Abstract
The mammalian adipose organ is composed of subcutaneous and visceral depots containing white and brown adipocytes. Cold acclimatisation induces an increase in the brown component without affecting the overall number of adipocytes; this form of plasticity is associated to obesity and diabetes resistance in experimental models. Cold activates the drive of the sympathetic nervous system to the adipose organ, where the vast majority of nerve fibers are in fact noradrenergic. However, it is unclear whether and how such fibers are involved in the plastic changes of the adipose organ. We thus conducted a systematic study of the distribution and number of sympathetic noradrenergic nerve fibers in the adipose organ of mice kept at different environmental temperatures. Adult Sv129 female mice were kept at 28 °C or 6 °C for 10 days. The density of tyrosine hydroxylase (noradrenergic)-positive nerve fibers (no. of fibers per 100 adipocytes) was calculated in the subcutaneous and visceral depots of the adipose organ, and a correlation was sought between fiber density and proportion of brown adipocytes. Tyrosine hydroxylase-positive parenchymal fibers were detected in all subcutaneous and visceral depots among white as well as brown adipocytes, the mediastinal depot displaying the densest innervation. Cold acclimatisation induced a threefold increase in the total number of TH fibers in the whole organ. The proportion of brown adipocytes positively correlated with noradrenergic fiber density in the organ. Taken together, these data suggest that cold acclimatisation induces noradrenergic fiber branching in the adipose organ of adult mice, and that such changes may be a precondition for its plastic transformation into a brown phenotype.
Keywords: brown adipocyte, obesity, sympathetic nervous system, tyrosine hydroxylase, UCP1, white adipocyte
Introduction
Mammals have two distinct types of adipocytes, white and brown, with different morphologies and functions. White adipocytes are unilocular cells that allow fatty acid accumulation after meals and distribute them to the body as a fuel source between meals. In addition, the white adipocyte mass controls food intake and energy expenditure behaviors through secretion of hormones such as leptin (Zhang et al. 1994). Brown adipocytes are multilocular cells rich in characteristic, large mitochondria containing uncoupling protein 1 (UCP1), which is uniquely expressed in this cell type and is responsible for uncoupling oxidative phosphorylation from electron transport. White and brown adipocytes are traditionally held to be organized into white and brown adipose tissue (WAT and BAT), respectively. As a consequence, mammalian WAT and BAT are described as separate entities, with different functions, and occupying discrete anatomical sites. In contrast, we showed in previous papers that both white and brown adipocytes are found mixed in all subcutaneous and visceral adipose depots; this led to the suggestion that the fat depots should in fact be viewed as the parenchyma of an actual multidepot organ, for which we proposed the name of adipose organ (Cinti, 2000, 2001a,b, 2002, 2005; Murano et al. 2005). In addition, contrary to the widely accepted definition of brown adipocytes as cells filled with UCP1-expressing mitochondria storing lipids in a multilocular fashion (Cannon et al. 1982; Ricquier et al. 1983), we described a large population of UCP1-negative multilocular adipocytes exhibiting the typical organelles of brown adipocytes on transmission electron microscopy that could represent adipocytes transdifferentiating to brown adipocytes (Cinti, 2000, 2001a,b, 2002, 2005; Murano et al. 2005). In this work, multilocular adipocytes will therefore be considered brown adipocytes, and their UCP1 immunoreactivity specified as required.
A remarkable feature of the adipose organ is its plasticity, i.e. its ability to undergo changes in cell composition and nerve and vascular supply to meet different metabolic requirements. Thus, the number of white and brown adipocytes in the adipose organ is not fixed, but varies as a function of physiological and pathological conditions. One of the best known of such conditions is cold acclimatisation, in which the phenotype of the organ turns from predominantly white to predominantly brown (Young et al. 1984; Cousin et al. 1992; Guerra et al. 1998). The transformation is of interest because most experimental conditions inducing the reverse situation (conversion of the brown phenotype into a white phenotype) are accompanied by obesity (Lowell et al. 1993; Bachman et al. 2002). Furthermore, treatment of obese animals with beta3 adrenoceptor agonists increases the number of brown adipocytes in the adipose organ and reduces insulin resistance and obesity (Champigny et al. 1991; Collins et al. 1997; Ghorbani & Himms-Hagen, 1997; Ghorbani et al. 1997).
Cold exposure activates the drive of the sympathetic nervous system (Cannon et al. 1986; Youngstrom & Bartness, 1995; Cinti, 1999), although it is still unclear whether and how local sympathetic noradrenergic fibers are involved in the plastic adjustments of the adipose organ.
Here we show for the first time that all adipose organ depots are provided with vascular and parenchymal sympathetic noradrenergic nerves, and that parenchymal noradrenergic nerves are found among both white and brown adipocytes. Notably, cold acclimatisation increases parenchymal noradrenergic nerve density, particularly in depots that at 28 °C are predominantly white. We also demonstrate a strong correlation between proportion of brown adipocytes and density of noradrenergic parenchymal nerves in mice kept at 28 °C and at 6 °C.
Taken together, these data suggest that innervation by the sympathetic nervous system is a common feature of the fat depots forming the mouse adipose organ, and that its plasticity may be a consequence of the plasticity of the peripheral sympathetic nervous system (i.e. its ability to induce an increase in noradrenergic fiber density in the parenchyma of the adipose organ).
Materials and methods
Animals
The mice studied in this work are the same as those used in a previous study (Murano et al. 2005). Two groups each of five 12-week-old Sv129 female mice (Charles River, Milan, Italy) were kept at 6 °C (cold-acclimated) or at 28 °C (warm-acclimated) for 10 days; the control temperature (28 °C rather than the usual 20–25 °C) was selected to minimize adrenergic stimulation of the adipose organ. The thermoneutral temperature for mice is around 30 °C (Trayhurn & James, 1978); 28 °C is thus close to thermoneutrality. Animals were housed singly with a 12-h light/dark cycle and free access to standard laboratory chow diet (2018 Teklad Global, containing a minimum of 18% protein and 5% fat; Harlan, Udine, Italy) and water. Care and handling were in accordance with institutional and national guidelines. Mice were sacrificed with an overdose of anesthetics, ketamine (Ketavet 100, Intervet, Milan, Italy; 100 mg kg−1, i.p.) in combination with xylazine (Rompum, Bayer, Milan, Italy; 10 mg kg−1, i.p.), and immediately perfused transcardially with 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4, for 5 min.
Immunohistochemistry
After perfusion, the main depots of the adipose organ (anterior and posterior subcutaneous, mediastinal, mesenteric, interrenal, parametrial and perivesical), described in detail elsewhere (Murano et al. 2005), were dissected under a Zeiss OPI1 surgical microscope (Carl Zeiss, Oberkochen, Germany). Each depot was fixed in 4% paraformaldehyde overnight at 4 °C and paraffin-embedded so that the widest surface area was exposed on the plane of section. The two largest, most representative consecutive sections of each depot were used for the immunohistochemical localization of UCP1 and tyrosine hydroxylase (TH) proteins. TH, the catecholamine-synthesizing enzyme, whose expression is related to the rate of noradrenaline synthesis and content in sympathetic nerves (Flatmark, 2000), is used as a marker of peripheral nervous system sympathetic noradrenergic nerves. Paraffin sections were processed according to the avidin-biotin-peroxidase (ABC) method (Hsu et al. 1981) with the following incubation steps: 0.3% H2O2in methanol, 30 min at room temperature (r.t.) to block endogenous peroxidase; two 15-min washes in 0.015 m phosphate-buffered saline (PBS), pH 7.4. Normal rabbit serum (UCP1) and normal goat serum (TH protein; both from Vector Laboratories, Burlington, CA, USA) were diluted 1 : 75 in PBS and incubated for 20 min at r.t. to block aspecific sites. A sheep anti-rat UCP1 antibody (kindly provided by D. Ricquier, Paris, France) was used at a final dilution of 1 : 4000 and a rabbit anti-TH polyclonal antibody (Chemicon; Temecula, CA, USA) at 1 : 200 in PBS. Sections were incubated overnight at high relative humidity at 4 °C, washed in PBS (2 × 15 min) and incubated with IgG biotinylated rabbit anti-sheep secondary antibody for UCP1 immunolocalization and with goat anti-rabbit secondary antibody (both from Vector Laboratories) for TH, both diluted 1 : 200 in PBS, for 30 min at r.t. They were then washed in PBS (2 × 15 min); incubated in ABC reagent (Vector Laboratories) for 60 min at r.t.; washed in PBS (2 × 15 min); incubated in 0.02% H2O2and 0.075% diaminobenzidine (Sigma, St. Louis, MO, USA) in 0.05 m Tris buffer, pH 7.6, for 3 min in a dark room; rinsed in tap water; counterstained with hematoxylin and finally mounted in Eukitt (Kindler GmbH & Co., Freiburg, Germany). The ability of the antibodies to specifically detect the antigens was evaluated in sections of tissues known to contain the antigens, such as interscapular BAT (IBAT), from mice kept at 6 °C for 10 days (De Matteis et al. 1998; Cinti et al. 2002). Negative controls were obtained in each instance by omitting the primary antibody and using pre-immune instead of primary antiserum.
Morphometric analysis
TH-positive parenchymal nerve fibers (i.e. fibers closely associated to adipocytes in the depots) were counted; perivascular fibers (those in contact with arterioles and venules) were not considered in the quantitative analysis.
Twenty randomly selected multilocular or prevalently multilocular and 20 unilocular or prevalently unilocular areas at high magnification per depot were studied using a Nikon Eclipse E800 light microscope (Laboratory Imaging, Prague, Czech Republic) with a ×100 oil immersion objective at ×1250 final magnification. The density of TH-immunoreactive (ir) fibers was calculated as the number of fibers per 100 adipocytes. The total number of fibers per depot was calculated based on the results of previous work (Murano et al. 2005), where we obtained the total number of adipocytes per depot in mice of the same strain, sex and age as the present animals.
Statistical analysis
Results are given as mean ± SE. Significance was analysed using an unpaired t-test (InStat, GraphPad, San Diego, CA, USA). Differences between groups were considered significant when P ≤ 0.05. Linear correlations were calculated by a nonparametric test (Spearman) using GraphPadPrism, version 3.00, for Windows.
Results
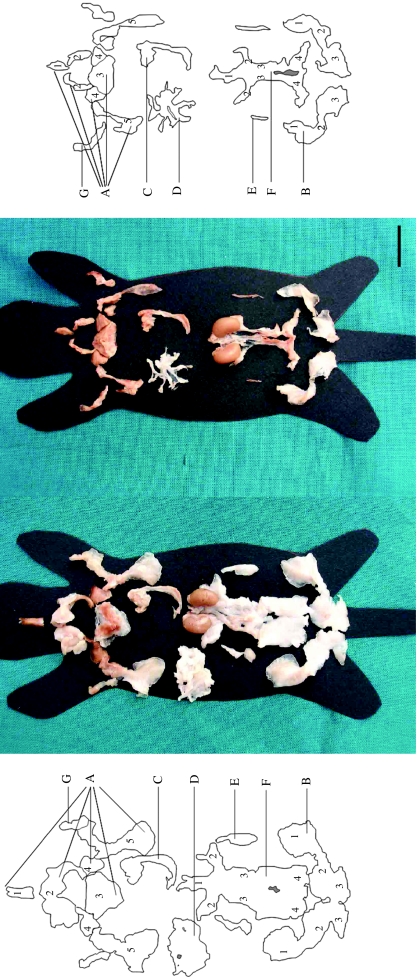
Anatomical dissection showed the adipose organ to be composed of subcutaneous and visceral depots having an identical shape in both experimental groups but a different size and color, as previously described in detail (Cinti, 1999, 2005; Murano et al. 2005) (Fig. 1). All depots except the mediastinal were significantly smaller in cold-acclimated animals than in control mice. In warm-acclimated mice the sole brown areas were the interscapular, subscapular, axillary and deep cervical regions of the anterior subcutaneous depot, the mediastinal depot, the interrenal depot, and parts of the parametrial portions of the abdomino-pelvic depot. In addition, nearly the whole organ of cold-acclimated mice had a reddish-brown color.
Fig. 1.
Multidepot adipose organ of adult Sv129 female mice kept at 28 °C (left) or at 6 °C (right) for 10 days. The organ was dissected under a surgical microscope and each depot was placed on a mouse template indicating its original anatomical position. Kidneys and ovaries were dissected together with the depots. The organ is made up of two subcutaneous depots, (A) anterior (1 – deep cervical portion, 2 – superficial cervical portion, 3 – interscapular portion, 4 – subscapular portion, 5 – axillo-thoracic portion) and (B) posterior (1 – dorsolumbar portion, 2 – inguinal portion, 3 – gluteal portion), and several visceral depots, (C) mediastinal, (D) mesenteric, (E) retroperitoneal and (F) abdomino-pelvic (1 – interrenal portion, 2 – periovarian portion, 3 – parametrial portion, 4 – perivesical portion). (G) Fat depots of anterior limbs. Fat depots of posterior limbs (thigh and popliteal) and omental fat depot not shown. Scale bar: 2 cm.
Previous research by our group showed that the vast majority of parenchymal fibers in the periovarian and interscapular fat depots are noradrenergic TH-ir fibers (Giordano et al. 1996; De Matteis et al. 1998; Giordano et al. 2005, 2006). Here we found that noradrenergic nerves are ubiquitous in all visceral and subcutaneous depots of the mouse adipose organ.
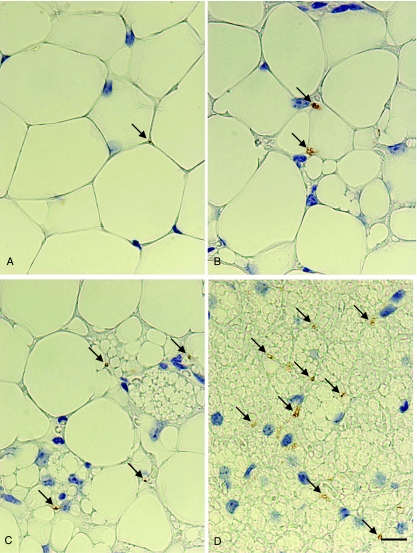
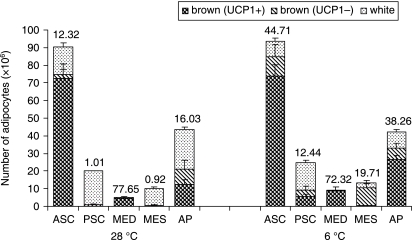
In line with previous investigations (Cannon et al. 1986; Giordano et al. 1996; De Matteis et al. 1998; Cinti 1999), we detected TH-ir nerve fibers around vessels and in close association to adipocytes (TH-ir parenchymal fibers, hereafter TH fibers) both in prevalently white and in prevalently brown areas, in either experimental condition (Fig. 2). TH fiber density and number of white and brown adipocytes (both UCP1-positive and UCP1-negative) per depot in the two groups of mice are shown in Fig. 3 and Table 1. TH fibers were more numerous in prevalently brown areas in both cold- and warm-acclimated animals.
Fig. 2.
Parametrial portion of the abdomino-pelvic depot of adult Sv129 female mice kept at 28 °C (A,B) or 6 °C (C,D) for 10 days. Representative picture (light microscopy) of TH-positive parenchymal nerve fiber morphology and immunoreactivity. Arrowheads indicate TH-positive parenchymal fibers. TH-positive fibers in the parenchyma were closely associated to adipocytes, both in areas made up of white (A) or prevalently white (B) adipocytes, and in prevalently brown (C) or exclusively brown (D) areas. In either experimental condition TH-positive fibers were more numerous in the areas containing a larger number of brown adipocytes. Scale bar: 10 µm.
Fig. 3.
Density of TH-immunoreactive parenchymal nerve fibers (no. of fibers per 100 adipocytes) in the adipose organ of adult Sv129 female mice kept at 28 °C or 6 °C for 10 days. Numbers above columns are the average TH-positive fiber density in each depot (see also Table 1). ASC, anterior subcutaneous; PSC, posterior subcutaneous; MED, mediastinal; MES, mesenteric; AP, abdomino-pelvic. Mean ± SE. The bars represent SEM.
Table 1.
Density (mean ± SE) of TH-immunoreactive parenchymal nerve fibers (no. of fibers per 100 adipocytes); no. of white adipocytes × 106; no. of brown adipocytes (UCP1-negative) × 106; no. of brown adipocytes (UCP1-positive) × 106 in the different depots of the adipose organ of adult Sv129 female mice kept at 28 °C or 6 °C for 10 days
| TH fiber density | No. of white adipocytes | No. of brown adipocytes (UCP1–) | No. of brown adipocytes (UCP1+) | |
|---|---|---|---|---|
| ASC | ||||
| 28 °C | 12.32 ± 7.28 | 15.64 ± 2.52 | 2.13 ± 0.25 | 72.52 ± 6 |
| 6 °C | 44.71 ± 7.85* | 8.66 ± 1.54* | 11.2 ± 2.96* | 73.57 ± 5.3 |
| PSC | ||||
| 28 °C | 1.01 ± 0.16 | 19.21 ± 0.23 | 0.94 ± 0.48 | 0.00 |
| 6 °C | 12.44 ± 1.79** | 15.69 ± 1.22* | 3.76 ± 1.05* | 5.52 ± 1.06*** |
| PSC | ||||
| 28 °C | 77.55 ± 1.55 | 0.14 ± 0.10 | 0.05 ± 0.03 | 4.71 ± 0.72 |
| 6 °C | 72.32 ± 25.61 | 0.00 | 0.13 ± 0.04 | 8.88 ± 2.19 |
| MES | ||||
| 28 °C | 0.92 ± 0.08 | 9.48 ± 0.79 | 0 | 0 |
| 6 °C | 19.71 ± 1.54** | 2.76 ± 1.55** | 10.43 ± 2.31** | 0.17 ± 0.03* |
| AP | ||||
| 28 °C | 16.03 ± 1.57 | 22.32 ± 1.35 | 8.9 ± 1.6 | 15.06 ± 3.27 |
| 6 °C | 38.26 ± 0.82** | 9.40 ± 1.17*** | 7.36 ± 0.96 | 24.63 ± 2.79* |
ASC, anterior subcutaneous; PSC, posterior subcutaneous; MED, mediastinal; MES, mesenteric; AP, abdomino-pelvic.
P < 0.05;
P < 0.01;
P < 0.001.
In cold-acclimated mice, TH fiber density, total number of brown adipocytes and proportion of UCP1-positive adipocytes increased in all subcutaneous and visceral depots (Table 1). The sole exception was the mediastinal depot, which was exclusively made up of brown adipocytes even in animals kept at 28 °C, and exhibited the highest density of TH fibers (Fig. 3).
In cold-acclimated mice the number of TH fibers calculated for the whole organ (see Materials and methods) increased threefold (21.32 ± 10.26 vs. 39.44 ± 8.5; P < 0.01), in parallel with a significant increase in brown adipocytes (P < 0.05), as described previously (Murano et al. 2005).
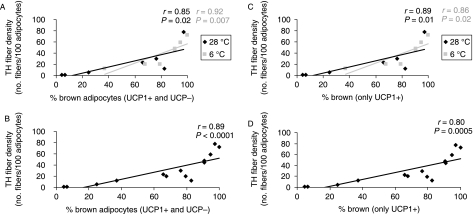
In both groups of animals and in all depots, TH fiber density correlated with the percentage of brown adipocytes, including both UCP1-positive and UCP1-negative multilocular adipocytes (Fig. 4A). The correlation was stronger when data from both experimental conditions were pooled (Fig. 4B).
Fig. 4.
Correlations between density of TH-immunoreactive parenchymal nerve fibers (no. of fibers per 100 adipocytes) and proportion of brown adipocytes (UCP1-positive and -negative) in the different adipose depots of adult Sv129 female mice kept at 28 °C or 6 °C for 10 days (A). Plot of data presented in (A) from all mice and depots (B). Correlations between TH-immunoreactive parenchymal nerve fiber density (no. of fibers per 100 adipocytes) and proportion of brown adipocytes (only UCP1-positive) in the different adipose depots of adult Sv129 female mice maintained at 28 °C or 6 °C for 10 days (C). Plot of data presented in (B) from all mice and depots (D). The mean density of TH-immunoreactive parenchymal nerve fibers and mean percentage of brown adipocytes (UCP1-positive and -negative) were determined for each depot or portion thereof: posterior subcutaneous, mesenteric, interrenal, parametrial, mediastinal, anterior subcutaneous and perivesical. The proportion of brown adipocytes was a strong predictor of fiber density. Linear correlations were calculated using Spearman's nonparametric test; r, Spearman coefficient; P, probability.
Positive correlations were also found when only UCP1-positive brown adipocytes in each experimental condition were considered (Fig. 4C) and when data from the two conditions were pooled (Fig. 4D).
Discussion
It is well established since Ashwell's 1984 pioneering work that cold acclimatisation induces the appearance of brown adipocytes in WAT (Young et al. 1984). The phenomenon has been described in several studies (Cousin et al. 1992; Guerra et al. 1998), some of which have also pointed to an important role for genetic background in its expression (Guerra et al. 1998). The adipose organ of adult Sv129 mice kept at 28 °C is composed of mixed white and brown adipocytes (Murano et al. 2005). In this obesity- and diabetes-resistant strain (Almind & Kahn, 2004), brown adipocytes are more numerous than their white counterparts (Murano et al. 2005). Previous work showed that cold acclimatisation, although not changing the total adipocyte number in the adipose organ, does significantly increase the number of brown adipocytes, with a similar, parallel reduction in white adipocyte numbers in all depots, suggesting a process of transdifferentiation of white to brown adipocytes in cold-acclimated mice (Murano et al. 2005). These data are in line with experimental data from our group (Himms-Hagen et al. 2000). Transformation into a more brown phenotype is related to adrenergic stimulation of beta3 adrenoceptors. Similar results have been obtained by administration of beta3 adrenoceptor agonists (Champigny et al. 1991; Ghorbani & Himms-Hagen, 1997; Ghorbani et al. 1997; Himms-Hagen et al. 2000; Granneman et al. 2005), whereas the phenomenon is blunted in cold-acclimated beta3 adrenoceptor knockout mice (Jimenez et al. 2003).
The vast majority of adipose organ parenchymal nerves are sympathetic noradrenergic nerves, few of which are likely to be sensory calcitonin gene-related peptide-containing nerves (Cui et al. 1990; Cui & Himms-Hagen, 1992a,b; Melnyk & Himms-Hagen, 1995; Giordano et al. 1996, 1998; Shi et al. 2005). Earlier research has shown that noradrenergic nerve density increases in cold-acclimated rat IBAT (De Matteis et al. 1998) and periovarian WAT (Giordano et al. 1996). However, quantitative immunohistochemical data for the whole adipose organ in cold-acclimated and control mice are not available. Here we document that all subcutaneous and visceral depots of the mouse adipose organ are provided with vascular and parenchymal noradrenergic nerves. In particular, parenchymal noradrenergic nerves are found in areas made up of both white and brown adipocytes, but they are more numerous among brown adipocytes. All depots share a similar innervation pattern, lending further support to the anatomical and functional concept of the mammalian adipose organ. In the adipose organ of cold-acclimated mice the total number of TH fibers increased threefold and was paralleled by an increase in brown adipocytes and in UCP1-positive adipocytes. As shown in previous papers, the total number of adipocytes does not differ significantly in the two experimental conditions (Murano et al. 2005), ruling out an ostensible increase in the number of TH fibers – calculated on the basis of density (fiber number per 100 adipocytes) – related to the volume change of adipocytes.
TH fiber density and proportion of brown adipocytes in the various depots were significantly correlated in both experimental conditions, even after the two datasets were pooled.
Several researchers believe brown adipocytes to be defined by the presence of UCP1. In our experience, all fully differentiated multilocular adipocytes have the characteristic organelles of brown adipocytes on electron microscopy; for this reason, and because multilocular adipocytes appearing in WAT after cold exposure express UCP1 only after 10 days in this condition, with a transient period in which only UCP1 mRNA and not the protein is expressed (Jimenez et al. 2003; Cinti, 2005, 2006a), we believe that multilocular UCP1-negative adipocytes are in fact transdifferentiating brown adipocytes in a transitional functional stage. However, exploration of the relationships between TH fiber density and proportion of UCP1-ir brown adipocytes in the different depots showed a correlation both in cold-acclimated mice and in pooled data from the two experimental conditions.
The varying nerve density and cell composition found in the different fat depots after cold exposure confirm previous reports of a different responsiveness of the individual depots to various challenges. However, the data also indicate that despite such variation, the adipose organ responds to cold acclimatisation as a whole, with a generalized branching of noradrenergic fibers in all depots. The phenomenon could be a precondition for the phenotypic transformation (i.e. increase in the amount of brown adipocytes) induced by cold acclimatisation.
Recent data support a mixed composition also for the depots of the human adipose organ (Oberkofler et al. 1997; Cinti, 2006b). In addition, recent radiological (positron emission tomography) findings, combined with immunohistochemistry and molecular biology data, suggest that BAT is well represented in adult humans, too (Tokuyama & Himms-Hagen, 1986; Hany et al. 2002; Tatsumi et al. 2004; Gelfand et al. 2005; Jan Nedergaard, 2007).
The ‘brown’ phenotype (i.e. a prevalence of brown adipocytes in the adipose organ) is likely to be responsible for obesity resistance in small mammals (Almind & Kahn, 2004; Almind et al. 2007), but some recent data seem to point to a similar mechanism also in humans, because UCP1 mRNA is reduced in the visceral adipose tissue of obese patients (Oberkofler et al. 1997) and genes expressed in the brown phenotype are underexpressed in subcutaneous WAT of overweight patients with insulin resistance (Yang et al. 2003).
Although the mouse strain investigated in this study is genetically resistant to obesity, thus preventing any generalization of our data, the present findings point to the need for further studies of drugs capable of inducing a brown phenotype (e.g. beta3 adrenoceptor agonists), despite the unsatisfactory results achieved to date in this field (Larsen et al. 2002; van Baak et al. 2002).
Acknowledgments
This work was supported by grants from the Italian Ministry of University (FIRB Internazionalizzazione 2005, to S.C.) and from Università Politecnica delle Marche (RSA 2007, to S.C.), and by TOBI EWO6-007 (Targeting Obesity-driven Inflammation).
References
- Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Cannon B, Hedin A, Nedergaard J. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 1982;150:129–132. doi: 10.1016/0014-5793(82)81319-7. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J, Lundberg JM, Hokfelt T, Terenius L, Goldstein M. ‘Neuropeptide tyrosine’ (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Exp Cell Res. 1986;164:546–550. doi: 10.1016/0014-4827(86)90052-2. [DOI] [PubMed] [Google Scholar]
- Champigny O, Ricquier D, Blondel O, Mayers RM, Briscoe MG, Holloway BR. Beta 3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc Natl Acad Sci U S A. 1991;88:10774–10777. doi: 10.1073/pnas.88.23.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The Adipose Organ. Milan: Kurtis; 1999. [Google Scholar]
- Cinti S. Anatomy of the adipose organ. Eat Weight Disord. 2000;5:132–142. doi: 10.1007/BF03354443. [DOI] [PubMed] [Google Scholar]
- Cinti S. The adipose organ: endocrine aspects and insights from transgenic models. Eat Weight Disord. 2001a;6:4–8. [PubMed] [Google Scholar]
- Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001b;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Cinti S. Cachexia and Wasting. Milan: Springer-Verlag Italia; 2006a. [Google Scholar]
- Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006b;16:569–574. doi: 10.1016/j.numecd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Cinti S, Cancello R, Zingaretti MC, et al. CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J Histochem Cytochem. 2002;50:21–31. doi: 10.1177/002215540205000103. [DOI] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. Pt 4. [DOI] [PubMed] [Google Scholar]
- Cui J, Himms-Hagen J. Rapid but transient atrophy of brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. 1992a;262:R562–567. doi: 10.1152/ajpregu.1992.262.4.R562. [DOI] [PubMed] [Google Scholar]
- Cui J, Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. 1992b;262:R568–573. doi: 10.1152/ajpregu.1992.262.4.R568. [DOI] [PubMed] [Google Scholar]
- Cui J, Zaror–Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol. 1990;259:R324–332. doi: 10.1152/ajpregu.1990.259.2.R324. [DOI] [PubMed] [Google Scholar]
- De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27:877–886. doi: 10.1023/a:1006996922657. [DOI] [PubMed] [Google Scholar]
- Flatmark T. Catecholamine biosynthesis and physiological regulation in neuroendocrine cells. Acta Physiol Scand. 2000;168:1–17. doi: 10.1046/j.1365-201x.2000.00596.x. [DOI] [PubMed] [Google Scholar]
- Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL. 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25:125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci. 1998;111:2587–2594. doi: 10.1242/jcs.111.17.2587. Pt 17. [DOI] [PubMed] [Google Scholar]
- Giordano A, Frontini A, Murano I, et al. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679–687. doi: 10.1369/jhc.4A6566.2005. [DOI] [PubMed] [Google Scholar]
- Giordano A, Song CK, Bowers RR, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006. [DOI] [PubMed]
- Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon T. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Barbatelli G, Allevi R, et al. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem. 2003;270:699–705. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- Larsen TM, Toubro S, van Baak MA, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Susulic VS, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Melnyk A, Himms-Hagen J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes Res. 1995;3:337–344. doi: 10.1002/j.1550-8528.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Murano I, Zingaretti CM, Cinti S. The adipose organ of Sv129 mice contains a prevalence of brown adipocytes and shows plasticity after cold exposure. Adipocytes. 2005;1:121–130. [Google Scholar]
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997;38:2125–2133. [PubMed] [Google Scholar]
- Ricquier D, Barlet JP, Garel JM, Combes-George M, Dubois MP. An immunological study of the uncoupling protein of brown adipose tissue mitochondria. Biochem J. 1983;210:859–866. doi: 10.1042/bj2100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- Tokuyama K, Himms–Hagen J. Brown adipose tissue thermogenesis, torpor, and obesity of glutamate-treated mice. Am J Physiol. 1986;251:E407–415. doi: 10.1152/ajpendo.1986.251.4.E407. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, James WP. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978;373:189–193. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- van Baak MA, Hul GB, Toubro S, et al. Acute effect of L-796568, a novel beta 3-adrenergic receptor agonist, on energy expenditure in obese men. Clin Pharmacol Ther. 2002;71:272–279. doi: 10.1067/mcp.2002.122527. [DOI] [PubMed] [Google Scholar]
- Yang X, Enerback S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res. 2003;11:1182–1191. doi: 10.1038/oby.2003.163. [DOI] [PubMed] [Google Scholar]
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol. 1995;268:R744–751. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]