous ossification (arrowhead). Large multinucleated cells (arrow) are visible in the medial part of the condylar cartilage (CC) but not in the lateral part of the cartilage. M, medial; L lateral. Bar: 100 µm.
Fig. 7.
(a) Human fetus Be-101 (65-mm CRL; 11 weeks of development). Frontal section. Azocarmine staining. In the medial part of the condylar cartilage there are multinucleated cells (arrows), along with vascular invasion and destruction of the condylar cartilage (arrowhead). CH, hypertrophic chondrocytes. Bar: 50 µm. (b) Magnification of (a). Multinucleate cell (arrow). Bar 15 µm.
Weeks 12 and 13
During weeks 12 and 13, the vascular canal in the posterolateral region acquired the shape of a deep canal, clearly visible in the 3D reconstruction (Fig. 8). Caudally, however, it is completely surrounded by the hypertrophic chondrocytes (Fig. 9a,b). The vessels of the posterolateral canal also participated in the vascular invasion and cartilage destruction (Fig. 10). The sagittal sections of the specimens from this period show the hypertrophic chondrocytes forming an irregular arrangement without columns (Fig. 11).
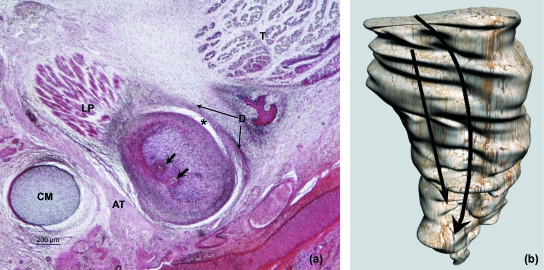
Fig. 8.
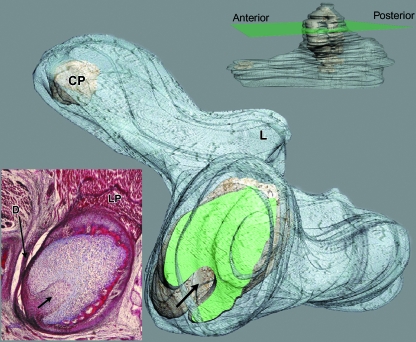
3D reconstruction of human fetus JR-6 (80-mm CRL; 12 weeks of development). The upper-right image indicates the section level (green plane). The reconstruction shows the arrangement of the condylar cartilage at the section level labelled in green (arrow, posterolateral vascular canal). The lower-left image shows the histological section that matches the section level pointed out in the reconstruction. L, lingula; CP, cartilage of the coronoid process; LP, lateral pterygoid muscle; D, articular disc. Bar: 200 µm.
Fig. 9.
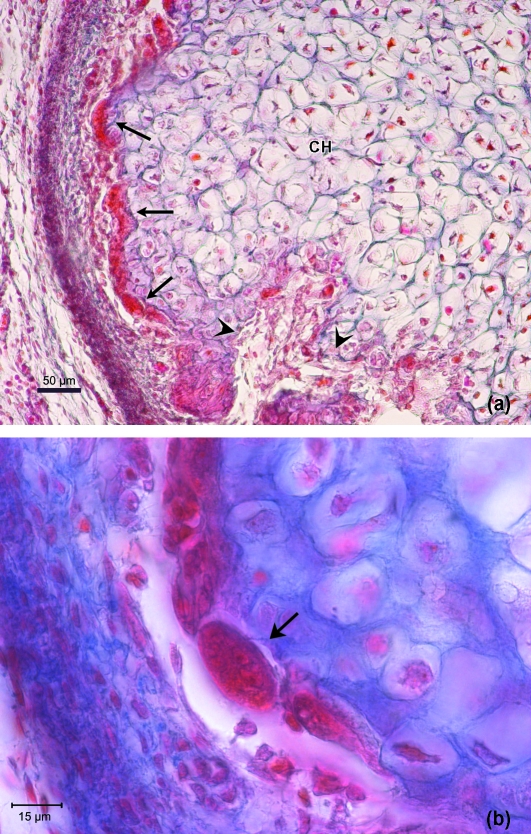
(a) Human fetus HL30 (74 mm CRL; 12 weeks of development). Transverse section. Haematoxylin-eosin staining. Caudally, the posterolateral vascular canal (CE) is a conduit inside the condylar cartilage. The canal is surrounded by a thick layer of cartilaginous tissue (arrows). The hypertrophic chondrocytes layer is diminished on the left-hand side of the figure due to the obliquity of the section. CH, hypertrophic chondrocyte. Bar: 100 µm. (b) Magnification of (a). Cuboid cells in band formation (arrows) on the periphery of the posterolateral vascular canal; vessels (V) appear on the inside. Some chondrocytes are visible in the cartilaginous intercellular matrix surrounding the canal. Bar: 50 µm.
Fig. 10.
Human fetus HL-30 (74-mm CRL; 12 weeks of development). Transverse section. Azocarmine staining. In the lower part of the condylar cartilage there are signs of vascular invasion and cartilage destruction (arrows). Some chondroclasts are visible in the medial part of the condylar cartilage (asterisk). D, articular disc. Bar: 100 µm.
Fig. 11.
Human fetus Be-516 (82-mm CRL; 13 weeks of development). Sagittal section. Haematoxylin-eosin staining. The different layers of the condylar cartilage are visible. A, articular layer; M, mesenchymal layer; B, chondroblastic layer; C, chondrocyte layer, CH, hypertrophic chondrocyte layer. Bar: 100 µm.
Weeks 14 and 15
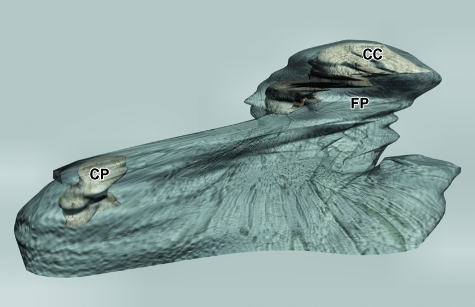
The destruction of the CC in the cranial region reached the area of insertion of the lateral pterygoid muscle. The 3D reconstruction shows the morphology of the CC and the pterygoid fovea in the anteromedial area (Fig. 12).
Fig. 12.
3D reconstruction of the mandibular ramus and condylar cartilage of human fetus B-29 (116-mm CRL; 15 weeks of development). Anteromedial view. The condylar cartilage (CC) and the cartilage of the coronoid process (CP) are visible. FP, pterygoid fossa.
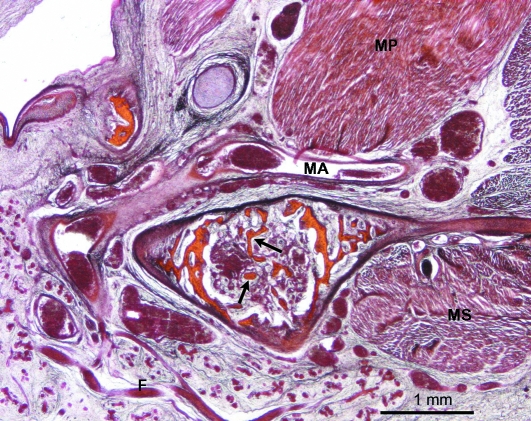
During week 15 of development, endochondral ossification of the CC was observed with the presence of osseous trabeculae in the area through which the maxillary vessels and the auriculotemporal nerve pass (Fig. 13).
Fig. 13.
Human fetus B-29 (116 mm CRL; 15 weeks of development). Transverse section. Azocarmine staining. Osseous trabeculae (arrows). F, facial nerve; MA, maxillary artery; MS, masseter muscle; MP, medial pterygoid muscle. Bar: 1 mm.
Discussion
It has been established that in human specimens, chondrification of the mandibular condyle commences in week 9 of development (Mérida Velasco el al. 1999; Suk et al. 2001). Our study of the CC therefore deals with specimens in at least week 10 week of development. At this time, the 3D reconstructions show that the CC has an inverted cone shape with the vertex located at the level of the future mandibular foramen. However, in the 3D reconstructions of the mandible by Radlanski et al. (2003), the CC appeared to acquire that shape during week 11 of growth. During the development period considered, the CC consists of five cell layers: the articular layer, the mesenchymal layer, the polymorphous cell layer with scanty cartilaginous intercellular matrix (chondroblastic layer), the chondrocyte layer, and the deeper layer of hypertrophic chondrocytes. Some authors suggest that the polymorphous cell layer corresponds to the cells known as skeletoblasts (Silbermann et al. 1987) or osteoprogenitor cells (Geneser, 2000). These are derived from the mesenchymal cells and possess the ability to form chondrocytes or osteocytes, depending on the biomechanical context. We therefore believe that the parallel growth of the CC and the TMJ is important, as noted in an earlier article (Mérida Velasco et al. 1999). Formation of the lower articular cavity commences during week 9 of development, coinciding with the onset of chondrogenesis, whereas organization of the upper articular cavity commences during week 11 of development. The movement of the TMJ during these growth phases presumably facilitates the differentiation of these cells into chondrocytes. It has been reported that joint movement stimulates condylar chondrification (Strauss et al. 1990; Ben-Ami et al. 1993; Habib et al. 2005); however, other studies (Glasstone, 1971; Vinkka-Puhakka & Thesleff, 1993) have reported CC development in the absence of mechanical stimulation. Also, the arrangement of the hypertrophic chondrocytes of the CC, which is clearly visible in the sagittal sections, is irregular. Nevertheless, the hypertrophic chondrocytes in the epiphyses of the long bones adopt a columnar arrangement in those areas associated with the metaphysary region during bone growth. These observations confirm earlier reports (Durkin, 1972; Thilander et al. 1976; Gómez de Ferraris & Campos, 1999). The irregular arrangement of the hypertrophic chondrocytes could favour multidirectional growth of the condyle, depending on external mechanical stimuli.
The VC in the CC were first described by Vinogradoff (1910), who used the term ‘crampons’ to denote them. Most authors consider the VC to derive from invaginations of the perichondral mesenchyme (Haines, 1933; Levene, 1964; Blackwood, 1965; Lufti 1970; Wilsman & van Sickle, 1970; Kugler et al. 1979; Chappard et al. 1986; Ganey et al. 1992, 1995; Burkus et al. 1993; Reidenbach & Schmidt, 1994; Cheng et al. 1997; Shapiro 1998; Blumer et al. 2006). However, some authors have suggested that the perichondrium is not necessary for development of the VC (Delgado-Baeza, 1991a,b). Like the studies on the CC carried out by Blackwood (1965) and Fuentes (1988), our own study confirms that the VC are derived from mesenchymal invaginations. The VC in the CC of human specimens appear during week 10 of development. Nonetheless, Symons (1952) reported that the VC appeared in the CC during week 19, whereas Blackwood (1965) and Fuentes (1988) reported its appearance in week 15 of development. The VC are located in the posteromedial and posterolateral areas of the CC. This arrangement was originally reported by us (Mérida Velasco, 2002) and is now confirmed by 3D reconstructions. Blackwood (1965) reported that the number of VC in the CC increased in the posterior and lateral region. A pattern of VC appearance has been reported in other studies in long bones (Brookes, 1958; Reidenbach & Schmidt, 1994) and in short bones (Cheng et al. 1997). Our results show that the VC in the CC of both regions present considerable differences. The VC in the posteromedial area are one to three times shallower. However, the 3D reconstructions show only one vascular canal in the posterolateral area of the CC; this becomes a genuine conduit inside the CC, reaching as far as the lower layer where the hypertrophic chondrocytes are located. In the latter layer, the posterolateral vascular canal presents chondroblasts aligned in the periphery, surrounded by cartilaginous intercellular matrix with few chondrocytes. This may suggest appositional growth towards the cartilage. In addition, the vessels of this canal participate in the invasion and destruction of the cartilage.
There have been reports of the presence of certain types of cells in the canals: chondroclasts (Kugler et al. 1979; Cole & Wezeman, 1985); multinucleated cells (Chandraraj & Briggs, 1988; Ganey et al. 1992, Burkus et al. 1993); multivacuolated cells (Rodríguez et al. 1985); monocytes/macrophages (Chappard et al. 1986); fibroblast, multinucleated cells and multivacuolated cells and histiocytes (Delgado-Baeza et al. 1991a). We suggest that cells of these types could be derived from mesenchymal cells. We found that the canal contained mesenchymal cells and vessels. During weeks 10 and 11 of development, cuboid cells were detected on the periphery of the VC, arranged in band formation and associated with the cartilaginous intercellular matrix. These banded cells were reported by Fuentes (1988) in human specimens of 212-mm CRL. We believe that these cells are chondroblasts that are derived from the mesenchymal cells in the canal and would produce chondrocytes.
Our results show that during week 11 of development, there was intramembranous ossification from the area of insertion of the lateral pterygoid muscle to the posteromedial area, coinciding with the appearance of the upper articular cavity of the TMJ. This ossification was continued by the bony tissue of the posterior edge of the mandible. Other authors have reported similar results (Perry et al. 1985; Berraquero et al. 1992; Radlanski et al. 1999).
During weeks 10–11 of development, large multinucleated cells appeared together with vascular invasion and cartilage destruction. These cells may be chondroclasts and they may locate in the hypertrophic chondrocyte layer. The chondroclasts have ultrastructural features similar to osteoclasts (Lewinson & Silbermann, 1992). However, the chondroclasts and osteoclasts differ not only in location but possibly also in mode of action (Nordahl et al. 1998). The location of the chondroclasts in the medial area of the CC would explain the description given by Thilander et al. (1976), who reported that in young specimens there was a lateral apposition but medial resorption.
The classical theory is that during development, the CC presents a kind of appositional growth (Durkin, 1972; Beresford, 1981; Copray et al. 1986). However, other factors may be involved in the growth of the condyle. For instance, the posterolateral vascular canal is important in that it influences the rearward and outward development of the CC and may also contribute to appositional growth. Moreover, the posterolateral vascular canal may be a relevant structure for the growth of the CC during a stage of development in which TMJ and its muscles are not yet developed, and the activity of the latter is very reduced. In addition, the vascularization by means of the VC has been suggested to play an important part in cartilage development, as damage to the VC may provoke severe pathologies (Visco et al. 1991; Carlson et al. 1995; Bravo et al. 1996). At the same time, the location of the chondroclasts, vascular invasion, and cartilage destruction in the medial area, all presumably favour a process of remodelling of the CC, causing it to grow outwards.
Acknowledgments
We wish to express our gratitude to the following for their technical assistance in the elaboration of this paper: Prof. Jesús Boya Vegue and Mrs Montserrat Juanilla Pérez.
References
- Ben-Ami Y, Lewinson O, Silbermann M. Structural characterization of the mandibular condyle in human fetuses: light and electron microscopy studies. Acta Anat. 1992;145:79–87. doi: 10.1159/000147346. [DOI] [PubMed] [Google Scholar]
- Ben-Ami Y, Mark K, Franzen A, Bernard B, Lunazzi GC, Silbermann M. Transformation of fetal secondary cartilage into embryonic bone in organ cultures of human mandibular condyles. Cell Tissue Res. 1993;271:317–322. doi: 10.1007/BF00318618. [DOI] [PubMed] [Google Scholar]
- Berraquero R, Palacios J, Rodríguez JI. The role of the condylar cartilage in mandibular growth. Am J Orthod Dentofac Orthop. 1992;102:220–226. doi: 10.1016/S0889-5406(05)81056-X. A study in thanatophoric dysplasia. [DOI] [PubMed] [Google Scholar]
- Blackwood H. Vascularization of the condylar cartilage of the human mandible. J Anat. 1965;99:551–563. [PMC free article] [PubMed] [Google Scholar]
- Blumer MJ, Longato S, Fritsch H. Cartilage canals in the chicken embryo are involved in the process of endochondral bone formation within the epiphyseal growth plate. Anat Rec. 2004;279A:692–700. doi: 10.1002/ar.a.20058. [DOI] [PubMed] [Google Scholar]
- Blumer MJ, Schwarzer C, Perez MT, Konakci K, Fritsch H. Identification and location of bone forming cells within cartilage canals on their course into the secondary ossification centre. J Anat. 2006;208:695–707. doi: 10.1111/j.1469-7580.2006.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo C, Kawamura H, Yamaguchi T, Hotokebuchi T, Sugioka Y. Experimental osteochondrosis dissecans. Fukuoka Acta Med. 1996;87:133–141. The role of cartilage canals in chondral fractures of young rabbits. [PubMed] [Google Scholar]
- Brookes M. The vascularization of long bones in the human foetus. J Anat. 1958;92:261–267. [PMC free article] [PubMed] [Google Scholar]
- Burkus JK, Ganey TM, Odgen JA. Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. Yale J Biol Med. 1993;66:193–202. [PMC free article] [PubMed] [Google Scholar]
- Carlson CS, Cullins LD, Meuten DJ. Osteocondrosis of the articular-epiphyseal cartilage complex in young horses: evidence for a defect in cartilage canal blood supply. Vet Pathol. 1995;32:641–647. doi: 10.1177/030098589503200605. [DOI] [PubMed] [Google Scholar]
- Chandraraj S, Briggs CA. Role of cartilage canals in osteogenesis and growth of the vertebral centra. J Anat. 1988;158:121–136. [PMC free article] [PubMed] [Google Scholar]
- Chappard D, Alexandre C, Riffat G. Uncalcified cartilage resorption in human fetal cartilage canals. Tissue Cell. 1986;118:701–707. doi: 10.1016/0040-8166(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Cheng X, Wang Y, Qu H. Intrachondral microvasculature in the human fetal talus. Foot Ankle Int. 1997;18:335–338. doi: 10.1177/107110079701800604. [DOI] [PubMed] [Google Scholar]
- Cole AA, Wezeman FH. Perivascular cells in cartilage canals of the developing mouse epiphysis. Am J Anat. 1985;174:119–129. doi: 10.1002/aja.1001740203. [DOI] [PubMed] [Google Scholar]
- Copray JCVM, Jansen HWB, Duterloo HS. Growth and growth pressure of mandibular condylar and some primary cartilages of the rat in vitro. Am J Orthod Dentofac Orthop. 1986;90:19–28. doi: 10.1016/0889-5406(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Delatte M, Von den Hoff JW, Van Rheden REM, Kuipers-Jagtman AM. Primary and secondary cartilages of the neonatal rat: the femoral head and the mandibular condyle. Eur J Oral Sci. 2004;112:156–162. doi: 10.1111/j.0909-8836.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Delgado-Baeza E, Gimenez M, Miralles C, Nieto A, Santos I. Relationship between the cartilage canal and the perichondrium in the rat proximal tibial epiphysis. Acta Anat. 1991a;141:31–35. doi: 10.1159/000147095. [DOI] [PubMed] [Google Scholar]
- Delgado-Baeza E, Gimenez M, Miralles C, Nieto A, Santos I. Morphogenesis of the cartilage canals: experimental approach in the rat tibia. Acta Anat. 1991b;142:132–137. doi: 10.1159/000147177. [DOI] [PubMed] [Google Scholar]
- Durkin JF. Secondary cartilage: A misnomer? Am J Orthod. 1972;62:15–41. doi: 10.1016/0002-9416(72)90122-4. [DOI] [PubMed] [Google Scholar]
- Enlow DH. Crecimiento Maxilofacial. Mexico: Interamericana McGraw-Hill; 1992. pp. 94–154. [Google Scholar]
- Fuentes A. Condyle mandibulaire. Processus évolutiff prénatal. Rev Stomatol Chir Maxillofac. 1988;89:299–305. [PubMed] [Google Scholar]
- Ganey TM, Love SM, Odgen JA. Development of vascularization in the chondroepiphysis of the rabbit. J Orthop Res. 1992;10:496–510. doi: 10.1002/jor.1100100405. [DOI] [PubMed] [Google Scholar]
- Ganey TM, Odgen JA, Sasse J, Neame PJ, Hilbelink DR. Basement membrane composition of cartilage canals during development and ossification of the epiphysis. Anat Rec. 1995;24:425–437. doi: 10.1002/ar.1092410318. [DOI] [PubMed] [Google Scholar]
- Geneser F. Histología. 3rd edn. Madrid: Editorial Panamericana; 2000. pp. 273–274. [Google Scholar]
- Glasstone S. Differentiation of the mouse embryonic mandible and squamous-mandibular joint in organ culture. Arch Oral Biol. 1971;16:723–729. doi: 10.1016/0003-9969(71)90117-8. [DOI] [PubMed] [Google Scholar]
- Gómez de Ferraris ME, Campos Muñoz A. Histología y Embriología Bucodental. Madrid: Editorial Panamericana; 1999. pp. 159–172. [Google Scholar]
- Habib H, Hatta T, Udagawa J, Zhang L, Yoshimura Y, Otani H. Fetal jaw movement affects condylar cartilage development. J Dent Res. 2005;84:474–479. doi: 10.1177/154405910508400514. [DOI] [PubMed] [Google Scholar]
- Haines RW. Cartilage canals. J Anat. 1933;68:45–64. [PMC free article] [PubMed] [Google Scholar]
- Kuettner KE, Pauli BU. Vascularity of cartilage. In: Hall BK, editor. Cartilage. Vol. 1: Structure, Function and Biochemistry. New York: Academic Press; 1983. pp. 281–341. [Google Scholar]
- Kugler JH, Tomlinson A, Wagstaff A, Ward SM. The role of cartilage canals in the formation of secondary centres of ossification. J Anat. 1979;129:493–506. [PMC free article] [PubMed] [Google Scholar]
- Levene C. The patterns of cartilage canals. J Anat. 1964;98:515–538. [PMC free article] [PubMed] [Google Scholar]
- Lewinson D, Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat Rec. 1992;233:504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- Livne E, Lewinson D, Dickson G, Silbermann M. Histochemical study of metrical components in the mandibular condyle of the neonatal mice. Acta Anat. 1990;138:32–41. doi: 10.1159/000146917. [DOI] [PubMed] [Google Scholar]
- Lufti AM. Mode of growth, tale and functions of cartilage canals. J Anat. 1970;106:135–145. [PMC free article] [PubMed] [Google Scholar]
- McManus JFA, Mowry RW. Técnica Histológica. Madrid: Atika S A; 1968. [Google Scholar]
- Mérida Velasco JR. Vascular canals. An R Acad Nac Med. 2002;121:379–385. A model for the mandibular condyle growth. [PubMed] [Google Scholar]
- Mérida Velasco JR, Rodríguez Vazquez JF, Mérida Velasco JA, Sánchez Montesinos I, Espin Ferra J, Jiménez J. Development of the human temporomandibular joint. Anat Rec. 1999;255:20–33. doi: 10.1002/(SICI)1097-0185(19990501)255:1<20::AID-AR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nordahl J, Andersson G, Reinholt FP. Chondroclasts and osteoclasts in bones of young rats: comparison of ultrastructural and functional features. Calcif Tissue Int. 1998;63:401–408. doi: 10.1007/s002239900548. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Human Embryology and Teratology. New York: Wiley-Liss; 1996. [Google Scholar]
- Perry HT, Yinghua XU, Forbes DP. The embryology of the temporomandibular joint. Cranio. 1985;3:125–132. doi: 10.1080/08869634.1985.11678094. [DOI] [PubMed] [Google Scholar]
- Proff P, Gedrange T, Franke R, Schubert H, Fanghänel J, Miehe B, Harzer W. Histological and histomorphometric investigation of the condylar cartilage of juvenile pigs after anterior mandibular displacement. J Dent Res. 2007;84:474–479. doi: 10.1016/j.aanat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Lieck S, Bontschev Ne. Development of the human temporomandibular joint. Eur J Oral Sci. 1999;107:25–34. doi: 10.1046/j.0909-8836.1999.eos107106.x. Computer-aided 3D-reconstructions. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Renz H, Klarkowki C. Prenatal development of the human mandible. Anat Embryol. 2003;207:221–232. doi: 10.1007/s00429-003-0343-4. 3D reconstructions, morphometry and bone remodelling pattern, sizes 12–117 mm CRL. [DOI] [PubMed] [Google Scholar]
- Reidenbach MM, Schmidt HM. Cartilage canals in the fetal elbow joint in man. Acta Anat. 1994;149:195–202. doi: 10.1159/000147576. [DOI] [PubMed] [Google Scholar]
- Roach HI, Baker JE, Clarke NMP. Initiation of the bony epiphysis in long bones: chronology of interactions between the vascular system and chondrocytes. J Bone Miner Res. 1998;13:950–961. doi: 10.1359/jbmr.1998.13.6.950. [DOI] [PubMed] [Google Scholar]
- Rodríguez JI, Delgado E, Paniagua R. Multivacuolated cells in human cartilage canals. Acta Anat. 1985;124:54–47. doi: 10.1159/000146096. [DOI] [PubMed] [Google Scholar]
- Shapiro F. Epiphyseal and physeal cartilage vascularization. Anat Rec. 1998;252:140–148. doi: 10.1002/(SICI)1097-0185(199809)252:1<140::AID-AR12>3.0.CO;2-O. A light microscopic and tritiated thymidine autoradiographic study of cartilage canals in newborn and young postnatal rabbit bone. [DOI] [PubMed] [Google Scholar]
- Shen G, Darendeliler MA. The adaptative remodelling of condylar cartilage. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. A transition from chondrogenesis to osteogenesis. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, Koyama E. Temporomandibular joint formation and condyle growth require Indian Hedgehog signalling. Dev Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, von der Mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Sperber GH. Hamilton, Ontario: BC Decker; 2001. Craniofacial development; pp. 126–138. [Google Scholar]
- Strauss PG, Closs EI, Schmidt J, Erfle V. Gene expression during osteogenic differentiation in mandibular condyles in vitro. J Cell Biol. 1990;110:1369–1378. doi: 10.1083/jcb.110.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk L, Yeon K, Hee O, Kyo Y, Eun K, Je C. Prenatal development of the human mandible. Anat Rec. 2001;263:314–325. doi: 10.1002/ar.1110. [DOI] [PubMed] [Google Scholar]
- Symons NBB. The development of the human mandibular joint. J Anat. 1952;86:326–332. [PMC free article] [PubMed] [Google Scholar]
- Takenoshita Y. Development with age of the human mandibular condyle: histological study. Cranio. 1987;5:317–323. doi: 10.1080/08869634.1987.11678206. [DOI] [PubMed] [Google Scholar]
- Thilander B, Carlsson GH, Ingervall B. Postnatal development of the human tempormandibular joint. Acta Odontol Scand. 1976;34:117–126. doi: 10.3109/00016357609026564. I. A histological study. [DOI] [PubMed] [Google Scholar]
- Vinkka-Puhakka H, Thesleff I. Initiation of secondary cartilage in the mandible of the Syrian hamster in the absence of muscle function. Arch Oral Biol. 1993;38:49–54. doi: 10.1016/0003-9969(93)90154-e. [DOI] [PubMed] [Google Scholar]
- Vinogradoff A. Development of l’articulation temporo-maxillaire chez l’homme dans la periode intra-uterine. Int Monatschr Anat Physiol. 1910;27:490–523. [Google Scholar]
- Visco DM, Hill MA, Van Sickle DC, Kincaid SA. Cartilage canals and lesions typical of osteochondrosis in growth cartilages from the distal part of the humerus of newborn pigs. Vet Rec. 1991;128:221–228. doi: 10.1136/vr.128.10.221. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Sickle DC van. The relationship of the cartilage canals to the initial osteogenesis of the secondary centers of ossification. Anat Rec. 1970;168:381–392. doi: 10.1002/ar.1091680305. [DOI] [PubMed] [Google Scholar]