Abstract
Comparative analysis of the anuran pelvic and thigh musculoskeletal system revealed that the thigh extensors, responsible for the initial phase of jump, the propulsive stroke in swimming and, if used asynchronously, also for walking, are least affected by the transformations observed between anurans and their temnospondyl ancestors (as reflected in contemporary caudates). The iliac shaft and urostyle, two of the most important anuran apomorphies, represent skeletal support for muscles that are mostly protractors of the femur or are important in attaining a crouching position, a necessary prerequisite for rapid escape. All of these muscles originate or insert on the iliac shaft. As the orientation of the pubis, ischium and ilium is the same in anurans, caudates and by inference also in their temnospondyl ancestors, it is probable that the pelvis was shifted from the sacral vertebra posteriorly along the reduced and stiffened tail (urostyle) by the elongation of the illiac shaft. Thus, the original vertical orientation of the ilium was maintained (which is also demonstrated by stable origins of the glutaeus maximus, iliofemoralis and iliofibularis on the tuber superius) and the shaft itself is a new structure. A review of functional analysis of anuran locomotion suggests some clear differences from that in caudates, suggesting that terrestrial jumping may have been a primary locomotor activity, from which other types of anuran locomotion are derived.
Keywords: Anura, anuran jumping, Caudata, comparative myology, evolution, functional anatomy, locomotion
Introduction
Jumping in frogs is associated with profound anatomical modification compared with the general structural scheme of tetrapods and includes an elongation of the hind limbs, a reduction and stiffening of the tail, a shortening of the presacral vertebral column, a shift of the acetabular portion of the pelvis posteriorly and a reduction of the ribs (see also Gans & Parsons, 1966). The origin of some of these anatomical modifications may be understood based on developmental data, e.g. the reduction of ribs and, consequently, the isolation of the pectoral girdle from the axial skeleton (Blanco & Sanchiz, 2000), the reduction of the number of presacral somites (Richardson et al. 1998), the transformation and displacement of the pelvis and fusion of the anterior caudal vertebrae, and the reduction of terminal tail somites (Hodler, 1949; Ročková & Roček, 2005; Handrigan & Wassersug, 2007). Other features associated with saltation may be inferred from the fossil record (e.g. shortening of the presacral vertebral column; Rage & Roček, 1989), although fossil evidence of the anatomical transition between the Permian/Triassic temnospondyls and the earliest true frogs from the early Jurassic (Prosalirus bitis; Jenkins & Shubin, 1998) is limited to Gerobatrachus (Anderson et al. 2008), still lacking any anuran features on its postcranial skeleton, and Triadobatrachus and Czatkobatrachus (Roček & Rage, 2000).
Whereas skeletal adaptations for saltatory locomotion in adult anurans are obvious, one crucial question still remains unanswered: was jumping the primary drive resulting in the profound transformation of the anuran skeleton (Noble, 1931; MacBride, 1932; Gadow, 1933; Romer, 1950; Reig, 1957; Schmalhausen, 1958; Eaton, 1959; Hecht, 1962; Inger, 1962; Gans & Parsons, 1966) or, alternatively, was synchronous swimming primary and terrestrial jumping only evolved later, as suggested by Böker (1935), Gans (1961) and Griffiths (1963)? Although this question is sometimes considered inappropriate (Peters et al. 1996), it is in fact crucial for understanding the origin of the anurans.
Most of the attempts to explain the anuran origin have been based on fossils and thus on osteology. Only in a few cases has the entire musculoskeletal system been considered (Jenkins & Shubin, 1998). Additionally, some comparative anatomical or developmental studies have been published on the musculoskeletal system, which may provide insights into the evolutionary transformations that occurred in the lineage leading to modern anurans (e.g. Gaupp, 1896; Green, 1931; Dunlap, 1960). Later workers used functional analyses of the different locomotor traits themselves to try to gain insights into the functional consequences of the unique morphology of frogs (Rand, 1952; Stokely & Barberian, 1953; Palmer, 1960; Whiting, 1961; Rand & Rand, 1966; Zug, 1972; Emerson, 1978, 1979, 1982; Emerson & de Jongh, 1980; Videler & Jorna, 1985; van Dijk, 2002; Lutz & Rome, 1996a,b; Olson & Marsh, 1998).
The aim of the current study was to analyse pelvic and thigh muscles related to locomotion in various taxa representing the principal anuran locomotor types (saltation, swimming, crawling and burrowing). Comparisons are made between highly derived taxa (e.g. Rana, which is often taken as the main source of the anatomical information on anurans), phylogenetically primitive contemporary anurans, such as Ascaphus and discoglossoids, and neotenic caudates, which are generally considered to reflect ancestral temnospondyl anatomy. From these comparisons we try to infer principal evolutionary transformations of the pelvic musculoskeletal system that were associated with the origin of Anura, in contrast to those that occurred only later during anuran phylogeny, and those purely related to locomotion and thus occurring across a wide range of anuran taxa, independent of their phylogenetic status.
Materials and methods
We chose nine anuran species representing the principal locomotor types and phylogenetic affinities for our analysis of the musculoskeletal system. For Rana esculenta (2 ♂♂, 1 ♀), Discoglossus pictus (4 ♂♂, 4 ♀), Bombina orientalis (2 ♂♂, 1 ♀), Bufo guttatus (2 ♂♂), Pelobates fuscus (2 ♂♂, 1 ♀) and Xenopus laevis (2 ♀♀) functional data are also available but were not included in this study. In addition, we investigated the muscle arrangements in Ascaphus truei (1 ♂, 1 ♀), Barbourula busagensis (1 ♀) and Pipa pipa (1 ♀) because of their phylogenetically primitive status.
We used Discoglossus pictus as a basis for a thorough description because anurans with discoglossoid skeletal features are known from as early as the Middle Jurassic (Evans et al. 1990). Rana, which is typically used as a model of anuran musculoskeletal anatomy (e.g. Gaupp, 1896; Dunlap, 1960), is highly derived (Frost et al. 2006) and thus not appropriate for our purposes. Description of other species is confined to differences from the basic scheme observed in Discoglossus.
Caudates were dissected as a baseline for comparison as their anatomy is thought to best represent the condition in ancestral temnospondyls. We dissected two neotenic caudates [Necturus (3) and Ambystoma (3)] and one adult Salamandra salamandra (1).
The functions of muscles were inferred from their origins and insertions, and confirmed based on a stimulation experiment in one subadult male Pyxicephalus adspersus (body length 72.36 mm, ilium length 22.37 mm, femur length 25.65 mm, tibia length 25.99 mm, foot length 27.06 mm, longest toe on the hind foot 17.81 mm). The animal was brought under deep anaesthesia using ketamine (225 mg/kg body mass) and pithed once anaesthetized. The muscles were exposed and twisted bipolar NiCr electrodes were inserted into each muscle in the middle of the respective muscle bellies and connected to a stimulator (Grass S48). The stimulation circuit was charge balanced by a coupling capacitor and bleed resistor (Loeb & Gans, 1986) to avoid muscle damage and undue fatigue. Muscles were stimulated at 12 V with a pulse train of 500 ms at 70 Hz and 3 ms pulse duration (see Manzano et al. 2008). The results of the stimulation experiment are represented in Table 1.
Table 1.
Review of pelvic and thigh muscles
| Name (and synonyms in caudates) | Origin | Insertion | Function | |
|---|---|---|---|---|
| Axial skeleton → axial skeleton | ||||
| 1 | Longissimus dorsi | Sacral and praesacral vertebrae | Urostyle | Dorsal rotation of the urostyle |
| 2 | Coccygeosacralis | Transverse process of sacral vertebra | Urostyle | Lateral bending of the body at the iliosacral joint (unilateral activity), stiffens trunk and moderately rotates urostyle dorsally (bilateral activity) |
| Axial skeleton → pelvis | ||||
| 3 | Iliolumbaris | Praesacral vertebrae | Iliac shaft | Lateral bending of the body at the iliosacral joint (unilateral activity), anterior gliding of the ilium along the sacral diapophysis (bilateral activity) |
| 4 | Coccygeoiliacus | Urostyle | Iliac shaft | Anterior gliding of the ilium along the sacral diapophysis, rotates urostyle ventrally or shifts pelvis posteriorly (both-side activity) |
| Axial skeleton → femur | ||||
| 5 | Pyriformis (coccygeofemoralis; caudalifemoralis Francis 1934) | Urostyle | Femur | Rotation of the urostyle towards the ilium of the active side in the horizontal plane and slight long axis rotation of the urostyle |
| 6 | Caudalipuboischiotibialis | Urostyle | Semimembranosus | |
| Pelvis → femur | ||||
| 7 | Iliacus externus | Iliac shaft | Femur | Protraction of the femur |
| 8 | Tensor fasciae latae | Iliac shaft | Fascia lata | Puts tension on the fascia lata, no obvious movements of the appendicular skeleton |
| 9 | Iliacus internus | Ilium | Femur | Protraction and abduction of the femur |
| 10 | Cruralis | Ilium | Knee aponeurosis | Knee extension |
| 11 | Glutaeus maximus (iliotibialis) | Ilium | Cruralis | Knee extension |
| 12 | Iliofemoralis | Ilium | Femur | Retraction and adduction of the femur |
| 13 | Adductor longus | Ilium and pubis | Femur | Adduction and protraction of the femur |
| 14 | Pectineus [puboischiofemoralis externus,Hoffmann (1873–1878), Iordansky & Morozov (1994); puboischiofemoralis internus, Noble (1922)] | Ischium | Femur | Fixation of femur in acetabular cavity |
| 15 | Adductor magnus | Ischium | Femur | Protraction and slight adduction of the femur. When the hip angle is greater than 90° the muscle causes adduction only |
| 16 | Obturator externus | Ischium | Femur | Retraction of the femur |
| 17 | Obturator internus | Ischium | Femur | Craniad long axis rotation of the femur |
| 18 | Quadratus femoris | Ischium | Femur | Was not stimulated |
| 19 | Gemellus | Ischium | Femur | Retraction of the femur |
| 20 | Semimembranosus [ischioflexorius, Hoffmann (1873–1878); caudalipuboischiotibialis, Francis (1934)] | Ilium and ischium | Knee aponeurosis | Retraction of the femur and slight knee flexion |
| 21 | Semitendinosus [ischioflexorius, Mivart (1869); puboischiotibialis, Hoffmann (1873–1878)] | Ischium | Knee aponeurosis | Knee flexion |
| 22 | Gracilis major [puboischiotibialis, Mivart (1869), Iordansky & Morozov (1994)] | Ischium | Knee aponeurosis | Retraction and adduction of the femur |
| 23 | Gracilis minor | Ischium | Knee aponeurosis | Retraction and adduction of the femur |
| Pelvis → tibiofibula | ||||
| 24 | Iliofibularis | Ilium | Fibula | Retraction of the femur and flexion at the knee |
| 25 | Sartorius [gracilis, Stannius (1856); pubotibialis, Mivart (1869), Iordansky & Morozov (1994)] | Ischium | tibia | Protraction and adduction of the femur |
Muscle nomenclature in frogs principally follows that of Gaupp (1896) and that of caudates follows Francis (1934).
Results
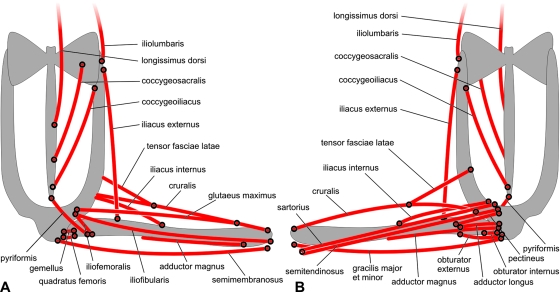
The pelvic muscular system may be divided into two groups (Fig. 1A and B). The first group, derived from epaxial trunk musculature, includes muscles responsible for rotation and sliding of the pelvis at the iliosacral articulation. These are the longissimus dorsi, iliolumbaris, coccygeosacralis and coccygeoiliacus. There are also two small muscles that belong to this group (caudalipuboischiotibialis and pyriformis), which are vestigial. The second group consists of muscles whose origins are on the pelvis and insertions on the limb. This group comprises all of the remaining muscles.
Fig. 1.
Diagrammatic representation of the pelvic and thigh muscles in (A) dorsal and (B) ventral views.
Discoglossus pictus (Figs 2–1 and 2–2)
Fig. 2–1.
Discoglossus pictus. (A) Pelvic region (dorsal view), skin removed. (B) Same as in A but muscles attached to the urostyle prepared on the left side. (C) Surface layer of thigh muscles (ventral view). (D) Surface layer of thigh muscles (dorsal view). (E) Same as in D but glutaeus maximus partly removed. (F) Same as in C but gracilis major partly removed (ventral view). (G) Same as in D but glutaeus maximus and gracilis major removed (dorsal view). (H) Deep layer of thigh muscles (ventral view). (I) Same as in G but semimembranosus removed. Origin of glutaeus maximus marked by arrow. (J) Femur (ventral view), with insertions of obturator externus and adductor magnus. (K) The deepest layer (except for the cruralis) of thigh muscles (dorsal view). (L) Pelvis (dorsolateral view). (M) Same as in L but iliofemoralis removed. E is male and all others are female.
Fig. 2–2.
Discoglossus pictus. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on the right lateral surface of the pelvic girdle. (C) Origins of muscles on the inner surface of the right ilium. Based on skeleton DPFNSP 6514a (sex not recorded).
The longissimus dorsi runs anteroposteriorly between the spinal processes and diapophyses of the vertebrae. In its presacral section it is segmented by transverse tendinous septa. In the postsacral region (Fig. 2–1A and B), the muscle tapers and inserts on the dorsal surface of the urostyle along its whole length, and on the lateral surface of the anterior half of the urostyle. This caudal portion of the muscle is not segmented.
The coccygeosacralis originates on the dorsal side of the sacral diapophysis (Fig. 2–1B) and inserts on the dorsolateral surface of the urostyle (its posterior two-thirds; Fig. 2–2A). It is partly covered by the longissimus dorsi (Fig. 2–1B). The coccygeoiliacus originates on the medial surface of the dorsal crista of the iliac shaft (Fig. 2–2C), from the level of the posterior margin of the sacral diapophysis to slightly posterior of the midlength of the shaft, and inserts onto the posterior two-thirds of the ventrolateral surface of the urostyle.
The pyriformis (Fig. 2–1E) is a short, slender muscle that originates on the ventral side of the tip of the urostyle. It is attached to the crista femoralis, proximal to the insertion of the iliofemoralis (see below).
The iliacus externus consists of two parts. The pars externa originates along the entire length of the lateral surface of the iliac shaft (Fig. 2–2B), the pars interna on the most anterior part of the inner surface of the iliac shaft (Fig. 2–2C) and runs beneath the ventral surface of the shaft. Both parts run more or less independently towards the proximal section of the femur where they may fuse with each other. However, they may also stay separated up to their insertion onto the anterior surface of the femur. Although the origins of the coccygeoiliacus and iliacus externus on the iliac shaft are isolated from each other by a thin dorsal crista, which is moderately declined medially, part of the iliacus externus extends dorsally such that both muscles meet and completely cover the iliac shaft.
The iliacus internus originates on the inner surface of the ilium, opposite to the anterior part of the acetabulum (Fig. 2–2C), and runs over the anterior margin of the acetabular portion of the ilium laterally towards the proximal section of the anteroventral surface of the femur (beneath the insertion of the iliacus externus) where it inserts along half to two-thirds of its length. Its origin on the ilium is variable – it can also arise on the anterior rounded margin of the acetabular portion of the ilium, rather than on its inner surface. Except for part of its distal insertion, it is covered by the iliacus externus (Fig. 2–1L).
The tensor fasciae latae is a thin superficial muscle on the anterior surface of the thigh. Its origin may vary considerably. It can originate within a small area on the inner surface of the iliac shaft (Fig. 2–2C) or on the ventral surface of the shaft. It then runs across the ventral surface of the shaft towards the anterior surface of the thigh where it is attached onto the fascia of the cruralis. This fascia (termed the fascia lata) continues towards the knee where it turns into a broad aponeurosis. The cruralis (proximally covered by the tensor fasciae latae) determines the shape of the thigh anteriorly. It originates on the anteroventral margin of the acetabulum and on the corresponding part of the articular capsule. It inserts on the distal part of the femur and partly on the knee aponeurosis. The glutaeus maximus is located on the dorsal surface of the thigh (Fig. 2–1B and D). It originates on the dorsolateral surface of the tuber superius (Fig. 2–2B) and inserts on the fascia of the distal part of the cruralis (Fig. 2–1E).
The iliofibularis and iliofemoralis are slender muscles that are closely related with one another in that they have a common origin on the lateral surface of the posterior part of the tuber superius (Fig. 2–2B). The iliofibularis is the dorsal of the two (Fig. 2–1G). It runs parallel to the semimembranosus and its proximal half is covered by the glutaeus maximus. Its distal half is exposed (Fig. 2–1D) and is attached into the knee aponeurosis (in part covering the head of the fibula). The iliofemoralis (Fig. 2–1G and L) inserts on the dorsal surface of the crista femoralis (which extends from the proximal section of the femur posteriorly).
The semimembranosus is a relatively robust muscle, the proximal part of which shapes the thigh posterodorsally (Fig. 2–1D). In its proximal section it is divided by a tendinous inscription. It originates on the pars ascendens ilii and on the adjacent part of the ischium and then runs parallel to the iliofibularis but its distal portion is located ventrally, so that it emerges on the ventral side between the sartorius and gracilis major (Fig. 2–1F). It inserts onto the posterior surface of the articular capsule of the knee and on the posterior surface of the proximal head of the tibiofibula.
The gracilis major (together with the slender gracilis minor) forms the posterior part of the thigh in its ventral aspect (Fig. 2–1C and F). It originates within a comparatively small area on the posterodorsal part of the ischium (Fig. 2–2B) and inserts, together with the semimembranosus, onto the posteroventral surface of the knee articular capsule. The gracilis minor is a very slender muscle, originating partly from the skin on the posterior surface of thigh and partly from the cloacal wall.
The most conspicuous muscle on the ventral surface of the thigh is the sartorius (Fig. 2–1C and F). It takes its origin by a flat tendon from the ventral surface of the ischium (Fig. 2–2B) and inserts into the ventral side of the articular capsule of the knee (Fig. 2–1C). It is very thin in its proximal portion but thicker distally. The semitendinosus is a slender muscle that originates by two heads, i.e. dorsal and ventral. The dorsal head originates on the posterior part of the ischium (between the gracilis major and adductor magnus; Fig. 2–2B) and the fibres of the ventral head take their origin from the adductor magnus. Its distal portion is made up of a long tendon (Fig. 2–1H) that joins the tendon of the sartorius in its insertion onto the articular capsule of the knee.
Below the sartorius is the adductor magnus (Fig. 2–1C), which takes its origin along the posteroventral margin of the ischium (Fig. 2–2B) and inserts on the posterodorsal surface of the distal section of the femur (Fig. 2–1J).
The adductor longus is a long muscle that originates on the pars descendens ilii and on the pubic cartilage (Fig. 2–2B), runs closely adjacent to the pectineus, and inserts on the ventral surface of the distal quarter of the femur (Fig. 2–1C and H). The pectineus is similar to the adductor longus but shorter (Fig. 2–1H). It originates on the ventral margin of the pubic cartilage and ischium (Fig. 2–2B), and inserts on the ventral surface of the crista femoralis.
The obturator externus is positioned adjacent to the pectineus posteriorly and in younger individuals it is difficult to recognize the border between them. It originates on the ischium, posterior to the pectineus (Fig. 2–2B), as a broad thin muscle but it tapers distally and inserts on the ventral surface of the crista femoralis, proximal to the insertion of the pectineus (Fig. 2–1J). The obturator internus is the deepest muscle of the pelvis. Its crescent-like origin on the ischium (Fig. 2–2B) surrounds the iliac joint posteriorly and inserts on the capsule of the joint.
The gemellus and quadratus femoris were not recognized as independent muscles. Notes on Discoglossus pictus can also be found in Dunlap (1960).
Ascaphus truei (Figs 3–1 and 3–2)
Fig. 3–1.
Ascaphus truei. (A) Pelvic region (dorsal view), skin removed. (B) Same as in A but right longissimus dorsi removed. (C) Pelvis (dorsolateral view). (D) Surface layer of thigh muscles (ventral view); arrow marks origin of sartorius-semitendinosus complex from obturator externus. (E) Surface layer of thigh muscles (dorsal view). (F) Same as in D but sartorius interrupted and partly raised, and gracilis-semimembranosus complex and cruralis partly removed. (G) Same as in E but glutaeus maximus partly removed. Arrow marks position of tendinous inscription of semimembranosus where caudalipuboischiotibialis is attached. (H) Same as in F but common insertion of sartorius-semitendinosus and gracilis-semimembranosus complexes cut and displaced to show their joint ligament. (I) Same as in G but iliofibularis and glutaeus maximus removed. (J) Same as in H but middle part of sartorius-semitendinosus removed and their proximal heads slightly raised to show their origins from obturator externus. (K) Middle layer of thigh muscles (dorsal view); cruralis and semimembranosus-gracilis complex partly displaced. (L) The deepest layer of thigh muscles (ventral view). (M) The deepest layer of thigh muscles (dorsal view); semimembranosus removed and distal part of caudalipuboischiotibialis detached. All illustrations represent male.
Fig. 3–2.
Ascaphus truei. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Attachments of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6538a (sex not recorded).
The longissimus dorsi inserts along the whole dorsolateral surface of the urostyle except for the tip, which remains uncovered (Fig. 3–1A). The coccygeoiliacus originates on the medial surface of the anterior part of the iliac shaft (some fibres seem to continue along the lateral surface of the iliosacral joint into the presacral region) and inserts on the ventrolateral surface of the distal third of the urostyle. It is difficult to separate the coccygeoiliacus from fibres that are attached to the posterior margin of the lateral part of the sacral diapophysis and that might be interpreted as the coccygeosacralis. Rather, it seems that this is a single fan-like muscle layer originating on both the iliac shaft and sacral diapophysis (Fig. 3–1B). Similarly, anterior to the sacral vertebra it is difficult to distinguish between the longissimus dorsi and iliolumbaris, except for the fact that the latter is attached to the anterior margin of the sacral diapophysis, whereas the longissimus dorsi continues onto the urostyle.
The other two epaxial muscles, i.e. the caudalipuboischiotibialis and pyriformis, originate on the ventrolateral surface of the tip of the urostyle. The caudalipuboischiotibialis originates posterior to the origin of the pyriformis and inserts on the tendinous inscription of the semimembranosus (Fig. 3–1E and G). The pyriformis penetrates between the iliofibularis and semimembranosus to insert on the posterior surface of the crista femoralis (Fig. 3–1I).
The iliacus externus arises from the posterior half of the lateral surface of the iliac shaft (Fig. 3–2B) and inserts on the posterior surface of the caput femoris (Fig. 3–2A). The iliacus internus originates on the anterior surface of the ilium and partly also on a small area on the ventral surface of the iliac shaft (Fig. 3–2B and C), turns, runs over the dorsal surface of the proximal section of the femur, and inserts on the crista femoralis. The origin of the tensor fasciae latae lies below the iliacus externus, on the ventral surface of the iliac shaft (Fig. 3–2C). The muscle inserts on the fascia of the cruralis close to the basis of the thigh. The cruralis originates from the lateral surface of the ilium and pubic cartilage along the acetabular margin, and from the adjacent portion of the joint capsule (Fig. 3–2B). It inserts into the knee aponeurosis. The glutaeus maximus originates on the dorsolateral surface of the proximal part of the iliac shaft (Fig. 3–2B) and inserts by means of a narrow tendon on the dorsal surface of the distal part of the cruralis (Fig. 3–1G). The iliofibularis and iliofemoralis originate by a common tendon just posterior to the glutaeus maximus (Fig. 3–2B). The iliofibularis inserts on the fibular head of the tibiofibula and the iliofemoralis inserts on the posterior surface of the crista femoralis.
The semimembranosus originates on the lateral surface of the dorsal portion of the ischium (Fig. 3–2B), runs across the posterior part of the thigh to the lower side of the knee and inserts onto the tibial head of the tibiofibula. About one-third from its origin it is divided by an oblique tendinous inscription into which the caudalipuboischiotibialis is inserted (see above). The most posterior portion of the thigh is occupied by the gracilis major, which arises on the ischium posterior to the acetabulum (Fig. 3–2B) and inserts on the proximal part of the tibial portion of the tibiofibula. At its distal third there is a more or less distinct oblique tendinous inscription. The gracilis minor originates on the fascia of the ventrolateral surface of the postpubic rod and inserts into the most ventral part of the tendinous inscription of the gracilis major. The inscription is continuous with that of the semimembranosus.
The sartorius-semitendinosus complex is a single muscle mass that, however, originates by means of two heads. The sartorial head arises from the fascia of the obturator externus and the semitendinosal head arises from the fascia of the adductor magnus (Fig. 3–1D and J). The heads are separated from one another by a shallow cleft. The complex inserts by means of a single tendon on the tibial head of the tibiofibula (Fig. 3–1J).
The adductor magnus consists of two heads. The ventral head, partly covered by the sartorius-semitendinosus complex, originates along the ventral margin of the ischium and adjacent part of pubis, and inserts on the crista femoralis at the end of the femur. The dorsal head originates on the posterior lateral margin of the ischium and inserts on the dorsal surface of the distal aspect of the femur where it fuses with the ventral head (Fig. 3–1K).
The deep layer of ventral muscles consists of the pectineus, which originates from the lateral surface of the pubis, from the praepubic plate and partly even from the most posterior segment of the rectus abdominis, and inserts on the anterior surface of the crista femoralis. Its proximal portion is exposed between the cruralis anteriorly and the obturator externus posteriorly (Fig. 3–1D and F). The obturator externus originates along the posterior margin of the ischium and its origin is continuous with the quadratus femoris. Both insert on the proximal section of the crista femoralis. The gemellus is located posterior to the quadratus femoris and its origin is on the dorsolateral surface of the ischium. It inserts on the posterior surface of the crista femoralis. The deepest is the obturator internus. It originates along the posterior margin of the acetabulum (Fig. 3–2B) and inserts on the posterior surface of the head of the femur.
A detailed account of the myology of Ascaphus truei can be found in Ritland (1955), van Dijk (1955) and partly also in Dunlap (1960).
Barbourula busagensis (Figs 4–1 and 4–2)
Fig. 4–1.
Barbourula busagensis. (A) Pelvic region (dorsal view), skin removed. Posterior margin of transversus marked by arrow. (B) Same as in A but fascia where transversus inserts partly removed to display longissimus dorsi-coccygeosacralis. (C) Same as in B but transversus on both sides detached from epaxial muscles to show both parts of iliacus externus. (D) Same as in C but in left dorsolateral view. (E) Surface layer of thigh muscles (ventral view); broken line marks origin of sartorius-semitendinosus on fascia of adductor magnus. (F) Surface layer of thigh muscles (dorsal view); posterior margin of transversus marked by arrow. (G) Same as in E but rectus abdominis and wall of abdominal cavity removed, posterior muscles partly isolated from each other. Arrow marks proximal margin of sartorius-semitendinosus. Inset illustrates outer portion of tensor fasciae latae. (H) Same as in F but fascia removed and muscles partly isolated from each other. Posterior margin of transversus marked by arrow. (I) Same as in G but dorsal head of semitendinosus partly removed. Arrow marks proximal margin of sartorius-semitendinosus. (J) Same as in H but glutaeus maximus partly removed to show its insertion. (K) Same as in I but gracilis major partly removed. Arrow marks proximal border of sartorius-semitendinosus. (L) Same as in J but glutaeus maximus removed. (M) Same as in K but sartorius-semitendinosus displaced and partly turned in order to expose ventral head of semitendinosus. Arrow marks tendon by which semitendinosus inserts in fascia separating both heads of adductor magnus. (N) Same as in L but iliofibularis removed. (O) Same as in M but sartorius-semitendinosus and semimembranosus removed. (P) Same as in N but gracilis major and minor removed. (Q) Same as in O but ventral head of adductor magnus detached and partly turned to show inner arrangement. (R) Same as in P but semimembranosus removed. (S) Same as in Q but adductor magnus removed. (T) Deep muscles of thigh (ventral view). (U) Deep muscles of thigh (dorsal view). (V) Deep proximal muscles of thigh (ventral view); tensor fasciae latae partly removed to show its origin.
Fig. 4–2.
Barbourula busagensis. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6554a (female).
A remarkable feature of the trunk muscles in this frog is that the transversus (the innermost sheet of hypaxial muscles with fibres oriented transversely) extends onto the dorsum so that it covers the iliolumbaris and is attached onto the dorsal fascia covering the longissimus dorsi. The posterior margin of the transversus is sharp and oriented vertically (Fig. 4–1A). The obliquus externus is restricted to the anteroventral portion of the flanks, leaving the posterodorsal part of the transversus exposed (Fig. 4–1B).
The longissimus dorsi inserts along the whole length of the urostyle. Posterior to the sacral diapophysis it extends as a single muscle mass laterally, leaving only the cartilaginous rim of the diapophysis uncovered (Fig. 4–1C). Hence, the coccygeosacralis is not differentiated from the longissimus dorsi. However, its lateral portion is divided into two layers, one attached to the sacral diapophysis dorsally and another (noticeably thinner) ventrally. Anterior to the sacral diapophysis, the lateral part of this muscle complex is confluent with the iliolumbaris. The iliolumbaris is separated from the longissimus dorsi. The coccygeoiliacus is covered dorsally by the longissimus dorsi-coccygeosacralis complex. It originates on the lateral surface of the posterior half of the urostyle, ventral to the origin of the longissimus dorsi-coccygeosacralis complex, and inserts on the anterior third of the inner surface of the iliac shaft (Fig. 4–2A).
The pyriformis originates on the ventrolateral surface of the tip of the urostyle (Fig. 4–1H,J,L,N,P,R and U) and inserts on the posterior surface of the proximal section of the femur.
The iliacus externus consists of two parts. The inner part (pars interna) originates both on the inner and outer surface of the posterior part of the iliac shaft (Figs 4–1C,D,J and R, and 4–2B), covers it dorsally and inserts on the dorsal surface of the acetabular capsule. The outer part (pars externa) originates on the lateral surface of the anterior half of the iliac shaft (Fig. 4–2B) and is confluent with the thin layer of the iliolumbaris covered by the transversus (see above). This thin muscle sheet (consisting of the iliacus externus and iliolumbaris) inserts posteriorly on the dorsal surface of the femur, parallel to the attachment of the iliacus internus (Figs 4–1C,D,J,L,N,P and R, and 4–2A). The iliacus internus originates on the anterior surface of the preacetabular portion of the ilium (Fig. 4–2C) from which it extends slightly on both the inner and outer surface. It inserts on the proximal half of the femur, ventral to the insertion of the outer part of the iliacus externus (Fig. 4–1L,N,P,R and U).
The anterior part of the thigh is formed by the cruralis, which originates on the anteroventral margin of the acetabulum, i.e. on the articular capsule (Figs 4–1S and 4–2B), and inserts onto the knee aponeurosis. The tensor fasciae latae is a slender muscle originating on the ventral surface of the iliac shaft (Figs 4–1G and V, and 4–2C) and inserting onto the fascia lata at the most proximal free part of the cruralis. It should be noted that the tensor fasciae latae and fascia lata are both covered by the fascia of the cruralis. The glutaeus maximus has a small accessory tendon attached to the lateral margin of the longissimus dorsi-coccygeosacralis complex (Fig. 4–1H). The main part of this muscle originates on the lateral side of the extensive but not prominent tuber superius and inserts as a thin sheet onto the dorsal fascia of the distal part of the cruralis (Figs 4–1J and 4–2B). The iliofibularis and iliofemoralis originate behind the glutaeus maximus (Fig. 4–2B). Below the iliofibularis runs the n. ischiadicus (Fig. 4–1N).
The semimembranosus is divided by an indistinct tendinous inscription located in its distal portion. It originates on the lateral surface of the dorsal part of the ischium (Fig. 4–2B) and inserts on the tibial head of the tibiofibula, covered by the common insertion of the sartorius-semitendinosus complex. The gracilis major originates on the most posterior part of the ischium (Figs 4–1G,I and K, and 4–2B) and inserts onto the knee aponeurosis. The gracilis minor originates from the skin of the posterior part of the thigh; distally it is fused to the gracilis major and both insert at the knee aponeurosis.
The sartorius and semitendinosus are not completely separated. The sartorius originates obliquely by means of a thin fascial sheet at the ventral head of the adductor magnus (Fig. 4–1E,G,I,K and M). The semitendinosus consists of two parts. The dorsal head originates on the lateral surface of the ischium between the gracilis major and the dorsal head of the adductor magnus (Fig. 4–2B). The ventral head is located below the posterior margin of the sartorius and originates at the fascia separating the ventral and dorsal heads of the adductor magnus (Fig. 4–1M). Both heads of the semitendinosus fuse together and with the dostal part of the sartorius. This complex inserts onto the knee aponeurosis (Fig. 4–1M).
The adductor magnus consists of two parts. The ventral head (caput ventrale) originates on the ventral part of the ischium and the dorsal head (caput dorsale) arises on the posterior part of the ischium (Fig. 4–2B). Both heads fuse distally with one another and surround ventrally, posteriorly and dorsally the distal part of the femur. The dorsal attachment is partly covered by the cruralis.
The adductor longus is separated from the pectineus at the free part of the thigh but their proximal sections (located in the abdominal cavity and covered only by the peritoneum) are undivided (Figs 4–1S and 4–2B). Distally, the adductor longus inserts onto the distal portion of the adductor magnus (Fig. 4–1O and Q). The pectineus is attached to the ventral surface of the femur (Fig. 4–1S).
The obturator externus and quadratus femoris are separated proximally but are undivided distally (Figs 4–1T and V, and 4–2B). The former originates along the ventral margin of the ischium and the latter next to it on the posteroventral part of the ischium. Their common distal attachment is on the posteroventral surface of the proximal section of the femur.
The gemellus is part of the deep layer on the posterodorsal side, originating from the dorsal part of the ischium and inserting on the proximal section of the femur (Fig. 4–1R). The obturator internus strengthens the articular capsule posteriorly (Fig. 4–2B).
Bombina orientalis (Figs 5–1 and 5–2)
Fig. 5–1.
Bombina orientalis. (A) Pelvic region (dorsal view), skin removed. (B) Pelvis (dorsal view); muscles originating on right side of urostyle prepared, coccygeoiliacus removed. (C) Pelvis and proximal part of left femur with deep muscles (dorsolateral view). (D) Surface layer of thigh muscles (ventral view). (E) Surface layer of thigh muscles (dorsal view). (F) Same as in D but gracilis major removed, semimembranosus partly removed and glutaeus maximus partly displaced. (G) Same as in E but glutaeus maximus partly removed. (H) Same as in F but semitendinosus (caput dorsale) displaced to demonstrate its attachments. (I) Same as in G but semimembranosus removed and iliofibularis partly removed to show position of adductor magnus. (J) Same as in H but adductor magnus displaced and turned. (K) Same as in I but semimembranosus partly removed to show positions of gemellus and iliofemoralis. (L) Same as in J but all muscles inserting on femur, except for iliacus internus, removed. (M) Same as in K but all muscles except for iliacus externus and iliacus internus removed. A–C, K and M are male; D–J and L are female.
Fig. 5–2.
Bombina orientalis. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Insertions of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6583 (male).
In contrast to Discoglossus, the iliacus externus in Bombina is not divided. It originates along the dorsal surface of the iliac shaft from where it moderately extends medially and laterally (Fig. 5–2B and C), and inserts on the anterior surface of the proximal third of the femur. The origin on the lateral side is longer than that on the medial side. The iliacus internus has its origin from the anterior part of the acetabular portion of the ilium (Fig. 5–2B and C) and inserts onto the anterior surface of the middle section of the femur (Fig. 5–1M). In some individuals, its origin may be divided into medial and lateral portions, separated by the anterior edge of the bone, so that two origins on the ilium, although close to each other, can be discerned.
The tensor fasciae latae is a very slender muscle that originates invariably on the medial surface of the iliac shaft (Fig. 5–2C). The cruralis has the same origin and insertion as in Discoglossus but seems to consist of two separate layers, one deeper, attached to the anterior surface of the distal third of the femur, and one superficial, which fuses to the deeper layer distally only.
The glutaeus maximus and the common tendon of the iliofibularis and iliofemoralis are proximally attached to an indistinct elevation on the supra-acetabular portion of the ilium, which therefore represents the tuber superius (Figs 5–1I and 5–2A and B). A well-distinguishable ligament spreads in a fan-like shape from the common tendon of the iliofemoralis and iliofibularis and fuses to the fascia covering the dorsal surface of the pelvic girdle. A similar ligament was not observed in Discoglossus.
The gracilis minor is a very slender muscle (Fig. 5–1D) that inserts together with the gracilis major on the femur. In contrast to Discoglossus pictus, it originates on the skin (Fig. 5–1G), rather than on the wall of the cloaca.
The semitendinosus originates on the ischium as two separate parts (termed the caput dorsale and caput ventrale). Both parts run independently towards the femur where they fuse (approximately at the level of the midlength of the bone). Their common attachment to the femur is identical to that in Discoglossus.
The adductor longus is not present as an independent muscle. In contrast, the pectineus is relatively robust (Fig. 5–1J) and its origin on the ilium and insertion on the femur are larger than in Discoglossus pictus. Similar to the adductor longus, which is not differentiated from the pectineus, the quadratus femoris also seems to be fused to the obturator externus. In contrast, the gemellus is well distinguishable among the deep muscles of the dorsal side (Fig. 5–1K). It originates on the supra-acetabular portion of the ischium (Fig. 5–2B) and inserts on the dorsal surface of the crista femoralis.
Notes on the myology of Bombina can also be found in Dunlap (1960).
Pelobates fuscus (Figs 6–1 and 6–2)
Fig. 6–1.
Pelobates fuscus. (A) Pelvis (dorsal view); most muscles originating on iliac shaft, except for coccygeoiliacus, removed. Note position and extent of sacral diapophyses. (B) Pelvis (dorsal view); superficial muscles around iliac joint removed. Note segmentation of longissimus dorsi. (C) Pelvis (left lateral view); muscles inserted on posterior part of iliac shaft removed. (D) Same as in C but some muscle insertions on posterior part of iliac shaft preserved, tensor fasciae latae partly removed. Sacral diapophysis and iliac shaft disarticulated. (E) Surface layer of thigh muscles (ventral view). (F) Surface layer of thigh muscles (dorsal view). Accessory head of plantaris longus marked by arrow. (G) Same as in E but gracilis major partly displaced to show origin of sartorius. (H) Same as in F but glutaeus maximus partly removed to show its insertion on cruralis, and two portions of cruralis. Plantaris longus removed. (I) Same as in G but sartorius and adductor magnus removed. (J) Same as in H but semimembranosus removed. (K) Deep layer of thigh muscles (ventral view). Pectineus partly displaced to demonstrate border with adductor longus. (L) Deep layer of thigh muscles (dorsal view). A, B–D, H and K are male; E–G, I, J and L are female.
Fig. 6–2.
Pelobates fuscus. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6333a (male).
The coccygeosacralis originates along the whole length of the urostyle, below the attachment of the longissimus dorsi, and is attached to the dorsal surface of the sacral diapophysis (Fig. 6–1A). The coccygeoiliacus takes its origin on the posterior half of the urostyle, thus covering the origin of the coccygeosacralis (Fig. 6–1A), and inserts on the ventral surface of the anterior part of the iliac shaft, both on its medial and lateral surface. Its medial area of attachment is longer than the lateral one (Fig. 6–2B and C). In some individuals, the medial and lateral portions of the muscle may be in contact with each other on the dorsal side of the shaft but do not fuse.
The pyriformis was absent in all specimens investigated by us.
The iliacus externus is divided into two parts that originate on the lateral and medial surface of the iliac shaft (Fig. 6–2B and C). The origin of the inner part of the muscle is in an oblique groove slanting anteroventrally from the dorsal surface of the bone. The origin of the outer part is more extensive, extending to the ventral margin of the shaft. The muscle then runs to the femur where it inserts on the dorsal surface of the proximal part. The iliacus externus and coccygeoiliacus surround the iliac shaft entirely. The iliacus internus originates only on the medial surface of the ilium (Fig. 6–2C), with a tendency to extend anteriorly onto the shaft.
The tensor fasciae latae (Fig. 6–1D) originates in a small area on the ventral side of the iliac shaft, slightly expanding on its lateral and medial surface (Fig. 6–2B and C). The cruralis consists of two parts. The proximal part originates on the anterior part of the acetabular margin and inserts in the articular capsule of the knee and the distal portion originates on the distal third of the femur and also attaches to the articular capsule of the knee. The distal part is partly covered by the proximal portion. Both parts are well distinguishable from one another (Fig. 6–1H and J).
Although the tuber superius on the dorsal surface of the iliac shaft is absent, the glutaeus maximus, iliofibularis and iliofemoralis originate at the typical area on the lateral and dorsolateral surface of the proximal portion of the shaft (Fig. 6–2B).
The sartorius is attached to the posteroventral rather than the anteroventral part of the pelvis (Figs 6–1G and 6–2B).
Only the dorsal head of the semitendinosus could be recognized in our specimens, located between the gracilis major and adductor magnus (Fig. 6–2B). Its distal insertion is the same as in Discoglossus.
The origin of the obturator externus on the pelvis, as indicated in Fig. 6–2B, is in fact a common origin of the obturator externus, quadratus femoris and gemellus because these muscles cannot be distinguished as separate units. This complex lies closely adjacent to the obturator internus.
Bufo guttatus (Figs 7–1 and 7–2)
Fig. 7–1.
Bufo guttatus. (A) Pelvis (dorsal view); skin removed. (B) Pelvis (dorsal view); right iliacus externus removed. (C) Pelvis (left dorsolateral view). (D) Pelvis (left dorsolateral view); only deep proximal muscles preserved. (E) Surface layer of thigh muscles in ventral view. The sartorius slightly raised to demonstrate its size. (F) Surface layer of thigh muscles (dorsal view). (G) Same as in E but sartorius partly removed (see arrow). (H) Same as in F but glutaeus maximus partly displaced to demonstrate extent of its fusion with cruralis. (I) Same as in G but gracilis major removed. (J) Same as in H but glutaeus maximus removed. (K) Deep proximal thigh muscles (ventral view). (L) Deep thigh muscles (dorsal view). All are female.
Fig. 7–2.
Bufo guttatus. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on the inner surface of the right ilium. Based on skeleton DPFNSP 6582 (male).
The coccygeosacralis originates on the posterior edge of the sacral diapophysis (Figs 7–1A and B, and 7–2A). The coccygeoiliacus runs from the urostyle onto the medial surface of the iliac shaft (Figs 7–1A and B, and 7–2A and C).
The iliacus externus originates mostly on the lateral surface of the iliac shaft with only its minor posterior part extending onto the medial surface of the latter (Figs 7–1L and 7–2A–C). The iliacus internus takes its origin both on the medial and lateral surface of the praeacetabular portion of the ilium (Fig. 7–2B and C) and inserts on the anterodorsal surface of the proximal half of the femur (Figs 7–1J and L, and 7–2A).
The tensor fasciae latae is tiny and takes its origin on the ventral (but partly also lateral and medial) surface of the iliac shaft (Fig. 7–2B and C) after which it runs along the anteroventral surface of the thigh (Fig. 7–1K). The glutaeus maximus is attached to the cruralis along the distal two-thirds of the latter (compare Fig. 7–1F and H).
The gracilis major (Fig. 7–1E and G) has its proximal origin markedly more extensive and more ventral than in Discoglossus pictus. In one of the investigated specimens it even partly covered the proximal origin of the ventral head of the adductor magnus.
The sartorius differs from that in Discoglossus in having its origin located more anteriorly (Figs 7–1E and 7–2B). The semitendinosus has two proximal heads (Fig. 7–2B). The dorsal head is larger and its origin is between the semimembranosus, gracilis major and dorsal head of the adductor magnus. The ventral head originates between the gracilis major, quadratus femoris, and between the dorsal and ventral heads of the adductor magnus. Both heads fuse with each other at about one-third of the femur.
The adductor magnus is proximally divided into two heads. The dorsal head originates on the posterior part of the ischium, between the origins of the dorsal head of the semitendinosus, and the gracilis major, and the ventral head of the semitendinosus and the quadratus femoris. The ventral head of the adductor magnus originates between the gracilis major and obturator externus (Fig. 7–2B). It is partly covered by the sartorius (Fig. 7–1E). Both heads fuse with each other at about one-third down the femur. The distal portion of the muscle turns onto the dorsal surface of the femur (Fig. 7–1L).
The pectineus has a larger origin (Figs 7–1G and 7–2B) than in Discoglossus, reaching onto the ilium. The obturator externus has a more confined origin than in Discoglossus, covering only the ventral margin of the acetabulum. It is positioned adjacent to the quadratus femoris posteriorly (Fig. 7–2B) and the gemellus, the latter being covered by the proximal origin of the semimembranosus.
Rana esculenta (Figs 8–1 and 8–2)
Fig. 8–1.
Rana esculenta. (A) Pelvis (dorsal view); fascia removed on right side. (B) Pelvis (dorsal view); right coccygeoiliacus removed. (C) Pelvis and proximal part of left femur with deep muscles (lateral view). (D) Same as in C but iliacus internus removed. (E) Surface layer of thigh muscles (ventral view). (F) Surface layer of thigh muscles (dorsal view). (G) Same as in E but gracilis minor removed; gracilis major displaced to show ventral head of semitendinosus. (H) Same as in F but glutaeus maximus cut off proximally to demonstrate its insertion on cruralis. Note a. circumflexa femoris lateralis between iliacus internus and cruralis. (I) Same as in G but distal section of sartorius raised to show its attachment to caput tibiae. (J) Same as in H but semimembranosus displaced to show deep layer of dorsal thigh muscles. (K) Same as in I but sartorius removed. (L) Same as in J but semimembranosus removed. (M) Same as in K but gracilis major removed. (N) Same as in L but adductor magnus partly removed. A and B are female; C–N are male.
Fig. 8–2.
Rana esculenta. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6298b (female).
The coccygeoiliacus inserts in the longitudinal depression on the medial surface of the iliac shaft (Fig. 8–2C). It does not extend onto its lateral surface because its insertion is separated from that of the iliacus externus by a prominent dorsal crista (the latter is protruding underneath the skin and may be detected by palpation). Consequently, the crista is exposed underneath the skin along its whole length (Fig. 8–1A and B).
The iliacus externus originates only on the lateral surface of the dorsal crista of the iliac shaft (Figs 8–1D and 8–2B) and may thus be considered a homologue of its pars externa in Discoglossus. It inserts on the anterior surface of the femur and the insertion never extends over the crista femoralis. The iliacus internus (Figs 8–1C,H,L and N, and 8–2C) always originates at the medial surface of the ilium and inserts on the anterior surface of the femur about midway down its length.
The tensor fasciae latae is comparatively robust (Fig. 8–1B and F) and originates at the ventral portion of the medial surface of the iliac shaft (Fig. 8–2C). The iliofibularis and iliofemoralis, as well as the glutaeus maximus, take their origin at the posterior end of the dorsal crista of the iliac shaft (Fig. 8–2B), which is therefore a homologue of the tuber superius.
The gracilis major can be robust to such a degree that, in some individuals, it exceeds beyond the semimembranosus in dorsal view. The gracilis minor originates by a ligament that comes from the wall of the cloaca, its distal insertion being the same as in Discoglossus.
The sartorius is robust (Fig. 8–1E and I) and takes its origin on the lateral surface of the anterior part of the acetabular portion of the ilium (Fig. 8–2B). The semitendinosus (Fig. 8–2B) originates by two thin proximal heads, the dorsal one being attached to the ischium between the semimembranosus and gemellus, and the ventral one between the ventral head of the adductor magnus and obturator internus (Fig. 8–2B). Both parts fuse with each other and insert into the aponeurosis of the knee as in Discoglossus.
The adductor magnus has two parts, one (caput dorsale) originates on the lateral surface of the posterior part of the ischium and the second (caput ventrale) on the lateral surface of the ventral part of the ischium. Both parts fuse with each other at about midway down the thigh. Distally, the muscle turns around the femur and inserts onto the dorsal side (Fig. 8–1L and N).
The gemellus originates at the dorsal margin of the ischium (Fig. 8–2B) and inserts on the dorsal surface of the femur, close to its proximal head. The quadratus femoris is continuous with the obturator externus (both are separated only by the origin of the caput ventrale of the semitendinosus) and covers the posterior part of the ischium (Fig. 8–2B).
The muscles of Rana have been described in detail by Gaupp (1896), Dunlap (1960) and partly also by Green (1931).
Xenopus laevis (Figs 9–1 and 9–2)
Fig. 9–1.
Xenopus laevis. (A) Pelvis (dorsal view); skin removed. Border between iliacus externus and longissimus dorsi marked by arrow. (B) Pelvis (dorsal view); right iliacus externus partly displaced to demonstrate its layers. (C) Pelvis (dorsal view); right iliacus externus removed. (D) Surface layer of thigh muscles (ventral view). (E) Surface layer of thigh muscles (dorsal view). (F) Same as in D but surface fascia and gracilis minor removed. Note extent of rectus abdominis. (G) Same as in E but glutaeus maximus partly displaced and semimembranosus removed. (H) Same as in F but sartorius removed and semitendinosus partly displaced to show its two parts. (I) Same as in G but glutaeus maximus and iliofibularis removed, and cruralis partly displaced to demonstrate extent of adductor magnus. (J) Deep thigh muscles (ventral view). (K) Insertion of iliacus externus on femur (dorsal view). All are females.
Fig. 9–2.
Xenopus laevis. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. Based on skeleton DPFNSP 6411a (sex not recorded).
A peculiar feature of Xenopus is the latissimus dorsi, which covers the suprascapula and extends posteriorly as far as the glutaeus maximus and cruralis where it fuses with their superficial layers (Fig. 9–1A and B). It is a superficial muscle that is also attached to a dorsal fascia. Although it inserts on the mentioned thigh muscles and also partly covers the pelvis, its main function is to rotate the front limb posteriorly.
The longissimus dorsi is located in a deeper layer below the latissimus dorsi where, as in Discoglossus, it is attached to the transverse processes of the vertebrae. Posteriorly, it inserts on the lateral surface of the anterior two-thirds of the urostyle (Fig. 9–1C).
Another peculiar feature is the iliacus externus muscle, which is split into three layers, i.e. the inner, middle and outer (Fig. 9–1B). The inner layer originates on the lateral, ventral and medial surface of the iliac shaft (Fig. 9–2B and C) and inserts on the anterior surface of the femur, along its proximal third (Fig. 9–1A). The middle layer also originates on the lateral, dorsal and medial surface of the iliac shaft (Fig. 9–2B and C) but inserts on the femur slightly more proximal than the inner layer (Fig. 9–1K). The origins of both layers of the iliacus externus join posteriorly on the lateral side of the iliac shaft (Fig. 9–2B). The outer layer originates on the dorsal fascia (Fig. 9–1C), namely on a ligamentous plate that extends from the anterior part of the iliac shaft to its counterpart on the opposite side as the thickened posterior margin of the dorsal fascia. It inserts on the crista femoralis on the dorsal surface of the proximal one-third of the femur.
The gracilis minor is thick (Fig. 9–1D) and has common attachments with the gracilis major. The proximal origin is partly on the wall of the cloaca.
The sartorius partly originates on the ischium but predominantly on the praepubis (Fig. 9–2B). It should be noted that de Sá & Hillis (1990) observed partial fusion of the sartorius and semitendinosus in Xenopus (and Silurana). The semitendinosus is, compared with other species, rather extensive and its origin is between the gracilis major and sartorius (Fig. 9–2B). Its anterior margin may even fuse with the surface of the sartorius (Grobbelaar, 1924).
The adductor magnus is divided proximally into two parts (Fig. 9–2B). The caput dorsale is covered by the gracilis major and the caput ventrale by the semitendinosus.
The obturator externus is confluent with the quadratus femoris. The gemellus takes its origin on the lateral surface of the dorsal part of the ilium and ischium and inserts on the femur close to its proximal head. It is covered by the semimembranosus.
The rectus abdominis (Fig. 9–1F) comes from the ventral surface of the body and is posteriorly attached, partly to the praepubis and partly to the ventral surface of the proximal one-third of the femur where it penetrates from the surface layer between the cruralis and sartorius [on the anterior margin of the sartorius, according to Grobbelaar (1924)].
The coccygeosacralis is absent. In contrast, Xenopus has a short muscle that takes its origin on the descending part of the ilium (not shown in Fig. 9–2C) and inserts on the ventral surface of the cartilaginous epipubis (see also Grobbelaar, 1924).
The muscles of Xenopus have been described in detail by Grobbelaar (1924) and partly also by Grobbelaar (1935), Millard (1941) and Dunlap (1960).
Pipa pipa (Figs 10–1 and 10–2)
Fig. 10–1.
Pipa pipa. (A) Pelvis (dorsal view); skin removed. (B) Right part of pelvis (dorsal view); longissimus dorsi and iliacus externus removed. Note structure of iliosacral joint. (C) Pelvis (left dorsolateral view). Note extent of longissimus dorsi and division of iliacus externus. (D) Pelvis (left lateral view). Pulmonum proprius removed, its insertion marked by arrow. (E) Surface layer of thigh muscles (ventral view). Posterior part of rectus abdominis raised to show its insertion between cruralis and sartorius. (F) Surface layer of thigh muscles (dorsal view). (G) Same as in E but rectus abdominis flapped posteriorly to show adductor longus. (H) Same as in F but longissimus dorsi partly removed to show three parts of iliacus externus. (I) Same as in G but rectus abdominis flapped anteriorly to show partly displaced semitendinosus. (J) Same as in H but glutaeus maximus removed and semitendinosus partly displaced. (K) Deep thigh muscles (ventral view). (L) Insertion of three parts of iliacus externus on femur (dorsal view).
Fig. 10–2.
Pipa pipa. (A) Semidiagram of some pelvic and thigh muscles (dorsal view). Internal part of iliacus externus not illustrated. (B) Origins of muscles on right lateral surface of pelvic girdle. (C) Insertions of muscles on inner surface of right ilium. All images represent female. Based on skeleton DPFNSP 6385a (male).
The latissimus dorsi (see also Xenopus) originates on the humerus and then runs on the flanks posteriorly towards its insertion on the posterior part of the lateral surface of the iliac shaft and on the dorsal fascia (Fig. 10–1A).
The structure of the iliosacral articulation differs from that in other anurans (including Xenopus) in that the iliac shaft is twisted along its long axis so that its originally inner surface has become the dorsal one (Figs 10–1B and 10–2A).
The longissimus dorsi (Fig. 10–1A) is a broad muscle covering the dorsum and part of the flanks. Posteriorly, it is attached to the urostyle and to the fascia of the coccygeoiliacus. The coccygeoiliacus originates on the posterior half of the urostyle (Fig. 10–2A) and inserts on the dorsomedial surface of the anterior portion of the iliac shaft and, to a lesser extent, also on its ventrolateral surface (Fig. 10–2B and C). The coccygeosacralis is absent.
The iliacus externus consists of two parts. The pars interna originates from the dorsal surface of the posterior part of the iliac shaft (Fig. 10–2B and C) and inserts on the proximal part of the femur close to its articular head (Fig. 10–1C and D). The pars externa takes its origin from the ventrolateral surface of the iliac shaft and inserts on the anterior surface of the femur (Fig. 10–1C and D). Lateral to the pars externa but well separated from it is a muscle termed the pulmonum proprius that originates on the dorsolateral surface of the lungs and inserts on the dorsal surface of the femur, close to but posterior to the insertion of the ventral part of the iliacus externus (Fig. 10–1C and D; see also Dunlap, 1960, Fig. 12). The iliacus internus originates on the anteromedial part of the acetabular portion of the ilium, as in most other anurans, but its distal insertion is confined to the anterodorsal crista of the femur.
The tensor fasciae latae is a slender muscle that, although its attachments are similar to other anurans, is located on the anteroventral surface of thigh.
The gracilis minor is comparatively robust (Fig. 10–1H) and has a common origin with the gracilis major (Fig. 10–2B). However, this origin is not associated with the cloaca. Insertions of both muscles are separate from one another.
The rectus abdominis is a thin flat muscle extending from the ventral surface of the trunk onto the ventral surface of the thigh where it dives in between the cruralis and sartorius and inserts on the ventral crista of the femur.
The sartorius has its origin partly covered by the ventral head of the semitendinosus, i.e. it is deeper than in other anurans (Figs 10–1E and 10–2B). Immediately posterior is the origin of the adductor magnus. The distal portion of the sartorius is comparatively robust and inserts, as in other anurans, at the articular aponeurosis of the knee. The semitendinosus arises proximally by two well-defined heads (Fig. 10–1E and I); the ventral head originates close to the ventral margin of the ischium and pubis, whereas the dorsal head covers the origin of the adductor magnus (Fig. 10–2B). Both heads fuse with each other distally (about midway down the femur). Its distal insertion is at the knee aponeurosis but, compared with other anurans, as far as the proximal section of the tibiofibula (Fig. 10–1I). Compared with Discoglossus, the semitendinosus of Pipa is located more superficially.
The adductor magnus originates on the ischium and, to a lesser degree, on the pubis. One can recognize its two proximal heads (caput ventrale and caput dorsale) but these fuse with each other at the level of the iliac joint and the muscle continues distally, turning onto the dorsal surface of the femur (Fig. 10–1K).
The adductor longus also inserts at the ventral crista of the femur between the insertions of the cruralis and rectus abdominis, in addition to its usual attachment to the proximal third of the femur. The pectineus is not adjacent to the adductor longus, as is the case in other anurans, but is separated from it along the whole length of the femur by the rectus abdominis (Fig. 10–1E and G). Distally, it inserts onto the ventral crista of the femur (Fig. 10–1K). Its origin is nearly confluent with that of the obturator externus. The gemellus originates on the dorsal part of the ischium where it is covered by the semimembranosus (Figs 10–1D and 10–2B). Its insertion is on the posterodorsal surface of the caput femoris. The quadratus femoris could not be recognized in our specimen and the coccygeosacralis and pyriformis are absent.
The muscles of Pipa pipa are also mentioned by Dunlap (1960).
Discussion
Comparative myology
The longissimus dorsi, in its presacral section, is subdivided by transverse tendinous septa that, in some taxa (e.g. Rana; Gaupp, 1896), may extend up to the postsacral section. The muscle originates on spinal and transverse processes of the presacral vertebrae, runs across the sacrum and inserts on the urostyle. Its insertion may be short, limited only to the anterior part of the urostyle (e.g. Rana), or long and extending almost to its tip (Ascaphus). It may even be inserted near the posterior end of the urostyle and partly replaced by a tendon (Kaloula; Emerson & De Jongh, 1980). Postsacral sections of this paired muscle may either be in contact with one another, such that the urostyle is overlain dorsally, or be isolated from one another by a crista (Rana). Usually, the postsacral section is well discernible from the coccygeosacralis and coccygeoiliacus (Fig. 15B). However, in some taxa (e.g. Barbourula, Pelobates and Xenopus) it is confluent with the coccygeosacralis or, as in Ascaphus, with the coccygeosacralis-coccygeoiliacus complex. In its presacral section it is usually well separated from the iliolumbaris (e.g. Ascaphus and Rana) and, in some taxa (Bufo marinus; Emerson & De Jongh, 1980), it is divided, from the level of the fourth presacral vertebra, into medial and lateral portions.
Fig. 15.
Diagrammatic representation of some pelvic and thigh muscles in caudates (A) and anurans (B), in dorsal view.
The latissimus dorsi, extending as far as the pelvic region, occurs only in the Pipidae (see also Grobbelaar, 1924). It originates on the humerus but extends as far as the thigh where in Xenopus it fuses with superficial layers of the cruralis and glutaeus maximus or, as in Pipa, inserts on the posterior part of the lateral surface of the iliac shaft. Also, the pulmonum proprius (Fig. 10–1C,D and K), in spite of its origin on the dorsolateral surface of the lungs, extends posteriorly where it enters between the dorsal thigh muscles to insert onto the femur (see also Dunlap, 1960). These two muscles may potentially play a role in swimming but no functional studies have been conducted.
The iliolumbaris is differentiated from the longissimus dorsi (Fig. 15B) and originates on the transverse processes of the presacral vertebrae. When undivided, it inserts on the anterior margin of the sacral diapophysis and on the anterior tip of the iliac shaft. However, it may also be divided in the medial and lateral portions (in Rana; Gaupp, 1896). The medial part may be continuous with the coccygeosacralis or separated from it by a tendinous inscription. The lateral part inserts on the articular capsule of the iliosacral joint (e.g. Pseudis; Manzano & Barg, 2005), inserts along the anterior third of the iliac shaft or is continuous with the coccygeoiliacus to a varied degree (Ascaphus). Its insertion on the iliac shaft varies significantly across a wide spectrum of anuran taxa (Emerson, 1982; Manzano & Barg, 2005), which suggests that its pattern is more related to function than to phylogeny.
The coccygeosacralis may originate along the posterior margin of the sacral diapophysis (Bufo guttatus; Emerson & De Jongh, 1980) or may be continuous with the medial portion of the iliolumbaris (Fig. 15B). In this case it may be separated only by a tendinous inscription (Rana) or may be confluent with the iliolumbaris such that the sacral diapophysis is completely enclosed in the muscle both dorsally and ventrally (Barbourula). It inserts on the lateral surface of the urostyle, either on its anterior (Rana) or posterior section (Ascaphus and Kaloula), and may be partly replaced by a tendon (Emerson & De Jongh, 1980). It is not separated from the longissimus dorsi in Barbourula and Xenopus (see also Grobbelaar, 1924; Whiting, 1961), and Pipa.
The coccygeoiliacus originates on the inner surface of the iliac shaft (along the anterior two-thirds in Rana) or on both its lateral and medial surface (Pelobates). It inserts on the lateral surface of the urostyle along almost its entire length (Rana) or only on its posterior part (Ascaphus, Bombina orientalis, Pelobates fuscus and Kaloula; Emerson & De Jongh, 1980). The attachment onto the urostyle may be partly overlain by the coccygeosacralis (Discoglossus pictus, Bufo guttatus and Bombina orientalis) or longissimus dorsi and coccygeosacralis (Pelobates), or may even be completely covered by the coccygeosacralis (Barbourula).
The pyriformis originates uniformly at the distal end of the urostyle (van Dijk, 1955, 1959;Dunlap, 1960; Emerson & De Jongh, 1980; Cannatella, 1985) and inserts on the dorsal surface of the crista femoralis between the gemellus and iliofemoralis (Dunlap, 1960; Emerson & De Jongh, 1980). It is absent in Pipa and Hymenochirus, and may be reduced or absent in Xenopus (Dunlap, 1960; Cannatella, 1985). It is also absent in Pelobates (but present in Megophrys and Scaphiopus; Dunlap, 1960).
The caudalipuboischiotibialis occurs only in Leiopelma and Ascaphus. It originates from the dorsolateral margin of the tip of the urostyle (sometimes even from the connective tissue between the tip of the urostyle and dorsal surface of cloaca; Ritland, 1955) and inserts into the tendinous inscription of the semimembranosus (Fig. 15B).
The iliacus externus originates by a single head from either the lateral surface of the iliac shaft (Ascaphus and Bombina), its lateral and ventral surface (Rana) or by two heads (Discoglossus, Barbourula, Alytes, Pelobates, Pipa and Megophrys; see also Dunlap, 1960). The medial head (pars interna) arises from the dorsolateral surface of the shaft and the lateral head (pars externa) arises from the ventral or ventrolateral surface of the shaft. The lateral head in Pipa originates in part on the dorsal and ventrolateral surface of the iliac shaft posterior to the sacral diapophysis and partly along the entire ventral surface of the shaft (Dunlap, 1960). In Xenopus, the iliacus externus is divided into layers rather than heads. The inner layer originates on the lateral, ventral and medial surface of the iliac shaft. The middle layer arises on the lateral, dorsal and medial surface, and the outer layer originates on the dorsal fascia. The inner layer of Xenopus may be considered a homologue of the lateral head (pars externa) of other anurans and the middle layer a homologue of the medial head (pars interna). The outer layer occurs only in Xenopus. In Pelobates, the medial head originates in an oblique groove slanting anteroventrally from the dorsal surface of the bone (Fig. 6–2C). It is obvious that the origin of the iliacus externus varies in both extent and location across a wide range of taxa; however, it uniformly inserts on the posterodorsal surface of the proximal section of the femur.
The iliacus internus is rather uniform. It originates from the medial or anterior surface of the acetabular portion of the ilium and inserts along the dorsal and medial proximal aspect of the femur.
There is only a single undivided iliacus muscle in Leiopelma. It originates from the lateral surface of the ilium ventral to the origin of the glutaeus maximus and its insertion is similar to that of the iliacus externus (Dunlap, 1960).
The tensor fasciae latae may vary considerably, even within a single species. It originates on the inner or ventral surface of the iliac shaft, either along its posterior third (Ascaphus, Leiopelma, Xenopus, Scaphiopus and Megophrys) or along the anterior third (Limnodynastes, Eleutherodactylus, Atelopus and Rheobatrachus; Dunlop, 1960, Davies & Burton, 1982). In Rana (but see our results on Rana esculenta), its origin may be divided into two heads, one on the ventrolateral aspect of the iliac shaft (just posterior to the midshaft) and one on the ventral surface of the iliacus externus (Dunlap, 1960). It inserts variably onto the cruralis, in the majority of taxa on its middle third but in Rhinophrynus it inserts at the distal third and in Helioporus at the proximal third (Dunlap, 1960). It may also insert in part on the dorsolateral surface of the glutaeus maximus and a portion may extend distally to fuse with the aponeurosis of the cruralis (Rana; Dunlap, 1960). According to Limeses (1964), it may be present or absent in closely related species.
The cruralis originates uniformly from the anterior surface of the acetabulum and the corresponding part of the articular capsule between the iliacus internus and pectineus (e.g. Rana), and inserts onto the knee aponeurosis. According to Dunlap (1960), it is not divided (Ascaphus and Leiopelma), divided to a variable degree (Discoglossus, Bombina, Alytes and Rana) or completely divided in proximal and distal portions, or deep and superficial layers (e.g. Pelobates, Scaphiopus, Rhinophrynus, Hyla and Eleutherodactylus).
The glutaeus maximus originates invariably from the dorsolateral border of the tuber superius (or from a corresponding part of the iliac shaft), just dorsolateral to the origin of the iliofibularis and iliofemoralis. It inserts onto the aponeurosis of the cruralis. In some taxa (Bombina, Barbourula, Xenopus, Pipa, Scaphiopus and Megophrys), there is an accessory tendon attached to the dorsal fascia of the sacral region. This tendon is absent in Ascaphus, Leiopelma, Discoglossus, Alytes and Rana (Dunlap, 1960; Emerson & De Jongh, 1980).
All of the three previous muscles (tensor fasciae latae, cruralis and glutaeus maximus) form a structural and functional unit, termed the m. triceps femoris. All of the three parts are attached to the ilium relatively close to the acetabulum, whereas their distal ends are united in the aponeurosis covering the knee joint and, by means of the insertion of the cruralis, they are attached to the distal part of the femur. However, their innervation suggests that originally these muscles were independent (the tensor fasciae latae is innervated by the n. cruralis and the other two from the n. ischiadicus).
The iliofibularis originates invariably on the posterolateral border of the iliac shaft, just ventral and somewhat posterior to the origin of the glutaeus maximus. It inserts onto the knee aponeurosis. As it varies little in origin and insertion, it is one of the most uniform muscles of the thigh.
The iliofemoralis originates from the posterolateral surface of the iliac shaft ventral to the origin of the iliofibularis and in part from the tendon of origin of the iliofibularis. It inserts along the proximal third of the dorsomedial border of the femur, between the insertion of the pyriformis and the iliacus internus. It is relatively uniform. The chief deviations are in Leiopelma and Rhinophrynus, in which it is independent of the iliofibularis (Dunlap, 1960). In contrast, both muscles originate by means of a single tendon in Discoglossus and Bombina.
The semimembranosus is located on the posterodorsal side of the thigh. Its characteristic feature (shared only with the gracilis major) is an oblique tendinous inscription. It originates on the supra-acetabular portion of the pelvis (both at the dorsal part of the ischium and/or the pars ascendens ilii) and inserts invariably on the posterior surface of the articular capsule of the knee.
The gracilis major has a tendinous inscription, similar to the semimembranosus. It originates at the posterolateral surface of the ischium and inserts onto the knee aponeurosis and the posterior surface of the tibiofibula just below its proximal head. These two muscles are generally rather uniform.
The gracilis minor originates from the ischium (e.g. Ascaphus, Xenopus, Pipa, Scaphiopus and Megophrys), the wall of the cloaca (Rana and Xenopus) or the skin (Leiopelma, Bombina, Discoglossus and Alytes; Dunlap, 1960). It unites with the gracilis major and inserts by the common gracilis tendon (Rana) or it may insert independently of the gracilis major (e.g. Discoglossus, Alytes and Scaphiopus). In Ascaphus (and Leiopelma; Dunlap, 1960), both unite near the tendinous inscription of the gracilis major. According to Dunlap (1960), the gracilis minor in Rhinophrynus originates as a thin, broad sheet from the skin of the ventral side in the posterior half of the body, extends laterally onto the ventral surface of the thigh and inserts widely at the knee aponeurosis. It is especially large in Xenopus, whereas in the discoglossids it is moderately developed, and the muscle is absent in Hymenochirus (Cannatella, 1985) and Rheobatrachus (Davies & Burton, 1982).
A well-developed muscle extending from the skin of the dorsal surface of the thigh to the posteroventral border of the ischium is sometimes present and may be a differentiated section of the gracilis minor. It is possible that this corresponds to the ‘accessory head’ of the gracilis minor as mentioned by Cannatella (1985).
The sartorius originates on the anterolateral and ventrolateral border of the pelvis, on the ilium (Rana), pubis (or prepubis in Xenopus) or ischium (Discoglossus). In addition, it may originate from the ventral surface of the proximal section of the adductor longus (Rana). It inserts onto the aponeurosis of the ventral part of the cruralis, the insertion of the gracilis major, semitendinosus and the aponeurosis of the dorsal part of the cruralis. In Discoglossus, the sartorius inserts on the ventral head of the semitendinosus in the distal third of the thigh (Dunlap, 1960).
The semitendinosus originates by one (Discoglossus) or two (Barbourula, Bombina, Bufo, Rana and Pipa) slender tendons, of which the ventral one originates on the posteroventral aspect of the ischium (this tendon of origin penetrates the adductor magnus). The dorsal one originates at the posterodorsal part of the ischium, just under the ventral edge of the semimembranosus; both tendons unite in the distal part of the thigh. The muscle inserts on the ventral surface of the proximal part of the tibiofibula.
Whereas in derived frogs (including Rheobatrachus; Davies & Burton, 1982) both muscles are largely separated and united only close to their insertion, in Ascaphus and Leiopelma, some Discoglossidae (Barbourula, Bombina and Alytes), Pipidae (Xenopus and Pipa) and Scaphiopus, the sartorius and semitendinosus are a single muscle (called the sartorius-semitendinosus complex) originating by two heads, one from the pubis and the other from the posteroventral border of the pelvis and partly from the ventral margin of the gracilis major (Dunlap, 1960). Moreover, the muscle may also originate from the surface of the obturator externus and adductor magnus (see van Dijk, 1955 and our observations on Ascaphus and Barbourula).
The adductor longus usually originates on the anteroventral rim of the ilium and inserts on the lateral surface of the adductor magnus, and in some individuals of Rana also by a strong tendon on the knee aponeurosis. It is present in Discoglossus, Barbourula and Pelobates but absent in Ascaphus, Leiopelma, Bombina, Alytes and Scaphiopus, and some Megophrys, Xenopus, Pipa, Rhinophrynus and Hyla(Dunlap, 1960; Cannatella, 1985). According to Dunlap (1960) it has probably never been differentiated from a pectineus-adductor longus complex in those taxa where the adductor longus is absent.
The pectineus originates from the lateral surface of the ilium and from the anterior part of the pubis, and inserts on the crista femoralis (Ascaphus and Rana). The pectineus and adductor longus may be separated distally, whereas their proximal parts are still continuous (Barbourula).
The adductor magnus is divided proximally into two heads that are separated by the ventral tendon of the semitendinosus. The caput ventrale originates from the ventral border of the pubis, the dorsal head from the ischium and is overlain by the origin of the gracilis major.
The ventral head may receive an insertion from the adductor longus and an accessory head may be derived from the semitendinosus. This accessory head (if present) originates from the pelvic rim, between the caput ventrale of the adductor magnus and gracilis major. The ventral head inserts onto the ventral surface of the femur. The dorsal head fuses distally with the ventral and acessory heads, and inserts along the medial surface of the distal third of the femur. The accessory head is absent in Ascaphus, Leiopelma, Discoglossus, Bombina, Alytes, Xenopus, Pipa, Rhinophrynus, Scaphiopus and Megophrys (see also Dunlap, 1960) but present in other anurans, including Rheobatrachus (Davies & Burton, 1982). In Ascaphus and Leiopelma, the ventral and dorsal heads are separated only along the proximal quarter (or less) of the thigh. In Discoglossus, Bombina, Xenopus, Pipa, Scaphiopus and Megophrys, and also in Bufo and Rana, they are separated more extensively (Dunlap, 1960).
The obturator externus is situated just posterior to the pectineus and originates from the ventrolateral surface of the pelvic rim. It inserts on the ventral surface of the proximal half of the femur, medial to the insertion of the pectineus.
The quadratus femoris originates from the ventrolateral border of the ischium just posterior to the origin of the obturator externus and is separated from that muscle by the tendon of the ventral head of the semitendinosus. It inserts on the ventromedial surface of the femur at the base of its articular head.
The previous two muscles may be continuous or divided only in their distal parts (Ascaphus, Discoglossus, Barbourula, Bombina, Xenopus, Pipa, Scaphiopus, Megophrys, Bufo and Rana; see also Dunlap, 1960).
The gemellus originates from the dorsolateral margin of the ischium dorsal to the origin of the quadratus femoris (Barbourula, Bombina, Xenopus, Pipa and Rana) and inserts on the medial surface of the caput femoris.
The previous three muscles are distinct in some species (e.g. Alytes, Pipa, Bufo and Rana) but may show little or no division in others (Ascaphus, Leiopelma, Discoglossus and Pelobates, and some specimens of Bombina; see also Dunlap, 1960).
The obturator internus originates along the entire lateral surface of the pelvic rim, from the pars descendens ilii to the dorsal part of the ischium, and inserts on the dorsomedial surface of the caput femoris.
The rectus abdominis extends among thigh muscles only in the Pipidae. It enters from the surface layer between the cruralis and sartorius and inserts on the proximal section of the femur.
Evolution of pelvic and thigh muscles in the Anura
It is generally agreed that the caudates are good representatives of the primitive tetrapod body plan and that their locomotor pattern probably reflects that of the earliest tetrapods (e.g. Edwards, 1977). Although only relatively few thorough descriptions of the caudate pelvic and thigh musculature are available (e.g. Francis, 1934; Iordansky & Morozov, 1994; Walthall & Ashley-Ross, 2006), it is apparent that the muscles of the caudate hind limb are much less differentiated than those in the anurans. Therefore, it can be reasonably inferred that, irrespective of the function of muscles, the more recalls the muscle pattern of the anurans that of the caudates, the more it may reflect the primitive condition in the temnospondyl ancestors of the Anura.
Patterns of the pelvic and thigh musculature in temnospondyl ancestors of the Anura, which may have been aquatic (Boy & Sues, 2000; Roček & Rage, 2000), may be best inferred from the neotenic caudates such as Necturus maculosus (Walker, 1970; Gilbert, 1973) and Ambystoma, and from the most primitive recent caudates such as the hynobiid Salamandrella keyserlingii (data given below are from Iordansky & Morozov, 1994). However, the muscular pattern of the thigh is basically similar to that in adult Salamandra salamandra (Francis, 1934) and, to a varied degree, also similar to that observed in other caudates (e.g. Taricha torosa, Walthall & Ashley-Ross, 2006).
Axial and pelvic muscles in caudates
In all caudates, irrespective of whether they are neotenic or metamorphosed, the musculature of the trunk consists of myomeres, separated by myosepta, as in fish. Dorsal portions of the myomeres form the dorsal, longitudinal bundle of the dorsalis trunci. Its deeper layers are attached to the neural arches of the vertebrae and its fibres run between the successive myosepta in an approximately anteroposterior direction. In the presacral region of the trunk (i.e. at the level of the coelomic cavity), the body walls consist of three layers of hypaxial muscles, the obliquus externus, obliquus internus and innermost transversus. The longitudinally oriented fibres along the midventral line form an incipient rectus abdominis (Fig. 12F) that is, however, not yet well differentiated from the rest of the hypaxial myomeres.
Fig. 12.
Thigh muscles in Necturus maculosus. (A–G) Ventral view; (H–K) dorsal view. (A) Superficial level of thigh muscles (ventral view). (B) Same as in A but caudalipuboischiotibialis and ischiocaudalis exposed. (C) Same as in B but puboischiotibialis slightly raised to demonstrate its extent. (D) Same as in C but puboischiotibialis removed. (E) Same as in D but ischiofemoralis partly removed. (F) Same as in E but anterior portion of puboischiofemoralis externus removed so inner surface of pubis is exposed. (G) Surface level of thigh muscles (dorsal view). (H) Same as in G but ischioflexorius (and puboischiotibialis from the ventral side) removed. Posterior part of the iliotibialis slightly raised to demonstrate its posterior margin. (I) Same as in H but posterior part of iliotibialis removed. (J) Same as in I but iliofibularis removed. (K) Same as in J but anterior part of iliotibialis removed to demonstrate deep layer of muscles attached to femur. In A–C, the tendinous inscription in the puboischiotibialis is marked by arrows.
The dorsal surface of the thigh is composed of several extensors that arise from the ilium just dorsal to the acetabulum (Fig. 16B), extend across the knee by means of a tendon and insert onto the tibia. The anteriormost one is the iliotibialis, which may originate by two small tendons, the anterior (from the anterior surface of the ilium), called the pars anterior of the iliotibialis (Fig. 13G and K), and the posterior (coming from the lateral surface of the ilium), called the pars posterior of the iliotibialis (Fig. 13G and K). Often these two parts appear as one muscle as the separation between them may be obscure (Figs 12G and 14G). Sometimes, the posterior part is termed the ilioextensorius (Noble, 1931). The muscle inserts into the knee aponeurosis and its main function is probably to extend the knee joint. The most posterior of the superficial extensors is the iliofibularis (Figs 12I, 13G,H and K, and 14J), which arises from the outer surface of the ilium, close to the origin of the posterior part of the iliotibialis (Fig. 16B), and partly also from the proximal surface of the latter, and inserts at the proximal or middle third of the fibula. It is thought to elevate the femur and bend the knee joint.
Fig. 16.
Origins of some muscles in anuran (A) and caudate (B) pelvis, seen in right lateral view.
Fig. 13.
Thigh muscles in larval Ambystoma. (A–F) Ventral view; (G–J) dorsal view; (K–L) right lateral view. (A) Surface layer of thigh muscles (ventral view). Tendinous inscription in puboischiotibialis marked by arrow. (B) Same as in A but caudalipuboischiotibialis and ischiocaudalis exposed. Tendinous inscription in puboischiotibialis marked by arrow. (C) Same as in B but puboischiotibialis detached proximally to show anterior attachment of ischiocaudalis. (D) Same as in C but puboischiotibialis removed. (E) Same as in D but puboischiotibialis removed to show ischiofemoralis. In E1pubotibialis is partly removed to show pubofemoralis (marked by arrow). (F) Deep muscles of thigh (ventral view). (G) Superficial level of thigh muscles (dorsal view). (H) Same as in G but dorsal trunk muscles removed to expose ilium. (I) Same as in H but posterior muscles detached proximally and partly displaced. (J) Same as in I but posterior muscles removed to show pattern of deep muscles. (K) Proximal attachment of thigh muscles (lateral view). (L) Same as in K but posterior muscles removed. Muscles in A–C slightly detached from each other.
Fig. 14.
Thigh muscles in adult Salamandra. (A–F) Ventral view; (G–K) dorsal view. (A) Surface layer of thigh muscles (ventral view). (B) Same as in A but caudalipuboischiotibialis and ischiocaudalis exposed. (C) Same as in B but ischioflexorius exposed. (D) Same as in C but puboischiotibialis detached proximally. (E) Same as in D but puboischiotibialis removed. (F) Same as in E but puboischiofemoralis removed to expose pelvis and proximal section of femur. (G) Surface layer of thigh muscles (posterodorsal view). (H) Same as in G but dorsal view. (I) Same as in G but hypaxial trunk muscles partly removed. (J) Same as in I but iliotibialis removed to show deep layer. (K) Same as in J but most anterior of deep muscles removed.
The deep layer of the dorsal thigh muscles consists of the iliofemoralis (Fig. 12J), which arises from the posterolateral surface of the ilium and partly also from the inner (dorsal) surface of the ischium (Fig. 16B); it inserts on the dorsal surface of the femur and is covered by the iliofibularis. Its main function is to retract the femur if it is in the anterior position. The femorofibularis (Fig. 13J) is also located ventral to the iliofibularis, extending from the distal section of the femur and inserting on the posterolateral surface of the proximal end of the fibula. Its function is to flex the knee joint.
On the ventral surface of the thigh, the anterior part is occupied by the pubotibialis (Figs 12A, 13A–C, 14A–D and 16B), which originates from the anteroventral edge of the pubic cartilage and inserts on the medial surface of the proximal one-third of the tibia. It is thought to cause protraction of the femur and flexion of the knee joint. A similar muscle on the posterior part of the thigh is the puboischiotibialis (Figs 12A–C, 13A–E, 14A–E and 16B). It originates on the puboischiadic plate along its ventrolateral edge and inserts on the anterior surface of the tibia. It has been proposed that this muscle supports the body during walking and causes flexion of the knee. A characteristic feature of this muscle is that about one-quarter from its origin it is divided by a tendinous inscription that is well distinguishable superficially (Figs 12A–C and 13A–C). A similar septum may be found in Salamandrella and Taricha, and probably also in other caudates but not in Salamandra (Fig. 14). It is worth noting that the septum dividing the puboischiotibialis in the neotenic Ambystoma seems to continue (although not as a fibrous partition) into the puboischiofemoralis externus (Fig. 13A and B). Both the pubotibialis and puboischiotibialis converge with each other distally and are in contact along their distal section. It should also be noted that in the neotenic Andrias davidianus (Cryptobranchidae) the pubotibialis and puboischiotibialis are not yet differentiated from each other (Noble, 1931). The triangular space under the two superficial flexors (pubotibialis and puboischiotibialis) is occupied by the puboischiofemoralis externus (Figs 12A–E, 13A–C, 14A–E and 16B). It originates, similar to the puboischiotibialis but in a deeper layer, on the puboischiadic plate along its ventrolateral edge. However, it tapers sharply and inserts on the trochanter of the femur. Its proposed function is to support the body during walking and retraction of the femur. Immediately anterior in this deep layer is the pubofemoralis (Figs 12D and 14D), which arises from the anteroventral surface of the pubis and inserts on the ventral surface of the femur. It is dorsally covered by the puboischiofemoralis internus. Similarly to the puboischiofemoralis externus, its function is thought to be to support the body during walking and retraction of the femur. The most posterior of the deep muscles of the proximal section of the thigh is the ischiofemoralis (Figs 12D, 13E, 14E and 16B), which arises from the lateral border of the ischium and inserts on the posterior surface of the proximal head of the femur. Its proposed function is to protract the femur from its posteriormost position.
The anterior surface of the thigh is occupied by the puboischiofemoralis internus (Figs 12A and B, 13A–D, 14E and F, and 16B), which arises on the dorsal surface of the pubis and on the medial surface of the basis of the ilium and ischium. In Salamandrella, it consists of three portions, the anterior portion inserting on the proximal third of the femur, the medial portion inserting onto the knee aponeurosis and the posterior portion inserting onto the distal third of the femur. Its proposed function is to protract and elevate the femur.
The posterior part of the thigh is formed by the ischioflexorius (Figs 12A–C and G, 13G–I, 14C and G, and 16B), which is partly covered by the puboischiotibialis. It originates from the posterolateral surface of the ischium and its distal end fuses into the plantar aponeurosis. At the level of the knee, it is interrupted by a tendinous inscription. Francis (1934) interpreted the two parts of the ischioflexorius in Ambystoma as two separate muscles (which is supported by their different innervation). Therefore, it is probable that only the proximal section is the true ischioflexorius. The muscle is thought to help to retract the limb.
Three muscles arise on the caudal vertebrae and insert on either pelvis or femur. The medial and dorsal one is the iliocaudalis, which inserts on the ilium and may pull the tail laterally. The lateral and dorsal one is the caudalifemoralis (Figs 12E–F and H–K, 13J, 14E,F and I–K, and 15A), which inserts on the proximal section of the femur (mostly on the posterior surface of the trochanter). Its proposed function is to retract the femur. The medial and ventral one is the ischiocaudalis (Figs 12B and C, 13B–D, 14B–F, and 16B), which inserts on the ischium and pulls the tail laterally. The lateral and ventral one is the caudalipuboischiotibialis (Figs 12B and C, 13B, 14B–D, and 15A), which inserts on the posterior surface of the puboischiotibialis in the region of the fibrous septum (if present) by means of a tendon, or inserts onto the posterior edge of the puboischiotibialis by means of a fibrous strip at approximately right angles (Fig. 12). It may help to retract the limb and flex the tail laterally.
Homologues in the anurans
There is no doubt about the homology of the longissimus dorsi of anurans with the dorsalis trunci of caudates (O’Reilly et al. 2000) (Fig. 15). This may be demonstrated by its location (origin on the neural arches and postzygapophyses of the posterior presacral vertebrae, and insertion on the lateral surface of the anterior section of the urostyle), presence of the intersepta even within its postsacral section in some anuran taxa and its innervation from corresponding spinal nerves.
The iliolumbaris is clearly a separated lateral presacral part of the longissimus dorsi (i.e. dorsalis trunci of caudates), still divided by intersepta. It is also innervated from the spinal nerves, as is the dorsalis trunci of the caudates. In more derived taxa (e.g. Rana) it is divided into medial and lateral portions (Fig. 15B), whereas in primitive anurans, e.g. Ascaphus, Leiopelma and Discoglossus, it is not divided.
The coccygeosacralis is the posterior continuation of the medial portion of the iliolumbaris from which it is separated only by a fibrous septum (Fig. 15B). Their common origin from the dorsalis trunci is shown by their innervation from the dorsal branches of the spinal nerves. Similarly, the coccygeoiliacus may be considered a posterior continuation of the lateral portion of the iliolumbaris (Fig. 15B) innervated by the ventral branches of the spinal nerves, and thus also a derivative of the dorsalis trunci.
The caudalipuboischiotibialis is present in the primitive anurans Ascaphus and Leiopelma; however, in contrast to the caudates, it is located along the proximal section of the posterior face of the thigh, between its origin on the tip of the urostyle and insertion on the tendinous septum of the semimembranosus (van Dijk, 1955). As the urostyle is considerably shortened compared with the tail of caudates, and given the elongation and posterior shift of the ilia, the caudalipuboischiotibialis changed its position from an originally anteroposterior (running between the fibrous septum of the puboischiotibialis in caudates and presumably also in ancestral tempospondyls; Fig. 15) to a lateromedial one (between the transverse fibrous septum of the semimembranosus and the tip of the urostyle). Undoubtedly, it is homologous with one of the caudal muscles of the caudates, as was suggested by Noble (1922, 1931).
Similar to the caudalipuboischiotibialis in Ascaphus and Leiopelma, the pyriformis in most anurans (judging by its origin on the posterior section of the urostyle and insertion on the dorsal surface of the crista femoralis; see van Dijk, 1955) has probably evolved from the caudalifemoralis. Its original anteroposterior course in caudates and presumably ancestral temnospondyls became mediolateral (Fig. 15) due to elongation of the ilia, such that the femur reached the level of the caudal vertebrae (Ročková & Roček, 2005).
The iliacus internus and externus are undivided in Leiopelma (Dunlap, 1960), which suggests that they are derivatives of a single primitive muscle mass (Noble, 1922, Ritland, 1955). The origin of this undivided muscle is on the anterior surface of the ilium, both on its inner and outer sides, and its attachment is on the dorsal surface of the proximal section of the femur, close to its caput. A similar position in caudates is occupied by the puboischiofemoralis internus. In all anurans except for Leiopelma, the iliacus is divided into the iliacus internus and externus, but their function is similar to that of the puboischiofemoralis internus, i.e. they rotate the femur anteriorly. A remarkable shift of the origin of the iliacus externus anteriorly from the iliac joint undoubtedly improved its ability to protract the femur. The course of the iliacus internus presumably indicates the original position of the puboischiofemoralis internus in ancestral temnospondyls (where it presumably originated from the anterior extension of the pubis; Fig. 16B), whereas the iliacus externus (either single-headed, as in Ascaphus, Leiopelma and Rhinophrynus, or double-headed, as in the discoglossoids and pipoids) may have been involved in anatomical transformations associated with the origin of frog locomotion.
The tensor fasciae latae could also have been part of these suggested transformations. Noble (1922, 1931) considered it a homologue of the iliotibialis, which has shortened in frogs such that it inserts onto the surface fascia (‘fascia lata’) of the cruralis. However, it is more probable that it is only the superficial part of the iliotibialis [or of its anterior portion; it should be noted that Noble (1922, 1931) considered its posterior portion as a separate muscle termed the ilioextensorius]. If this is correct, then the cruralis would be a homologue of the remaining parts of the anterior portion of the iliotibialis. This seems to be supported by the origin of the anterior portion of the iliotibialis on the dorsolateral surface of the ilium and by the origin of the cruralis on the anterolateral surface of the ilium (Fig. 16). Moreover, the cruralis is still undivided in Ascaphus and Leiopelma, but can be divided in more derived anurans. The glutaeus maximus may then be considered a homologue of the posterior portion of the iliotibialis. A common origin of all three muscles is expressed in the term ‘triceps femoris’ or ‘extensor cruris communis’ (Gaupp, 1896).
The iliofibularis and iliofemoralis are two muscles that maintain a similar position and function in caudates and anurans (Fig. 16), and their homology is clear.
It has been suggested that the semimembranosus in the anurans may be a homologue of the caudate caudalipuboischiotibialis (e.g. Mivart, 1869, followed by some others, e.g. Iordansky & Morozov, 1994), which is, however, contradicted by the condition in Ascaphus and Leiopelma in which both the semimembranosus and caudalipuboischiotibialis are present. Alternatively, it has been suggested that it may be homologous to the ischioflexorius (Noble, 1922). However, our data suggest that neither of these two interpretations may be correct. The semimembranosus is most probably derived from the dorsal part of the puboischiotibialis, which is shown by: (i) the nearly invariable presence of its fibrous septum in the anurans (except for the Pipidae), (ii) the close topographical relations between the gracilis and semimembranosus (see also van Dijk, 1955, Fig. 34) and (iii) the fact that the caudalipuboischiotibialis in Ascaphus and Leiopelma inserts on the semimembranosus in the region of the septum, as is the case in the caudates (Figs 12–15).
The posterior part of the anuran thigh is occupied by the gracilis major, which is divided to various degrees by the oblique fibrous septum. According to our observations, the septum is well developed in both males and females of Rana and Pelobates, but restricted only to the gracilis major (see also Gaupp, 1896; Dunlap, 1960). It is well developed in both the gracilis major and minor of Xenopus and Pipa. In contrast, it is indistinct in the gracilis major of Discoglossus and Bombina. The septum suggests that the gracilis (comprising both its major and minor parts) may be homologous with part of the puboischiotibialis (see van Dijk, 1955, Fig. 34), rather than with the ischioflexorius as suggested by Noble (1922). This is also supported by the topographic relations of their origins (Fig. 16A).
Both the location and functions of the sartorius in frogs suggest that its evolutionary origins should be sought in the pubotibialis. Moreover, it can be inferred from the condition in the most primitive contemporary anurans (Ascaphus, Leiopelma, Bombina, Alytes, Xenopus, Pipa, Scaphiopus and some Megophrys) in which the sartorius and semitendinosus are not yet divided and where this muscle is only partly separated into two heads (Dunlap, 1960), that both the sartorius and semitendinosus took their origin from the ancestral pubotibialis. In contrast, in advanced anurans (e.g. Rana and Hyla) the sartorius is almost completely separated from the semitendinosus, and the latter muscle originates by two heads. As suggested by Dunlap (1960), the intermediate condition may be represented in Discoglossus where the sartorius-semitendinosus complex is subdivided into three points of origin. The homology of the pubotibialis and sartorius-semitendinosus also seems to be supported by the fact that their function is nearly identical.
There is no indication that the adductor magnus of the primitive frogs Ascaphus and Leiopelma is divided into two heads. Therefore, it seems improbable that the adductor magnus was originally composed of two muscles, one of them (represented by the ventral head of the adductor magnus) being homologous with the pubotibialis and the other supposedly derived from the puboischiofemoralis externus as suggested by Noble (1922) and later followed by Green (1931). However, its precise homologue in caudates remains obscure.
In contrast, the pectineus and adductor longus form a complex that can be recognized in contemporary caudates. Both muscles are represented by a single muscle mass in the most primitive contemporary anurans (Ascaphus, Leiopelma), Bombina, Alytes (but not in Discoglossus), Scaphiopus, some Megophrys, Xenopus, Pipa, Rhinophrynus and others. Dunlap (1960) explained the occurrence of this pattern by the fact that in primitive anurans the adductor longus is not yet differentiated from the common pectineus-adductor longus muscle, whereas in some more derived anurans (Atelopus, Polypedates and Chiromantis) it could have been secondarily lost. The common pectineus-adductor longus muscle originates from the anteroventral margin of the pelvic girdle and appears as a deeper layer in a triangular space between the cruralis and gracilis major, overlain by the sartorius. Its homology with the puboischiofemoralis externus was suggested by Hoffmann (1873–1878) and is supported by the similar origins and insertions of the muscles. However, it should be noted that Noble (1922) considered the pectineus homologous to the puboischiofemoralis internus.
The pectineus in some anurans (e.g. Phyllobates) is only partly differentiated from the obturator externus (Dunlap, 1960). In Ascaphus and Leiopelma, the most primitive contemporary anurans, the obturator externus cannot be separated from the gemellus and quadratus femoris, and therefore these three muscles presumably evolved from a single muscle present in the temnospondyl ancestors. It could be inferred from the location of the undifferentiated muscle relative to the pectineus that it might be a homologue of the ischiofemoralis. This is also supported by its innervation; both the ischiofemoralis of the caudates and the obturator externus, quadratus femoris and gemellus are innervated by the n. ischiadicus. Gradual differentiation of these three muscles may be followed during the development of Rana, in which the obturator externus and gemellus remain undivided until the metamorphosis (the complex is sometimes called the adductor brevis; in earlier developmental stages it is even difficult to distinguish it from the pectineus) (Green, 1931).
Anatomical transformations associated with anuran locomotion
The most striking difference between frogs and their temnospondyl ancestors is probably the type of locomotion, which is reflected in the structure of the pelvis. The pelvis of larval temnospondyls was triradiate, with the puboischiadic plate oriented anteroposteriorly and the ilium vertically, as is the case in contemporary caudates (Fig. 16B). The pubis was the last pelvic element to ossify (Boy, 1974). In adult frogs, the situation is different in that the ilium is protruding anteriorly (i.e. the long iliac shaft), the ischium is reduced to a narrow vertical lamina rimming the posteroventral edge of the acetabulum and the pubis is reduced to a narrow triangular, mostly cartilaginous, plate between the ventral parts of the ilium and ischium (Fig. 16A).
Possible transformations between these two types of pelvis are suggested by anuran metamorphosis. At the beginning of metamorphosis one can recognize a horizontal puboischiadic plate and a vertical element that is the rudiment of the ilium (Ročková & Roček, 2005). During subsequent development, the triradiate pelvis with the ilium situated dorsal to the head of the femur is transformed into the structure whose dominant part (i.e. the iliac shaft) is located horizontally along the caudal part of the vertebral column and whose acetabular portion is shifted posteriorly. If this process is observed in cleared-and-stained metamorphosing tadpoles, it can be understood as posterior rotation from the originally vertical location to the ultimate horizontal position.
However, the situation becomes more complex when considering the muscles associated with the pelvic girdle. First of all, most of the muscles present in caudates maintain approximately the same location of their origins relative to the acetabulum (e.g. the iliofibularis, iliofemoralis, pars posterior of the iliotibialis, pars anterior of the iliotibialis, puboischiofemoralis externus, pubotibialis and ischiofemoralis; see Fig. 16 for their synonyms in the anurans). This suggests that the anteroposterior orientation of the pelvis and ischium did not change, and the same holds for the supra-acetabular portion of the ilium. If this is true, then the iliac shaft appears to be a new structure that expanded from the relatively stable ilium anteriorly. Among the evidence supporting such an explanation belongs the insertion of the obliquus externus, which maintains its original attachment to the anterior part of the ilium. One may thus infer that the anuran pelvis did not arise by a rotation of a gradually posteriorly elongating iliac shaft (which would result in a dorsal position of the ischium and anterior position of the acetabular portion of the ilium), which would involve a corresponding rotation of muscle origins (unless one would admit that muscles could have shifted their origins dramatically). Rather, it seems that the acetabular portion of the pelvis maintained its original orientation and was shifted posteriorly due to elongation of the shaft from the iliosacral articulation. The tip of the ancestral ilium is then represented by the area of the tuber superius of the anuran ilium (cf. Fig. 16A and B).
Previous interpretations emphasized the role of the iliac shaft in the displacement of the acetabular joint posteriorly along the shortened and rigid tail (urostyle). However, the shaft is also an important skeletal support for muscles that are new in frogs and those that acquired a new function. One of them is the coccygeoiliacus, originally a part of the epaxial musculature important for bending and strenghtening the vertebral column. In frogs, however, it is positioned between the urostyle and the iliac shaft (Fig. 15B) and in good jumpers it even attains a nearly transverse position (Fig. 8–2A). Its new function (in cooperation with the coccygeosacralis) is to rotate the urostyle ventrally, relative to the main axis of the vertebral column (as in jumping Rana), to rotate the pelvis in the horizontal plane relative to the urostyle (as in burrowing Pelobates; Fig. 11), or to produce, together with the iliolumbaris, gliding movements of the pelvis in some specialized swimmers (Xenopus and Pipa). The contralateral use of the coccygeoiliacus and iliolumbaris is also important for walking, which is profoundly different from the same type of locomotion in caudates because it employs horizontal glinding of the iliac shaft along the expanded edges of the sacral diapophyses to compensate for the restricted flexion capacity of the vertebral column (in caudates, the active protraction of the femur is accompanied by flexion of the vertebral column; Edwards, 1977).
Fig. 11.
Pelobates fuscus. Extreme positions of the pelvis during burrowing.
Another important muscle originating on the iliac shaft is the iliacus externus, although the area of its origin varies in both extent and position. In some taxa (e.g. Ascaphus and Rana) it has a single but extensive origin and in others it originates by two heads, of which the external one is located far anteriorly, below the iliosacral joint. The iliacus externus inserts onto the anterodorsal surface of the proximal section of the femur and its main function appears to be the protraction of the femur, as is the case with caudates. However, in caudates the homologue of this muscle (puboischiofemoralis internus) originates at the anterior part of the pubis (Fig. 16B). A shift of the origin of this muscle from the anteriorly expanded pubis onto the similarly expanded iliac shaft may be explained by the decreased size of the pubis.
The tensor fasciae latae also originates on the iliac shaft. If examined as an isolated muscle it has no obvious locomotor function (Table 1) but its significance may increase in synchronous activity with the cruralis, when both muscles work as a single unit. Then, the tensor fasciae latae together with the cruralis protracts the femur anteriorly, similar to the iliacus externus (and probably also the pulmonalis proprius in Pipa). As a result, the femur is flexed anteriorly into the crouched position (with the knee touching the flanks of the body), which is important in the preparatory phase of a jump, in the recovery stroke of the swimming and, asynchronously, during anuran walking (Peters et al. 1996).
It should be emphasized that the thigh extensors, which have an important role in the initial phase of the jump (semimembranosus and gracilis, i.e. muscles derived from the puboischiotibialis), did not change their position in comparison to caudates. Thus, the most significant anatomical changes occurred in the anterior part of the pelvis, resulted in the resorption of the pubis and expansion of the iliac shaft, and were accompanied by corresponding changes in the muscles, which, however, maintain their original function. Both in ancestral temnospondyls as well as in the anurans, they work as protractors of the femur. In frogs, they probably facilitate the preparatory phase of the jump but are not crucial during the jump itself.
Another conclusion that follows from comparisons between caudates and anurans is that the pelvic and thigh muscles were originally organized in fewer but larger units (Table 2). Consequently, the transition from ancestral temnospondyls to frogs was accompanied by increasing diversification of the muscular system. For instance, the dorsalis trunci, which is rather compact along its course across the pelvic region in the caudates, splits into smaller units (longissimus dorsi and iliolumbaris) in the presacral region, and even more (longissimus dorsi, coccygeosacralis and coccygeoiliacus) in the caudal region (Fig. 15B). Diversification of epaxial muscles in the pelvic region seems to have continued also in the course of anuran phylogeny, as can be judged by the fact that they are still little differentiated in Ascaphus and discoglossids such as Barbourula, whereas they are well differentiated, for example, in Rana and Bufo.
Table 2.
Possible differentiation of pelvic and thigh muscles in anuran evolution
 |
Differentiation of muscles in the transition from ancestral temnospondyls to anurans also occurred in some of the thigh muscles. For instance, the puboischiotibialis split into two separate muscles, the semimembranosus and gracilis (well illustrated in van Dijk, 1955, Fig. 34) and the anterior portion of the iliotibialis differentiated into the cruralis and tensor fasciae latae.
Other muscles, such as the puboischiofemoralis internus (iliacus in the anurans), pubotibialis and puboischiofemoralis externus, obviously diversified only during anuran phylogeny and not in the transition from ancestral temnospondyls to anurans. This is demonstrated by the fact that the iliacus is still a single muscle in the primitive anuran Leiopelma, whereas it is divided into its external and internal portions in all other anurans. Also the sartorius-semitendinosus, adductor longus-pectineus and obturator externus-quadratus femoris-gemellus are only partly separated (which may be incorrectly reported as the absence of some of them) in primitive frogs, whereas they are well differentiated in derived taxa.
However, there are also muscles that seem to have maintained their original function and whose identity probably remained unchanged in comparison to ancestral temnospondyls. The most conspicious of these are the glutaeus maximus, the iliofibularis and the iliofemoralis. All three retained their origins and insertions.
Among the epaxial trunk muscles there are two muscles (caudalifemoralis = pyriformis and caudalipuboischiotibialis) that persist, although only vestigial, and their locations and functions were modified. The caudalipuboischiotibialis was lost in the majority of contemporary anurans and the pyriformis is reduced or absent in the Pipidae and Pelobates. This loss may be related to the marked shortening of the urostyle and its fusion with the sacral vertebra in a firm unit that prevents independent movements of the urostyle relative to the sacral vertebra.
Thus, it may be summarized that, in transition from ancestral temnospondyls to anurans, the morphological transformations of the pelvis (elongation of the ilia that caused the shift of the acetabulum posteriorly along the tail, combined with transformation of the tail in the urostyle) were combined with only partial transformation of the associated muscular system. The most important modifications occurred in the epaxial trunk muscles of the pelvic region, which diversified in accordance with the new role of the tail (= urostyle). Flexors (= protractors) of the femur were modified to a lesser degree; parts of the origins of the iliacus and cruralis, represented by the iliacus externus and tensor fasciae latae, shifted onto the iliac shaft. Curiously, the least affected were extensors of the femur, important in producing the propulsive force during jumping and swimming. Even though the ancestral puboischiotibialis was split into the semimembranosus and gracilis, its original position and function did not change. Entirely unchanged were three muscles on the dorsal side of the femur (glutaeus maximus, iliofibularis and iliofemoralis).
In the course of anuran phylogeny, some muscles diversified into smaller units (Table 2) (iliolumbaris, coccygeosacralis, pubotibialis, puboischiofemoralis externus and deep muscles around acetabulum). However, the principal flexors (= protractors) and extensors of the femur remained unchanged, except for the split of the iliacus externus in two parts that vary in their origins on the iliac shaft, and some variation in the origin of the tensor fasciae latae, suggesting that these protractors are responsible for the various types of locomotion.
Conclusions
Comparisons between caudate amphibians, considered to reflect the primitive tetrapod body plan and type of locomotion, and anurans revealed that most of the anuran thigh muscles maintain their original pattern of origins and insertions. This holds especially true for the muscles powering jumping (e.g. semimembranosus and gracilis).
In contrast, the most derived from the original caudate pattern and, at the same time, the most variable are muscles originating on the outer surface of the iliac shaft (which is an anuran apomorphy). Generally, these muscles are responsible for protractions of the femur.
Similar are the presacral and caudal epaxial muscles that insert on the urostyle and on the inner surface of the iliac shaft. Although vestigial, they gained a significant role in preparatory phase of the jump (crouched position).
Judging from the similar positions of the origins of the iliofibularis, iliofemoralis and glutaeus maximus of the anurans, and of their homologues on the caudate ilium, the anuran tuber superius may correspond to the dorsal end of the caudate ilium. If this is true, the iliac shaft of the anurans would be a neomorph that provided a sufficient area for attachment of the protractors of the femur (its outer surface) and of epaxial muscles rotating the urostyle and pelvis (its inner surface). At the same time, the iliac shaft shifted the pelvis posteriorly along the stiffened tail.
As revealed from comparisons between caudate and anuran musculoskeletal patterns, the transition from the ancestral temnospondyls and anurans was associated with diversification of muscles and this trend also continued within the radiation of the anurans.
As walking in anurans is performed by muscles associated with the iliac shaft, it can be considered a derived mode of locomotion and not transitional between the original locomotion of ancestral temnospondyls and present-day frogs. The same holds true for swimming in pipids, which may be considered derived because of the fused sacral and the urostyle. Jumping may thus be considered a primary type of locomotion in the anurans.
Acknowledgments
We are indebted to Eddie Van Dijk and Juri van den Heever (University of Stellenbosch, South Africa) for providing us with the Barbourula busagensis specimen, and to Vicky Schaerlaeken (University of Antwerp) for her kind help with the stimulation experiments. The project was supported by funds from the bilateral scientific cooperation between Belgium and the Czech Republic, and by a grant (AVOZ301230516) to the Geological Institute of the Academy of Sciences, Prague.
References
- Anderson JS, Reisz RR, Scott D, Fröbisch NB, Sumida SS. A stem batrachian from the early Permian of Texas and the origin of frogs and salamanders. Nature. 2008;453:515–518. doi: 10.1038/nature06865. [DOI] [PubMed] [Google Scholar]
- Blanco MJ, Sanchiz B. Evolutionary mechanisms of rib loss in anurans: a comparative developmental approach. J Morphol. 2000;244:57–67. doi: 10.1002/(SICI)1097-4687(200004)244:1<57::AID-JMOR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Böker H. Einführung in Die Vergleichende Biologische Anatomie der Wirbeltiere. Vol. 1. Jena: Gustav Fischer; 1935. [Google Scholar]
- Boy JA. Die Larven der rhachitomen Amphibien (Amphibia: temnospondyli; Karbon-Trias) Paläont Z. 1974;48:236–268. [Google Scholar]
- Boy JA, Sues H-D. Branchiosaurs: larvae, metamorphosis and heterochrony in temnospondyls and seymouriamorphs. In: Heatwole H, Carroll R, editors. Amphibian Biology – Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1150–1197. [Google Scholar]
- Cannatella DC. A Phylogeny of Primitive Frogs (Archaeobatrachians) Lawrence, Kansas: University of Kansas; 1985. PhD Thesis. [Google Scholar]
- Davies M, Burton TC. Osteology and myology of the Gastric Brooding Frog Rheobatrachus silus Liem (Anura: Leptodactylidae) Austr J Zool. 1982;30:503–521. [Google Scholar]
- van Dijk DE. The ‘tail’ of Ascaphus: a historical résumé and new histological-anatomical details. Ann Univ Stellenbosch. 1955;31A:1–71. [Google Scholar]
- van Dijk DE. On the cloacal region of Anura in particular of larval Ascaphus. Ann Univ Stellenbosch. 1959;35A:169–249. [Google Scholar]
- van Dijk DE. Longitudinal sliding articulations in pipid frogs. S Afr J Sci. 2002;98:555–556. [Google Scholar]
- Dunlap DG. The comparative myology of the pelvic appendage in the Salientia. J Morphol. 1960;106:1–76. doi: 10.1002/jmor.1051060102. [DOI] [PubMed] [Google Scholar]
- Eaton TH. The ancestry of modern Amphibia: A review of evidence. Univ Kansas Publ, Mus Nat Hist. 1959;12:155–180. [Google Scholar]
- Edwards JL. The evolution of terrestrial locomotion. In. In: Hecht MK, Goody PC, Hecht BM, editors. Major Patterns in Vertebrate Evolution. New York: Plenum Press; 1977. pp. 553–577. [Google Scholar]
- Emerson SB. Allometry and jumping in frogs: helping the twain to meet. Evolution. 1978;32:551–564. doi: 10.1111/j.1558-5646.1978.tb04598.x. [DOI] [PubMed] [Google Scholar]
- Emerson SB. The ilio-sacral articulation in frogs: form and function. Biol J Linn Soc. 1979;11:153–168. [Google Scholar]
- Emerson SB. Frog postcranial morphology: identification of a functional complex. Copeia. 1982;1982:603–613. [Google Scholar]
- Emerson SB, de Jongh HJ. Muscle activity at the ilio-sacral articulation of frogs. J Morphol. 1980;166:129–144. doi: 10.1002/jmor.1051660202. [DOI] [PubMed] [Google Scholar]
- Evans SE, Milner AR, Musset F. A discoglossid frog from the Middle Jurassic of England. Palaeontology. 1990;33:299–311. [Google Scholar]
- Francis ETB. The Anatomy of the Salamander. Oxford: Clarendon Press; 1934. [Google Scholar]
- Frost DR, Grant T, Faivovich J, et al. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297:1–370. [Google Scholar]
- Gadow H. The Evolution of the Vertebral Column. Cambridge: Cambridge University Press; 1933. [Google Scholar]
- Gans C. A bullfrog and its prey. A look at the biomechanics of jumping. Nat Hist. 1961;52:26–37. [Google Scholar]
- Gans C, Parsons TS. On the origin of the jumping mechanism in frogs. Evolution. 1966;20:92–99. doi: 10.1111/j.1558-5646.1966.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Gaupp E. Anatomie des Frosches. I. Lehre vom Skelet und vom Muskelsystem. Braunschweig: Friedrich Vieweg und Sohn; 1896. [Google Scholar]
- Gilbert SG. Pictorial Anatomy of the Necturus. Seattle: University of Washington Press; 1973. [Google Scholar]
- Green TL. On the pelvis of the Anura: a study in adaptation and recapitulation. Proc Zool Soc Lond. 1931;1931:1259–1291. [Google Scholar]
- Griffiths I. The phylogeny of the Salientia. Biol Rev. 1963;38:241–292. doi: 10.1111/j.1469-185x.1963.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Grobbelaar CS. Beitrage zu einer Monographie von Xenopus laevis(Daud) Z Anat Entwicklungsgesch (Abt.1) 1924;72:131–168. [Google Scholar]
- Grobbelaar CS. The musculature of the pipid genus Xenopus. S Afr J Sci. 1935;32:395. [Google Scholar]
- Handrigan GR, Wassersug RJ. The anuran Bauplan: a review of the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol Rev. 2007;82:1–25. doi: 10.1111/j.1469-185X.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- Hecht MK. A reevaluation of the early history of the frogs. Part I. Syst Zool. 1962;11:39–44. [Google Scholar]
- Hodler F. Untersuchungen über die Entwicklung von Sacralwirbel und Urostyl bei den Anuren. Ein Beitrag zur Deutung des Anuren Amphibientypus. Rev Suisse Zool. 1949;56:747–790. [Google Scholar]
- Hoffmann CK. Amphibien. In: Bronn HG, editor. Klassen und Ordnungen des Thier-Reichs. Leipzig and Heidelberg: Winter; 1873. pp. 1–726. –1878. [Google Scholar]
- Inger RF. On the terrestrial origin of frogs. Copeia. 1962;1962:835–836. [Google Scholar]
- Iordansky NN, Morozov NP. Musculature. In: Kuzmin SL, Ishchenko VG, editors. Siberian Newt (Salamandrella keyserlingii Dybowski, 1870) Moscow: Nauka; 1994. pp. 159–182. [Google Scholar]
- Jenkins FA, Jr, Shubin NH. Prosalirus bitis and the anuran caudopelvic mechanism. J Vert Paleo. 1998;18:495–510. [Google Scholar]
- Limeses CE. La musculatura del muslo en los ceratofrinidos y formas afines. Contrib Científ, Fac Cienc Exact Natural, Univ Buenos Aires, Ser Zool. 1964;1:191–245. [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: University of Chicago Press; 1986. [Google Scholar]
- Lutz GH, Rome LC. Muscle function during jumping in frogs. I. Sarcomere length change, EMG pattern and jumping performance. Am J Physiol. 1996a;271:C563–C570. doi: 10.1152/ajpcell.1996.271.2.C563. [DOI] [PubMed] [Google Scholar]
- Lutz GH, Rome LC. Muscle function during jumping in frogs. II. Mechanical properties of muscle: implications for system design. Am J Physiol. 1996b;271:C571–C578. doi: 10.1152/ajpcell.1996.271.2.C571. [DOI] [PubMed] [Google Scholar]
- MacBride EW. Some recent work on the development of the vertebral column. Biol Rev. 1932;7:108–148. [Google Scholar]
- Manzano A, Abdala V, Herrel A. ) Morphology and function of the forelimb in arboreal frogs: specializations for grasping ability? J Anat. 2008;213:296–307. doi: 10.1111/j.1469-7580.2008.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano AS, Barg M. The iliosacral articulation in Pseudinae (Anura: Hylidae) Herpetologica. 2005;61:259–267. [Google Scholar]
- Millard N. The vascular anatomy of Xenopus laevis(Daudin) Trans Roy Soc S Afr. 1941;28:387–439. [Google Scholar]
- Mivart SG. Notes on the myology of Menopoma alleghaniense. Proc Zool Soc Lond. 1869;1869:254–261. [Google Scholar]
- Noble GK. The Phylogeny of the Salientia. Bull Am Mus Nat Hist. 1922;46:1–87. [Google Scholar]
- Noble GK. The Biology of the Amphibia. New York: McGraw-Hill Book Co; 1931. [Google Scholar]
- O’Reilly JC, Summers AP, Ritter DA. The evolution of the functional role of trunk muscles during locomotion in adult amphibians. Am Zool. 2000;40:123–135. [Google Scholar]
- Olson JM, Marsh RL. Activation patterns and length changes in hindlimb muscles of the bullfrog Rana catesbeiana during jumping. J Exp Biol. 1998;201:2763–2777. doi: 10.1242/jeb.201.19.2763. [DOI] [PubMed] [Google Scholar]
- Palmer M. Expanded ilio-sacral joint in the toad Xenopus laevis. Nature. 1960;187:797–798. doi: 10.1038/187797a0. [DOI] [PubMed] [Google Scholar]
- Peters SE, Kamel LT, Bashor DP. Hopping and swimming in the leopard frog, Rana pipiens. 1. Step cycles and kinematics. J Morphol. 1996;230:1–16. doi: 10.1002/(SICI)1097-4687(199610)230:1<1::AID-JMOR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Rage J-C, Roček Z. Redescription of Triadobatrachus massinoti(Piveteau 1936) an anuran amphibian from the Early Triassic. Palaeontographica Abt A. 1989;206:1–16. [Google Scholar]
- Rand AS. Jumping ability of certain anurans, with notes on endurance. Copeia. 1952;1952:15–20. [Google Scholar]
- Rand AS, Rand PJ. The relation of size and distance jumped in Bufo marinus. Herpetologica. 1966;22:206–209. [Google Scholar]
- Reig OA. Los anuros del Matildense. Acta Geol Lilloana. 1957;1:185–297. [Google Scholar]
- Richardson MK, Allen SP, Wright GM, Raynauld A, Hanken J. Somite number and vertebrate evolution. Development. 1998;125:151–160. doi: 10.1242/dev.125.2.151. [DOI] [PubMed] [Google Scholar]
- Ritland RM. Studies on the postcranial morphology of Ascaphus truei. II. Myology. J Morphol. 1955;97:215–282. [Google Scholar]
- Roček Z, Rage J-C. Anatomical transformations in transition from temnospondyl to proanuran stages. In: Heatwole H, Carroll R, editors. Amphibian Biology – Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1276–1284. [Google Scholar]
- Ročková H, Roček Z. Development of the pelvis and posterior part of the vertebral column in the Anura. J Anat. 2005;206:17–35. doi: 10.1111/j.0021-8782.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS. Vertebrate Paleontology. Chicago: University of Chicago Press; 1950. [Google Scholar]
- de Sá R, Hillis DA. Phylogenetic relationships of the pipid frogs Xenopus and Silurana: an integration of ribosomal DNA and morphology. Mol Biol Evol. 1990;7:365–376. doi: 10.1093/oxfordjournals.molbev.a040612. [DOI] [PubMed] [Google Scholar]
- Schmalhausen II. Die Entstehung der Amphibien im Verlauf der Erdgeschichte. Naturwiss Beitr. 1958;9:941–961. [Google Scholar]
- Stokely PS, Barberian JF. On the jumping ability of frogs. Copeia. 1953;1953:187. [Google Scholar]
- Videler JJ, Jorna JT. Functions of the sliding pelvis in Xenopus laevis. Copeia. 1985;1985:254–257. [Google Scholar]
- Walker WF. Vertebrate dissection. 4th Ed. Philadelphia: W.B. Saunders; 1970. [Google Scholar]
- Walthall JC, Ashley-Ross MA. Postcranial myology of the California Newt, Taricha torosa. Anat Rec, Part A. 2006;288A:46–57. doi: 10.1002/ar.a.20279. [DOI] [PubMed] [Google Scholar]
- Whiting HP. Pelvic girdle in amphibian locomotion. Symp Zool Soc Lond. 1961;5:43–57. [Google Scholar]
- Zug GR. Anuran locomotion: Structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia. 1972;1972:613–624. [Google Scholar]