Abstract
Posterior lingual glands consist of two sets of minor salivary glands that serve important functions in oral physiology. To investigate the hypothesis that the hypoglossal nerve provides sympathetic innervation to the posterior lingual glands, we examined ultrastructural changes in the glands following hypoglossal denervation. In the posterior deep lingual glands (of von Ebner), the serous acinar cells showed a decrease in the number of secretory granules and an increase in lipofuscin accumulation. The ratios of cells containing lipofuscin granules were 11.39, 36.49 and 50.46%, respectively, of the control, 3- and 7-day post-axotomy glands (P < 0.001). Intraepithelial phagocytotic activity was increased. The mucous acinar cells in the posterior superficial lingual glands (of Weber) also showed degenerative changes after hypoglossal denervation. One week after nerve transection, marked cytoplasmic vacuolation and fragmentation of organelles were frequently observed. Degenerative changes were also found in unmyelinated axons associated with the glands. We provide the first evidence of the structural and functional connections between the sympathetic component of the hypoglossal nerve and posterior lingual glands.
Keywords: hamster, hypoglossal nerve, posterior lingual glands, sympathetic, von Ebner's gland, Weber's glands
Introduction
The posterior portion of mammalian tongues include two sets of minor salivary glands: the posterior deep and superficial lingual glands. The posterior deep lingual glands, also known as von Ebner's glands, are a group of tubulo-acinar serous glands located beneath the circumvallate and foliate papillae of the tongue and their ducts open into the trough at the base of the papillae. The saliva from posterior deep lingual glands is known to modulate the microenvironment at taste reception sites, and affect chemoreception (Gurkan & Bradley, 1988) and it is an important source of growth factors for taste receptor cells (Morris-Wiman et al. 2000). Previous studies have demonstrated that posterior deep lingual glands produce lingual lipase, which hydrolyzes triacylglycerols in the acidic gastric lumen, the first step in the digestion of dietary fat (Hamosh & Scow, 1973; Hamosh & Burns, 1977). In view of their close proximity to the lingual lymphatic tissues, the glands were also thought to be involved in antimicrobial action and local immune response (Nair & Schroeder, 1983). The posterior superficial lingual glands (of Weber) are located on the lateral margin, at the level of the foliate papillae and in the root of the tongue behind the circumvallate papillae. In human beings, the ducts of posterior superficial lingual glands open into the crypts of lingual tonsils. Mucus supplied by these glands may help to cleanse the crypts and aid in deglutition (Tandler & Riva, 1986).
Although important physiological functions have been attributed to posterior lingual glands, the neural circuit that controls their secretion has not been well studied. Gurkan & Bradley (1987) found that secretion of posterior deep lingual glands is under both sympathetic and parasympathetic control. By using retrograde transport of horseradish peroxidase (HRP) in rats, Bradley et al. (1985) showed that preganglionic parasympathetic axons originating in the inferior salivatory nucleus travel via the glossopharyngeal nerve to the intralingual ganglia (Remak's ganglia) in the posterior tongue, from which postganglionic fibers innervate the posterior deep lingual glands. As for the source of sympathetic innervation, it is well established that the postganglionic neurons in the superior cervical ganglion (SCG) send sympathetic postganglionic fibers to the cranial regions via the arterial nerve plexuses along the branches of internal and external carotid arteries (Bowers & Zigmond, 1979; Marfurt et al. 1986). On the other hand, morphological and electrophysiological studies have provided evidence that some cranial nerves, including trigeminal (Matthews & Robinson, 1980), facial (Thomander et al. 1984; Matthews & Robinson, 1986), vagus (Ling et al. 1990) and hypoglossal (O’Reilly & Fitzgerald, 1990; Fukui et al. 1992), also contain a sympathetic component. However, the functional significance of the sympathetic fibers in cranial nerves remains obscure.
Our earlier studies on the hypoglossal nerve of hamsters found no parasympathetic component, but confirmed the presence of sympathetic fibers (Tseng et al. 2001, 2005). Interestingly, we observed that most of the sympathetic fibers in hypoglossal nerve originate from large multipolar neurons in SCG (Tseng et al. 2001), which have been found to innervate salivary glands rather than vascular beds (Lahtivirta et al. 1995; Gibbins et al. 1996). Moreover, our topographic analysis of the hypoglossal nerve demonstrated that most of the unmyelinated fibers are located close to the endoneurium and not in the proximity of blood vessels (Tseng et al. 2005). Finally, the sympathetic axons in the hypoglossal nerve travel only via its medial branch (Tseng et al. 2001), which is known to innervate genioglossus and intrinsic muscles of the tongue. Taken together, these findings suggest that the sympathetic fibers in hypoglossal nerve may innervate intralingual visceral targets. Therefore, we examined the changes in posterior lingual glands, the largest glandular complex in the tongue, after transection of the hypoglossal nerve in hamsters, to determine whether there is a functional connection between the hypoglossal nerve and posterior lingual glands.
Materials and methods
Animals and anesthesia
Ten adult hamsters (4–6 months) of both sexes weighing 120–150 g were used. The experimental protocol was approved by the Center of Laboratory Query answered. College of Medicine, National Taiwan University, and the animals were maintained following the Guide to Management and Use of Experimental Animals, National Science Council, Taiwan. Surgery was performed under anesthesia achieved with an intraperitoneal injection of 7% chloral hydrate (0.6 mL 100 g−1 body weight).
Transection of the hypoglossal nerve
With an aseptic surgical technique, the digastric muscle on the right side was removed and the trunk of the hypoglossal nerve was exposed. The hypoglossal nerve bifurcates into medial and lateral branches at this level. A segment of the exposed nerve, 1 mm in length, was severed and removed proximal to the bifurcation. A sham operation was performed on the contralateral side but the hypoglossal nerve was kept intact as control. After nerve transection, the incision was closed and the animals were allowed to survive for 3 or 7 days (five animals in each group).
Perfusion and fixation
All animals were starved for 12 h before sacrifice. Following deep anesthesia, the animals underwent intracardiac perfusion with Ringer's solution, followed by an aldehyde mixture containing 1.25% glutaraldehyde and 1% paraformaldehyde in 0.1 m phosphate buffer at pH 7.4. After perfusion, the posterior portion of the tongue including the circumvallate and foliate papillae was removed carefully. Tissue specimens were postfixed in the same fixative for another 2 h and then stored in 10% sucrose buffer overnight at 4 °C.
Light and electron microscopic study
The specimen was cut into 200-µm-thick sections on a vibratome. The sections were trimmed to 3 × 3 mm and postfixed with 1% osmic acid in 0.1 m phosphate buffer, pH 7.4, for 1 h, dehydrated in a graded alcohol series and embedded in Epon-Araldite mixture. Semithin sections 1-µm-thick were cut and stained with toluidine blue for light microscopic study. Ultrathin sections were collected on 100-mesh grids, double-stained with saturated uranyl acetate and 1% lead citrate, and examined in a JEOL 2000EX electron microscope.
To compare quantitative differences of lipofuscin between the control and denervated acinar cells, 11, 23 and 18 Epon blocks, respectively, from the control, post-axotomy 3- and 7-day specimens were randomly selected. In each block, an ultrathin section was randomly selected and studied in the electron microscope at a magnification of 5000×. Only acinar cells presenting nuclei in the section were counted. The number of cells with or without lipofuscin in the cytoplasm was recorded.
Statistical analysis
The morphometric data were studied using a one-way analysis of variance test. Differences between the control and post-axotomy specimens were evaluated. P values were two-sided and the significant level was 0.05.
Results
All animals survived the operation and recovered from anesthesia uneventfully. In the following days after the surgery, no change was found in the general appearance and activity of any animal. The amount of food intake and body weight were also similar to those of the unoperated animals.
Light microscopy of posterior lingual glands following hypoglossal denervation
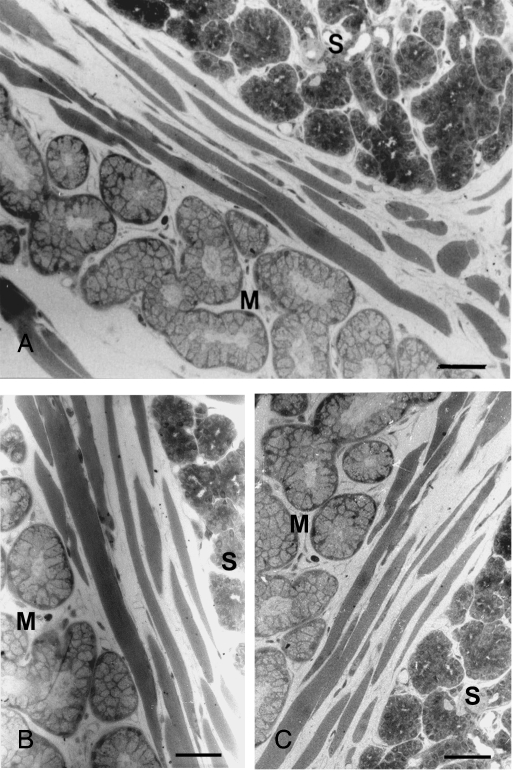
The posterior deep lingual glands were typically serous and located beneath the circumvallate papilla between the bundles of striated muscle. The posterior superficial lingual glands were tubulo-acinar glands consisting mostly of mucous secretory cells and intermingled with von Ebner's glands on their anterior aspect (Fig. 1A). The serous acinar cells were pyramidal in shape. Their cytoplasm was deeply stained with toluidine blue and their nuclei were round. In contrast, the mucous cells were more columnar and weakly stained. Their nuclei were flattened, and located at the bases of the cells. The mucous alveoli generally had larger and more apparent lumens. By light microscopy of both the posterior deep and superficial glands, no significant change was noted in either the overall structure or the morphology of individual acinar cells after 3 (Fig. 1B) or 7 days (Fig. 1C) of hypoglossal denervation.
Fig. 1.
Light microscopy showed no significant morphological changes in posterior deep and superficial lingual glands after hypoglossal denervation. (A) Normal serous and mucous glands beneath the circumvallate papilla between striated muscle fibers on the control side. No significant morphological alteration in the glands 3 (B) or 7 days (C) after hypoglossal denervation. S, serous glands; M, mucous glands. Toluidine blue. Bars = 150 µm.
Ultrastructure of normal acini in posterior deep lingual glands
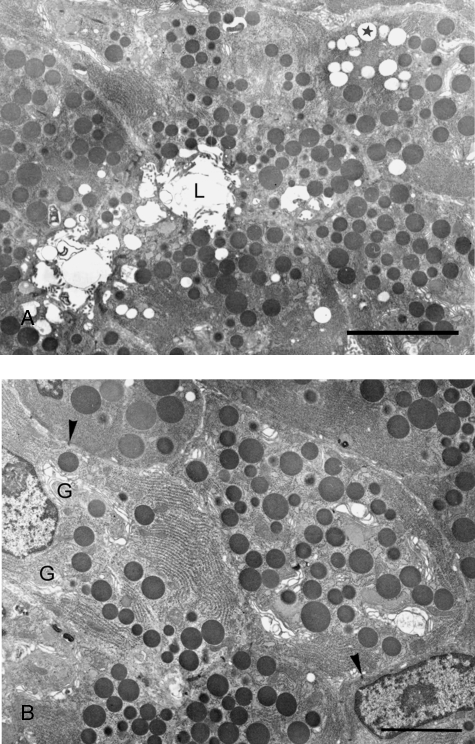
By electron microscopy, the acinar cells of von Ebner's glands were pyramidal in shape with parallel lateral borders exhibiting thin interdigitating folds which lined narrow intercellular spaces. Intercellular canaliculi separated from the intercellular spaces by junctional complexes were sometimes found. The nuclei were round, and cisternae of rough endoplasmic reticulum, which were present throughout the cytoplasm, were organized in highly ordered parallel arrays (Fig. 2A,B). The perinuclear regions contained a series of well-developed Golgi complexes (Fig. 2B) and small mitochondria were scattered throughout the cells. Electron-opaque secretory granules were the most distinctive feature of the acinar cells (Fig. 2A,B). Occasionally the spherical granules occupied most of the cytoplasm, but in other cases they were present only in the apical area. They were homogeneous, membrane-bound, and 0.5–1 µm in diameter. Electron-lucent droplets of various sizes, suggestive of lipid, were sometimes encountered (Fig. 2A). Myoepithelial cells were seen within the basement membrane surrounding the acinar cells. They contained myofilaments and their general structure was similar to that described previously in other salivary glands.
Fig. 2.
Ultrastructures of normal acinar cells in posterior deep lingual glands. (A) Normal acinar cells filled with electron-dense secretory granules. Lipid droplets (asterisk) are occasionally found. Intercellular canaliculi and acinar lumen (L) are evident. Bar = 10 µm. (B) Higher magnification of normal acinar cells showing parallel arrays of endoplasmic reticulum and well-developed Golgi complexes (G) in perinuclear regions. Arrowheads: macula adherens. Bar = 3.4 µm.
Ultrastructural changes of posterior deep lingual glands following hypoglossal denervation
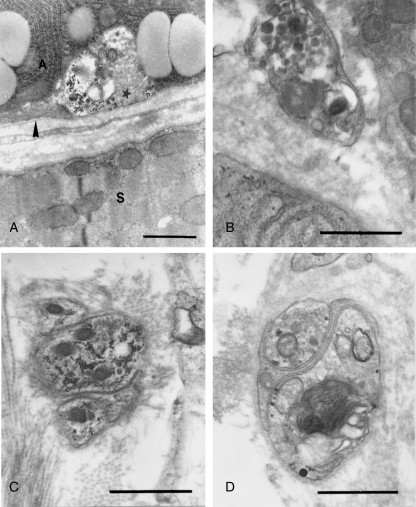
As compared to controls, the myelinated axons among the muscle fibers of the denervated side of the tongue showed cytoplasmic vacuolation and myelin disorganization, which confirmed successful denervation. Three days after axotomy, fewer secretory granules were found in acinar cells and lipid droplets increased dramatically, occupying a large portion of the cytoplasm (Fig. 3A). Lipofuscin granules scattered in the cytoplasm were frequently noted. Seven days following hypoglossal denervation, accumulation of large bodies containing dense materials and numerous vesicles of variable size (Fig. 3B) and lipofuscin granules (Fig. 3C) were the most striking ultrastructural features of the acinar cells. The proportions of cells containing lipofuscin granules were 11.39%, 36.49% and 50.46%, respectively, in the control, 3- and 7-day post-axotomy groups (Table 1). The increase of lipofuscin in acinar cells after hypoglossal denervation was statistically significant (P < 0.001).
Fig. 3.
Ultrastructural changes of posterior deep lingual glands after hypoglossal denervation. (A) Three days after hypoglossal denervation, lipid droplets (asterisk) increased dramatically at the expense of secretory granules. Bar = 3.4 µm. (B) Seven days following axotomy, large bodies containing dense materials and numerous vesicles (arrowheads) are prominent. Bar = 4.0 µm. (C) Lipofuscin granules (arrowhead) are frequently found. Also note chromatin condensation and nuclear vacuolation (arrows). Bar = 2.5 µm. (D) The intercellular spaces (IS) around some acinar cells are dramatically enlarged. A: acinar cell; C: intercellular canaliculi; M: myoepithelial cell. Bar = 2.5 µm. (E) Numerous lipofuscin granules (arrowhead) are scattered in myoepithelial cells. M: myoepithelial cell nucleus. Bar = 2.0 µm. (F) Active phagocytes invading the acini. Note the lipofuscin granules (arrow) and large bodies containing dense materials and numerous vesicles (arrowhead) in the cell. A: acinar cell; N: nucleus of phagocyte. Bar = 6.5 µm.
Table 1.
Percentages of von Ebner's acinar cells containing lipofuscin granules before and after hypoglossal denervation
| Control | 3 day post-axotomy | 7 days post-axotomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Block number | L | N | % | L | N | % | L | N | % |
| 1 | 5 | 51 | 9.80 | 85 | 210 | 40.48 | 47 | 86 | 54.65 |
| 2 | 19 | 97 | 19.59 | 6 | 27 | 22.22 | 70 | 141 | 49.65 |
| 3 | 9 | 86 | 10.47 | 52 | 159 | 32.70 | 47 | 74 | 63.51 |
| 4 | 21 | 154 | 13.64 | 40 | 153 | 26.14 | 53 | 108 | 49.07 |
| 5 | 11 | 90 | 12.22 | 43 | 101 | 42.57 | 40 | 81 | 49.38 |
| 6 | 8 | 115 | 6.96 | 96 | 226 | 42.48 | 89 | 173 | 51.45 |
| 7 | 9 | 62 | 14.52 | 31 | 116 | 26.72 | 51 | 115 | 44.35 |
| 8 | 11 | 104 | 10.58 | 62 | 219 | 28.31 | 4 | 12 | 33.33 |
| 9 | 7 | 95 | 7.37 | 55 | 159 | 34.59 | 73 | 142 | 51.41 |
| 10 | 4 | 31 | 12.90 | 54 | 174 | 31.03 | 73 | 124 | 58.87 |
| 11 | 7 | 96 | 7.29 | 35 | 108 | 32.41 | 79 | 182 | 43.41 |
| 12 | 47 | 118 | 39.83 | 67 | 143 | 46.85 | |||
| 13 | 16 | 66 | 24.24 | 94 | 181 | 51.93 | |||
| 14 | 50 | 94 | 53.19 | 86 | 153 | 56.21 | |||
| 15 | 57 | 118 | 48.31 | 115 | 218 | 52.75 | |||
| 16 | 50 | 164 | 30.49 | 149 | 266 | 56.02 | |||
| 17 | 64 | 159 | 40.25 | 104 | 221 | 47.06 | |||
| 18 | 43 | 92 | 46.74 | 61 | 126 | 48.41 | |||
| 19 | 13 | 42 | 30.95 | ||||||
| 20 | 78 | 174 | 44.83 | ||||||
| 21 | 15 | 33 | 45.46 | ||||||
| 22 | 75 | 174 | 43.10 | ||||||
| 23 | 29 | 90 | 32.22 | ||||||
| Average percentage (mean ± SD) | 11.39 ± 3.76 | 36.49 ± 8.54a | 50.46 ± 6.62a,b | ||||||
L, number of cells containing lipofuscin granules; N, total number of cells counted.
P < 0.001 vs. control.
P < 0.001 vs. 3 days post-axotomy.
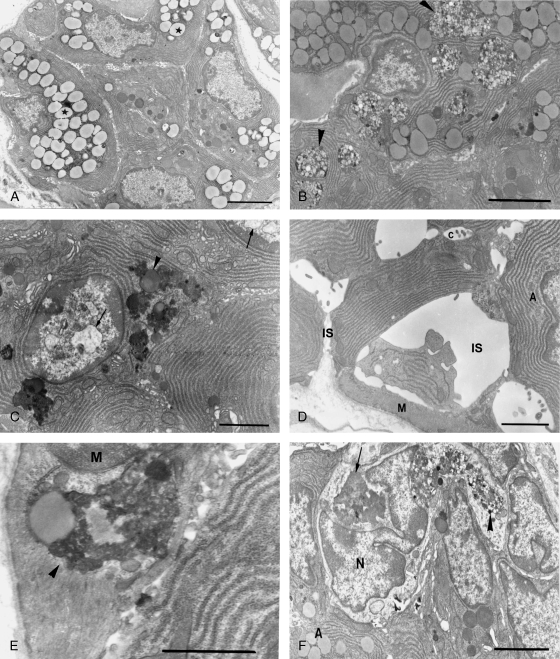
Besides the accumulation of lipofuscin, hypoglossal denervation also resulted in vacuolation of cytoplasmic organelles and dilation of intercellular spaces. Seven days after nerve transection, dramatic enlargement of the intercellular spaces was noted around some acinar cells (Fig. 3D). Chromatin condensation and nuclear vacuolation not found in control cells were occasionally observed after axotomy (Fig. 3C).
Significant ultrastructural changes were also noted in myoepithelial cells, numerous lamellar bodies, multivesicular bodies and lipofuscin granules were scattered in the cytoplasm (Fig. 3E). Some acini were invaded by phagocytic cells with their pseudopodia protruding between the degenerating secretory cells. The phagocytes were also filled with large bodies containing numerous vesicles and dense materials and lipofuscin granules (Fig. 3F).
Ultrastructure of normal acini in posterior superficial lingual glands
The nuclei of normal mucous acinar cells were flattened and located near the bases of the cells, and the cytoplasm was occupied mostly by the mucigen droplets. The remaining cytoplasm was restricted to thin basal and lateral zones containing RER, Golgi complexes and mitochondria. Regular intercellular spaces with narrow interdigitating cytoplasmic processes were noted at the basolateral borders of the cells (Fig. 4A).
Fig. 4.
Normal ultrastructure and changes following hypoglossal denervation in posterior superficial lingual glands. (A) Normal mucous acinar cells. Note the flattened nuclei and prominent mucigen droplets. S: intercellular spaces. Bar = 4.0 µm. (B) Degenerating mucous acinar cells three days after denervation. Note the enlarged intercellular spaces (double arrowheads), dilated cisternae of endoplasmic reticulum (arrowhead), edematous mitochondria (arrows) and autophagic bodies (asterisk) containing secretory granules and cytoplasmic debris. Bar = 3.4 µm. (C and D) Degenerated mucous acinar cells 1 week after denervation. Note the obvious cytoplasmic vacuolation and fragmentation of organelles. Bars = 3.4 µm.
Ultrastructural changes of posterior superficial lingual glands following hypoglossal denervation
Degenerative changes in the mucous posterior lingual glands were also observed after hypoglossal denervation. Three days after axotomy, the mucous acinar cells showed disorganized basolateral interdigitations and enlarged intercellular spaces. Dilated cisternae of endoplasmic reticulum, edematous mitochondria and autophagic bodies were frequently found (Fig. 4B). One week after nerve transection, marked cytoplasmic vacuolation and fragmentation of organelles were observed. In some acinar cells most of the cytoplasm was occupied by irregular vacuoles and amorphous debris (Fig. 4C,D).
Ultrastructural changes of axonal terminals following hypoglossal denervation
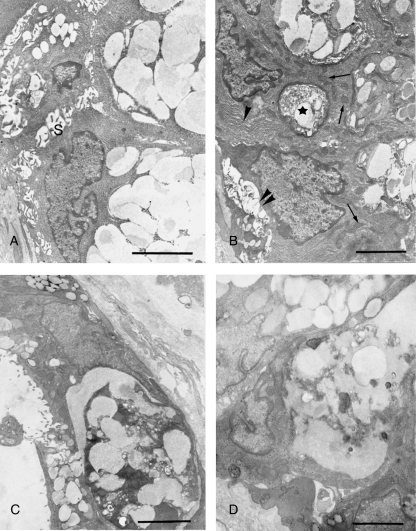
Most of the unmyelinated axon terminals associated with posterior lingual glands were extraparenchymal, situated in the connective tissue spaces outside the basement membrane. Intraparenchymal terminals, enclosed in depressed areas of the secretory and myoepithelial cells, were found less frequently. Three days after hypoglossal denervation, ultrastructural changes in axonal terminals of both types were obvious (Fig. 5). An increase in electron density was noted in the cytoplasm and the mitochondria were dilated and vacuolated. Accumulation of glycogen granules and lamellar bodies among the mixed population of clear and dense cored vesicles was observed (Fig. 5A–D). Similar but more severe degenerative changes were observed in the axonal terminals 1 week after axotomy.
Fig. 5.
Ultrastructural changes of axonal terminals associated with posterior lingual glands following hypoglossal denervation. (A) Three days after axotomy, a degenerating intraparenchymal terminal (asterisk) shows mitochondrial disruption and accumulation of glycogen granules. A: acinar cell; S: skeletal muscle cell; Arrowhead: basement membrane. Bar = 2.0 µm. (B–D) Degenerating extraparenchymal terminals observed 3 days after denervation, showing swollen mitochondria and vesicles (B), accumulation of glycogen granules (C) and lamellar body (D). Bars = 2.0 µm.
Discussion
The posterior deep lingual glands are unique salivary glands situated within the tongue that drain directly into the trench of circumvallate and foliate papillae. Secretion from posterior deep lingual glands appears to be regulated differently than the major salivary glands (Field et al. 1987) but the neural circuit underlying their control is not clear. Our previous experiments in hamsters (Tseng et al. 2001, 2005) have shown that hypoglossal nerve contains postganglionic sympathetic fibers from SCG. In the present study, we provide the first evidence of a functional connection between the sympathetic component of hypoglossal nerve and posterior deep lingual glands.
The normal ultrastructure of posterior deep lingual glands in hamster is similar to that described for rat (Hand, 1970) and human (Testa Riva et al. 1985). Following transection of hypoglossal nerve, obvious ultrastructural changes were observed in acinar cells of posterior deep lingual glands, which included a decrease in the number of secretory granules, and an increase of lipid droplets and accumulation of lipofuscin. The reason for the decrease of secretory granules is unknown, but one possibility is autophagic destruction of the granules, which may give rise to the large vesicle-containing bodies and possibly the lipofuscin granules. Another possibility is that the sympathetic fibers provide a trophic influence on the cells; after denervation, the synthesis of secretory protein declines. On the other hand, lipid droplets may accumulate due to increased uptake of lipid for use as an energy source, or by decreased use for the synthesis of secretory granules and other plasma-membrane materials.
The most striking feature in the posterior deep lingual glands after hypoglossal denervation was a rapid accumulation of lipofuscin granules. It is interesting to note that an increase of lipofuscin granules was previously observed in salivary acinar cells from aged rats (Kim, 1984) and mice (Meisel et al. 1988) and also from animals intoxicated with ethionine (Oliver et al. 1979) and isoproterenol (Boshell, 1981). Lipofuscin represents an intralysosomal polymeric material that cannot be degraded or exocytosed. Its continuous accumulation over time within postmitotic cells, such as neurons and cardiac myocytes, has been recognized as a sign of aging, and it was suggested that amassed lipofuscin may be hazardous to cellular functions (Brunk & Terman, 2002). Recently, Powell et al. (2005) have shown that in rat cardiomyocytes, lipofuscin accumulation results in inhibition of the proteasome, which initiates an apoptotic cascade as a result of dysregulation of several proapoptotic proteins. It is not known whether the accumulation of lipofuscin in von Ebner's acinar cells will trigger apoptosis. However, raised phagocytotic activity in von Ebner's glands in the absence of inflammatory reaction is compatible with increased macrophage recognition of apoptotic cells (Duvall et al. 1985). The fate of the lipofuscin-laden acinar cells and the mechanism underlying the rapid accumulation of lipofuscin after hypoglossal denervation deserves further study.
In addition to ultrastructural alterations of von Ebner's acinar cells, cytoplasmic vacuolation and fragmentation of organelles were observed in the adjacent posterior superficial lingual glands after hypoglossal denervation. Using light microscopy, Snell & Garrett (1958) found that sympathectomy in rats caused an increase in cytoplasmic vacuolation in the mucous acinar cells of submandibular gland. Alterations were observed in nerve terminals in and around the posterior lingual glands, which may indicate that they are terminals from the hypoglossal nerve. It is noteworthy that the unmyelinated extraparenchymal and intraparenchymal nerve fibers of the glands contain a mixed population of clear and dense cored vesicles, which implies that they have a dual function, both adrenergic and cholinergic (Potter et al. 1980). Taken together, our findings clearly indicate that, in the hamster, glandular structures in the posterior portion of tongue are visceral targets of the sympathetic fibers in hypoglossal nerve.
In conclusion, hypoglossal denervation caused degenerative changes of posterior lingual glands in hamster which are thought to be the result of transection of sympathetic fibers carried in the nerve. This is the first demonstration of a structural and functional connection between hypoglossal nerve and posterior lingual glands. Besides being the motor supply to the extrinsic and intrinsic muscles of the tongue, the significance of hypoglossal nerve in other aspects of oral physiology should not be ignored.
References
- Boshell JL. Effects of isoproterenol on the ultrastructure of pig parotid gland. Acta Anat (Basel) 1981;109:270–274. doi: 10.1159/000145392. [DOI] [PubMed] [Google Scholar]
- Bowers CW, Zigmond RE. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol. 1979;185:381–392. doi: 10.1002/cne.901850211. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Mistretta CM, Bates CA, Killackey HP. Transganglionic transport of HRP from the circumvallate papilla of the rat. Brain Res. 1985;361:154–161. doi: 10.1016/0006-8993(85)91285-5. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death. Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Field RB, Dromy R, Hand AR. Regulation of secretion of enzymes from von Ebner's gland of rat tongue. J Dent Res. 1987;66:586–587. doi: 10.1177/00220345870660023501. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Hayakawa T, Itoh M, Fujimoto Y, Nishimura Y, Takeuchi Y. The superior cervical ganglion: origin of sympathetic fibers in the facial and hypoglossal nerves in the cat. Brain Res Bull. 1992;28:811–815. doi: 10.1016/0361-9230(92)90265-y. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Mattew SE, Bridgman N, Morris JL. Sympathetic vasoconstrictor neurons projecting from the guinea-pig superior cervical ganglion to cutaneous or skeletal muscle vascular beds can be distinguished by soma size. Neurosci Lett. 1996;213:197–200. doi: 10.1016/0304-3940(96)12885-8. [DOI] [PubMed] [Google Scholar]
- Gurkan S, Bradley RM. Autonomic control of von Ebner's lingual salivary glands and implications for taste sensation. Brain Res. 1987;419:287–293. doi: 10.1016/0006-8993(87)90595-6. [DOI] [PubMed] [Google Scholar]
- Gurkan S, Bradley RM. Secretions of von Ebner's glands influence responses from taste buds in rat circumvallate papilla. Chem Senses. 1988;13:655–661. [Google Scholar]
- Hamosh M, Burns WA. Lipolytic activity of human lingual glands (Ebner) Lab Invest. 1977;37:603–608. [PubMed] [Google Scholar]
- Hamosh M, Scow RO. Lingual lipase and its role in the digestion of dietary lipid. J Clin Invest. 1973;52:88–95. doi: 10.1172/JCI107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand AR. The fine structure of von Ebner's gland of the rat. J Cell Biol. 1970;44:340–353. doi: 10.1083/jcb.44.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK. Changes in the secretory acinar cells of the rat parotid gland during aging. Anat Rec. 1984;209:345–354. doi: 10.1002/ar.1092090313. [DOI] [PubMed] [Google Scholar]
- Lahtivirta S, Koistinaho J, Hervonen A. A subpopulation of large neurons of the sympathetic superior cervical ganglion innervates the NGF-rich submandibular salivary gland in young adult and aged mice. J Auton Nerv Syst. 1995;50:283–289. doi: 10.1016/0165-1838(94)00099-6. [DOI] [PubMed] [Google Scholar]
- Ling EA, Shieh JY, Wen CY, Chan YG, Wong WC. Degenerative changes of neurons in the superior cervical ganglion following an injection of Ricinus communis agglutinin-60 into the vagus nerve in hamsters. J Neurocytol. 1990;19:1–9. doi: 10.1007/BF01188435. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Zaleski EM, Adams CE, Welther CL. Sympathetic nerve fibers in rat orofacial and cerebral tissues as revealed by the HRP-WGA tracing technique: a light and electron microscopic study. Brain Res. 1986;366:373–378. doi: 10.1016/0006-8993(86)91322-3. [DOI] [PubMed] [Google Scholar]
- Matthews B, Robinson PP. The course of post-ganglionic sympathetic fibers distributed with the trigeminal nerve in the cat. J Physiol. 1980;303:391–401. doi: 10.1113/jphysiol.1980.sp013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B, Robinson PP. The course of post-ganglionic sympathetic fibers distributed with the facial nerve in the cat. Brain Res. 1986;382:55–60. doi: 10.1016/0006-8993(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Meisel DL, Skobe Z, Prostak KS, Shklar G. A light and electron microscope study of aging parotid and submandibular salivary glands of Swiss-Webster mice. Exp Gerontol. 1988;23:197–210. doi: 10.1016/0531-5565(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Morris-Wiman J, Sego R, Brinkley L, Dolce C. The effects of sialoadenectomy and exogenous EGF on taste bud morphology and maintenance. Chem Senses. 2000;25:9–19. doi: 10.1093/chemse/25.1.9. [DOI] [PubMed] [Google Scholar]
- Nair Pnr, Schroeder HE. Retrograde access of antigens to the minor salivary glands in the monkey. Arch Oral Biol. 1983;28:145–152. doi: 10.1016/0003-9969(83)90121-8. [DOI] [PubMed] [Google Scholar]
- O’Reilly PMR, Fitzgerald MJT. Fiber composition of the hypoglossal nerve in the rat. J Anat. 1990;172:227–243. [PMC free article] [PubMed] [Google Scholar]
- Oliver C, Auth RE, Hand AR. Formation and fate of ethionine-induced cytoplasmic crystalloids in rat parotid acinar cells. Am J Anat. 1979;155:185–199. doi: 10.1002/aja.1001550204. [DOI] [PubMed] [Google Scholar]
- Potter DD, Landis SC, Furshpan EJ. Dual function during development of rat sympathetic neurons in culture. J Exp Biol. 1980;89:57–71. doi: 10.1242/jeb.89.1.57. [DOI] [PubMed] [Google Scholar]
- Powell SR, Wang P, Divald A, et al. Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic Biol Med. 2005;38:1093–1101. doi: 10.1016/j.freeradbiomed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Snell RS, Garrett JR. The effect of post-ganglionic sympathectomy on the structure of the submandibular and major sublingual salivary glands of the rat. Z Zellforsch Mikrosk Anat. 1958;48:639–652. doi: 10.1007/BF00398652. [DOI] [PubMed] [Google Scholar]
- Tandler B, Riva A. Salivary glands. In: Mjör IA, Fejerskov O, editors. Human Oral Embryology and Histology. Copenhagen: Munksgaard; 1986. pp. 243–284. [Google Scholar]
- Testa Riva F, Cossu M, Lantini MS, Riva A. Fine structure of human deep posterior lingual glands. J Anat. 1985;142:103–115. [PMC free article] [PubMed] [Google Scholar]
- Thomander L, Aldskogius H, Arvidsson J. Evidence for a sympathetic component in motor branches of the facial nerve: a horseradish peroxidase study in the cat. Brain Res. 1984;301:380–383. doi: 10.1016/0006-8993(84)91108-9. [DOI] [PubMed] [Google Scholar]
- Tseng CY, Lue JH, Lee SH, Wen CY, Shieh JY. Evidence of neuroanatomical connection between the superior cervical ganglion and hypoglossal nerve in the hamster as revealed by tract-tracing and degeneration methods. J Anat. 2001;198:407–421. doi: 10.1046/j.1469-7580.2001.19840407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CY, Wei IH, Chang HM, Lue JH, Wen CY, Shieh JY. Ultrastructural identification of a sympathetic component in the hypoglossal nerve of hamsters using experimental degeneration and horseradish peroxidase methods. Cells Tissues Organs. 2005;180:117–125. doi: 10.1159/000086752. [DOI] [PubMed] [Google Scholar]