Abstract
Previous work has reported non-adultlike distortion product otoacoustic emission (DPOAE) suppression in human newborns at f2 =6000 Hz, indicating an immaturity in peripheral auditory function. In this study, DPOAE suppression tuning curves (STCs) were recorded as a measure of cochlear function and acoustic admittance/reflectance (YR) in the ear canal recorded as a measure of middle-ear function, in the same 20 infants at birth and through 6 months of age. DPOAE STCs changed little from birth through 6 months, showing excessively narrow and sharp tuning throughout the test period. In contrast, several middle-ear indices at corresponding frequencies shifted systematically with increasing age, although they also remained non-adultlike at 6 months. Linear correlations were conducted between YR and DPOAE suppression features. Only two correlations out of 76 were significant, and all but three YR variables accounted for <10% of the variance in DPOAE suppression tuning. The strongest correlation was noted between admittance phase at 5700 Hz and STC tip-to-tail (R=0.49). The association between middle-ear variables and DPOAE suppression may be stronger during other developmental time periods. Study of older infants and children is needed to fully define postnatal immaturity of human peripheral auditory function.
I. INTRODUCTION
For over a decade, studies into the maturation of distortion product otoacoustic emissions (DPOAE) suppression characteristics in human infants have reported age differences (Abdala et al., 1996; Abdala, 1998). Iso-suppression tuning curves (STCs) from infants are non-adultlike in width and steepness on the low-frequency side when recorded at f2 =6000 Hz. The growth of suppression for low-frequency suppressor tones is shallow in infants relative to adults as well. These results clearly suggest immaturity in the peripheral auditory system of human newborns. More recently, DPOAE-based immaturities have been documented through at least 3 months of age (Abdala, 2004). It is not clear when these aspects of auditory function become adultlike in human infants because the timeline for maturation of DPOAE suppression tuning has not been completely defined. Additionally, the source of this immaturity is not clear.
Non-adultlike DPOAE suppression in infants was initially thought to arise from a purely cochlear immaturity. Several cochlear sources have been hypothesized and some have been systematically investigated, such as outer hair cell (OHC) function. The OHCs are morphologically developed early in gestation (Lavigne-Rebillard and Pujol, 1987, 1988) although their functional status in humans just before and after birth is not known. When aspirin is used to reversibly impair OHC motility in normal-hearing adults, DPOAE STCs become abnormal in morphology; however, they do not become like neonatal tuning curves. Rather, they become broad, bowl-shaped, and lose their tip region (Abdala, 2005). This finding does not support the hypothesis that OHC motility is the source of non-adultlike DPOAE suppression in human infants.
It is also possible that functional immaturities exist in the descending efferent system known to modulate OHC function. When the medial olivocochlear (MOC) reflex is evoked by contralateral noise, DPOAE amplitude is altered in the opposite ear (Puel and Rebillard, 1990). There are few published reports of the MOC reflex in human infants, but the existing studies suggest that MOC function remains immature just before and around the time of birth (Abdala et al., 1999; Morlet et al., 1993; Ryan and Piron, 1994). It is possible that an MOC immaturity contributes to non-adultlike DPOAE suppression tuning during maturation.
Noncochlear factors must also be considered as potential explanations for DPOAE-based immaturities. Although OAEs measure cochlear function, stimuli presented to evoke OAEs, as well as the emission itself, must travel the conductive pathway, and therefore are subject to immaturities in this pathway. The outer and middle ear can filter both the stimulus and the DPOAE emerging from the cochlea and measured in the car canal (Keefe, 2007). Recent work from our lab has shown that the infant middle ear attenuates the stimulus level during forward transmission to the cochlea more than does the adult middle ear, while the reduced ear canal area in infants boosts the DPOAE during reverse transmission from cochlear origin to microphone (Abdala and Keefe, 2006; Keefe and Abdala, 2007). The attenuation in forward transmission is particularly relevant because DPOAE STCs evoked with lower levels of stimuli are sharper and narrower than STCs evoked with high-level stimulation (Abdala, 2001), most likely due to initiation of low-level active processes that enhance frequency resolution.
Other acoustic ear-canal measures such as admittance and reflectance may encode aspects of middle-ear functioning that could influence DPOAEs as well. These measures indicate how the outer and middle ear absorbs and/or reflects sound. Maturation of reflectance and admittance characteristics in human infants shows a broad pattern of postnatal variation with age through 24 months (Keefe et al., 1993). These variations are relevant to the present study at measurement frequencies near the primary tones (5000–6000) and DPOAE frequency (4000 Hz). Keefe found that admittance levels in infants (1–24 months of age) were significantly different from adult levels at these measurement frequencies. Additionally, admittance levels at 1 month of age differed from infant admittance at 3–24 months of age, and level at 3 months differed from level measured at ages between 12 and 24 months. These differences may be due to age-dependent growth of the cross-sectional area of the ear canal. Admittance is not yet mature at age 11 years (Okabe et al., 1988). Energy reflectance shows mixed age effects. At ages 1, 6, and 12 months it differs from that of adults at 5040 Hz but no differences are observed at 4000 Hz for any age. Only 1-month-olds differ from adults at 6000 Hz. The energy reflectance at age 1 month is significantly different at 6350 Hz for ages 12 and 24 months, but, otherwise, no reflectance differences between infant age groups were observed.
When combined, independent research on maturation of DPOAE suppression tuning and maturation of middle-ear reflectance/admittance properties indicates that both are developing into the postnatal period. The purpose of the present study was to investigate: (1) the time period during which DPOAE suppression tuning becomes adultlike by testing infants from birth through 6 months of age using a longitudinal design and (2) the relationship between the maturation of DPOAE suppression tuning and maturation of middle-ear reflectance/admittance properties in the same group of infants during the first 6 postnatal months of life. By addressing these objectives, the present study sought to define when the cochlea becomes functionally mature in humans and examine the extent to which middle-ear functioning can account for DPOAE suppression immaturities and their changes over time.
II. METHODS
A. Subjects
Ten normal-hearing adults and 20 healthy infants participated as subjects in this study. Different auditory measures from this same group of infants have been previously analyzed to address related questions (Abdala and Keefe, 2006; Keefe and Abdala, 2007). The 10 adults had a mean age of 27.5 years (range=19–35 years) and audiometric thresholds <15 dB HL for frequencies from 250–8000 Hz. Four right and six left ears were tested. Six subjects were female and four were male. All adult subjects had negative histories of hearing loss and otologic pathology.
Of the 20 infant subjects, 11 were term born and nine were infants born prematurely but tested once they reached termlike status, i.e., 37–41 postconceptional weeks. Prematurely born infants were included in this study to address the question of whether they develop along a normal maturational time line once their age is corrected for premature birth. Infants had a mean birth weight of 2853 grams (range=1470–3960 grams) and mean 1- and 5-min Apgar scores of 7.7 and 8.7. There were 9 females and 11 male infants, 8 right and 12 left ears. Other than a stay in the Neonatal Intensive Care Unit of >48 h for observation (pre-mature infants only), none of the infants had high-risk factors for hearing loss as defined by the Joint Committee on Infant Hearing (JCIH, 2000). All infant subjects passed a hearing screening at 35 dB HL with a click-evoked auditory brainstem response (ABR) and a DP-gram (f2 frequencies ranging from 1500 to 8000 Hz).
B. Instrumentation and signal processing
A custom-designed DPOAE acquisition system (SupprDP) was used to generate stimuli and acquire data under the control of custom software using a 48 000-Hz sampling rate. The data acquisition hardware was based on an audio processor developed by House Ear Institute Engineering Department. The hardware includes 2-channel D/A, 2-channel A/D, and a DSP processor (all 24-bit), as well as an analog high-pass filter (12-dB/oct.; 700-Hz high-pass cutoff). The A/D converter received the electrical output signal from an Etymotic Research ER-10C probe microphone. The ER-10C probe contains two output transducers and a low-noise microphone. The two primary tones and suppressor tone were generated by the DSP processor. The primary tone at f1 was generated by one D/A converter and delivered via one transducer. The primary tone at f2 and the suppressor tone were produced by the second D/A converter and output through the second transducer. The microphone signal was high-pass filtered before being sampled by the A/D converter.
C. Data acceptance criteria
Twenty sweeps of the microphone signal were averaged by the DSP processor and comprised one block of data. A block of data was only accepted into the grand average if the noise measurements for three frequency bins (11.7 Hz wide) on either side of the 2f1-f2 frequency were <5 dB SPL. This ensured adequate subject state.
The grand DPOAE average had to meet the following criteria: (1) the measured DPOAE level was at least 5 dB above the average noise in these six bins around 2 f1-f2 and (2) averaged noise measurements for the six frequency bins were not greater than 0 dB SPL. A minimum of 6 and a maximum of 12 acceptable blocks of data comprised the final DPOAE grand average. If the maximum number of blocks was collected (12 blocks or 240 sweeps) without meeting these criteria, no DPOAE response was accepted and the program moved on to the next test condition.
D. Calibration
Intermodulation distortion produced by the recording system at 2 f1-f2 was measured with the probe in a Zwislocki coupler for all test conditions. The mean level of distortion was -25 dB SPL. The recording system noise floor was determined using a similar method with no tones present. The level of system noise floor ranged between −23 and −30 dB SPL. An in situ calibration procedure was conducted on both output transducers before each subject was tested. A chirp tone (swept-frequency signal from 10 to 10 000 Hz) with fixed voltage was presented to the transducer and the resulting SPL of the tone recorded in the ear canal. Based on this information, an equalization of output levels was performed for each subject to achieve target stimulus levels across test frequencies.
E. Procedure
The most marked age effects for DPOAE suppression have been previously observed at f2 =6000 Hz (Abdala, 1998, 2001), and for this reason, it was selected as the primary test frequency to enhance the probability of detecting maturational shifts. At the beginning of this study, attempts were also made to collect low-frequency data. Thirty-eight newborns were tested at f2 =1500 Hz, but noise in this frequency range was excessive at the older ages (3–6 months) and precluded successful completion of the protocol. Three infants were successfully tested at f2 =2000 Hz and, although the group data were too few to analyze, they are described in the Results section.
Infant subjects were tested once informed parental consent had been obtained. The first test occurred within 72 h of birth if subjects were term-born and once their corrected age reached term at 37–40 weeks postconceptional age (PCA) if they were born prematurely. All infants were then tested again at 3 months (mean=85.4 days), 4 months (mean =117.4 days), 5 months (mean=148.7 days), and 6 months (mean=182.9 days) of age ± 1 week.
Following the initial test, families received phone calls from the research audiologist within 2 weeks of the target age to schedule a test session. Once the session had been scheduled, the families were mailed reminder postcards 1 week prior to the appointment date. By using this method, success rate for appointment participation was 96%. All infant testing took place at the Infant Auditory Research Laboratory on the University of Southern California + Los Angeles County (USC+LAC) campus, Women’s and Children’s Hospital, in a quiet room away from the normal nursery and neonatal intensive care unit. Infants were tested in their hospital isolettes whenever possible, or occasionally tested while held in the parent’s arms or, at older ages, while sleeping in a car seat. All infants were tested during natural sleep and an infant test session was typically 2 to 2.5 h in duration. Adult subjects were tested within an IAC sound-attenuated booth at the House Ear Institute while sitting comfortably in a padded easy chair, reading or resting. One 1.5–2 h session was required for each adult.
All DPOAE STCs were generated with fixed stimulus tones of 65–55-dB SPL and an f2/f1 ratio of 1.2. In order to record a 6000-Hz STC, 15 different suppressor tones were presented at frequencies around f2 ranging from 3047 to 7239 Hz. Suppressor tones were presented at intervals of 25–150 cents (1 octave=1200 cents) with smaller intervals in the tip region. To record a 2000-Hz STC in three infant subjects and five adults, 12 different suppressor tones were presented at frequencies around f2, ranging from 1125 to 2520 Hz. The suppressor tones were presented ipsilaterally with the primary tones and increased in 5-dB intervals from 30 to 85 dB SPL. Unsuppressed DPOAE amplitude was measured at the start of data collection and prior to the presentation of each new suppressor frequency. STCs were generated with 2- and 6-dB suppression criteria. The suppressor levels producing criterion suppression were calculated for each suppressor tone by linear interpolation and plotted as a function of suppressor frequency
Acoustic admittance/reflectance (YR) measurement procedures are briefly summarized here and explained in more detail elsewhere (Keefe and Simmons, 2003; Keefe and Abdala, 2007). For this study, YR responses were analyzed at octave frequencies from 250 to 8000 Hz and two interoctave frequencies: 2800 and 5700 Hz. An Etymotic ER10C probe was used with a microphone and +20-dB additional gain to each of two receivers in the probe. This probe was the same probe used by Abdala and Keefe (2006). DPOAE and YR responses were acquired, whenever possible, based on the same ear-canal insertion. Receiver 1 delivered a brief “click” approximating a bandlimited impulse from 0.25 to 8 kHz. Receiver 1 was driven by the output of a D/A converter of a computer sound card (CardDeluxe) using custom-written software. The microphone output was synchronously recorded using an A/D converter on the sound card. The sample rate was 22.05 kHz using a D/A and A/D buffer length of 1024 samples.
Admittance/reflectance data were eliminated if the DPOAE in the same subject dropped by 10 dB or greater from one test session to another and parent reported the infant to be sick or congested, which indicated a possible upper respiratory infection (5% of the data). Data were also eliminated if the energy reflectance was close to zero and/or if the equivalent volume was extremely negative at low frequencies (≤1 kHz). The presence of either or both conditions indicated a likely leak of the probe in the ear canal (Keefe et al., 2000). Data were dropped if the energy reflectance at high frequencies (6–8 kHz) exceeded 1.2. Excessively high reflectance values (and corresponding abnormal admittance values) may have been due to a partially blocked probe tip by cerumen or resulting from placement near the ear-canal wall, or reflectance calibration errors at high frequencies. In total 30.9% of reflectance/admittance records were eliminated to ensure data quality, using the guidelines above.
F. Data analysis
1. DPOAE
DPOAE STCs generated with 2- and 6-dB suppression criteria were analyzed in the following manner: (1) the STC width was measured 10 dB above the tip, and the tip frequency was divided by this width to obtain a Q10 measure; if the STC was too broad to calculate a Q10, a value 2 standard deviations below the mean Q10 was assigned; (2) the slope of the low- and high-frequency flank of the STC was quantified by fitting regression lines from the tip of the STC to the lowest and highest frequencies on the flanks of the tuning curve; (3) the tip-to-tail value was measured by subtracting the suppressor level at the tip of the STC from the level at the lowest suppressor frequency which was approximately one octave below f2 (3047 Hz); (4) the STC tip frequency and tip level were measured; (5) DPOAE suppression growth was calculated by measuring slope of the DPOAE amplitude x suppressor level function using a linear regression equation. Any initial amplitude plateau was eliminated in this calculation and only the linear portion of this function was included. The measured segment began 1–2 dB down from the unsuppressed value and included all points with sufficient SNR.
Three statistical analyses were conducted on DPOAE data: First, one-way analyses of variance (ANOVAs) were conducted between infants born prematurely (but tested at term-equivalent corrected age) and those born after term birth on each DPOAE feature measured. These established whether a premature birth influenced DPOAE suppression measurements. Second, unpaired t-tests were conducted between adults and infants at two ages: (1) Newborn vs adult (to confirm previous research published on immaturities in DPOAE suppression at birth), and (2) Six-month-old vs adult (to assess whether infant suppression responses had become more adultlike over time). A significant age difference at birth and the absence of age differences at 6 months would be indicative of some maturational shift. Conversely, significant age differences at both birth and 6 months of age would indicate continued immaturity of the response throughout the test period. Third, repeated-measures one-way ANOVAs (repeated variable=age) were also conducted on infant data for each DPOAE suppression variable that was non-adultlike at birth, to more directly test for change as a function of age. A Bonferroni factor was applied to adjust the alpha level when multiple comparisons were conducted. The alpha level was p=0.05.
2. Relationships between middle ear and DPOAE suppression variables
Half-octave averaged admittance and reflectance responses assessed middle-ear functioning at three frequencies (approximately 2800, 4000, and 5700 Hz). The three frequencies were chosen for analysis because 2800 Hz is in the region of the STC “tail” and closest to the suppressor frequency used in making tip to tail measurements (3047 Hz), 4000 Hz is at the DPOAE frequency, and 5700 Hz is nearest the f2 frequency of 6000 Hz. Reflectance and admittance variables have significant correlations with one another across frequency. Therefore, either a subset of variables at particular frequencies is selected for detailed analysis, as in the present study, or new factor variables are defined as linear combinations of the original admittance and reflectance variables.
Admittance can be represented in terms of magnitude (YM) and phase (YP) or, alternatively, in terms of real and imaginary parts. The real part of admittance is the conductance (G), which can be expressed as a conductance level (LG=10 log10 G, with 0 dB for G =1 mmho). For a DPOAE experiment with fixed SPLs at the primary frequencies, the power level absorbed by the middle ear is proportional to LG. The imaginary part of admittance is susceptance (S). The correlation analyses included admittance variables in both level/phase and real/imaginary component representations. In addition, the acoustic estimate of ear-canal area was calculated for each ear and its level (in dB, normalized to the adult ear-canal area) was included in correlations. Keefe and Abdala (2007) reported that reverse DPOAE transmission is sensitive to ear-canal area, and Keefe and colleagues (1993) showed that ear-canal area was an important contributor to maturational differences in ear-canal impedance, and thus, admittance.
To assess the relationship between DPOAE suppression and YR responses measured at the ear-canal probe microphone during repeated test sessions from birth through 6 months of age, Pearson linear correlations were calculated between four DPOAE STC variables that are non-adultlike in newborns and seven middle-ear variables. The DPOAE suppression variables were (1) STC Q10; (2) slope of the STC low-frequency flank; (3) tip-to-tail level; and (4) tip level. The YR variables were (1) energy reflectance (ER); (2) reflectance phase (RP); (3) admittance magnitude (YM); (4) admittance phase (YP); (5) conductance (LG); (6) susceptance (S); and (7) an acoustic estimate of ear-canal area level calculated for each ear (in dB).
Correlations are shown in terms of their Pearson correlation (R) (Table I) and discussed in terms of their squared correlation (R2) values. Correlations were tested for significance; however, it is important to distinguish between statistical significance, which only indicates whether a nonrandom association is present between two variables, and the strength of association, which is assessed by R2. The R2 assessed how much variance in any given DPOAE suppression feature could be accounted for by variance in a particular YR variable. This index best addressed our research question, i.e., to evaluate the predictive power of YR variables on DPOAE suppression. A multiple regression was also conducted to investigate whether some of the variance in DPOAE suppression features could be explained using two or more YR variables producing the strongest correlations with that feature. Given that many correlations were conducted, a more rigorous p=0.01 alpha level was applied.
TABLE I.
Correlation coefficients (R) between DPOAE STC features (f2 =6000 Hz) and middle-ear admittance and reflectance features at three frequencies. Ear canal area was calculated as described in the text and although listed under 2800 Hz, estimates of ear canal area are independent of frequency. Significant correlations are in bold.
| Frequency (Hz) |
||||
|---|---|---|---|---|
| ME feature | STC feature | 2800 | 4000 | 5700 |
| Admittance magnitude (YM) | Tip-to-tail | −0.25 | −0.27 | −0.08 |
| LF slope | 0.22 | 0.10 | 0.11 | |
| Q | −0.19 | −0.26 | −0.13 | |
| Tip level | 0.12 | 0.26 | 0.24 | |
| Admittance phase (YP) | Tip-to-tail | 0.02 | 0.14 | 0.49a |
| LF slope | 0.17 | 0.10 | 0.24 | |
| Q | −0.20 | −0.02 | 0.20 | |
| Tip level | 0.10 | 0.09 | −0.20 | |
| Susceptance (S) | Tip-to-tail | 0.04 | 0.16 | 0.42a |
| LF slope | 0.18 | 0.07 | 0.23 | |
| Q | −0.21 | −0.10 | 0.26 | |
| Tip level | 0.05 | −0.02 | −0.23 | |
| Conductance level (LG) | Tip-to-tail | −0.31 | −0.26 | 0.08 |
| LF slope | 0.19 | 0.10 | 0.19 | |
| Q | −0.13 | −0.25 | −0.07 | |
| Tip level | 0.16 | 0.25 | 0.13 | |
| Energy reflectance (ER) | Tip-to-tail | 0.34 | 0.28 | −0.22 |
| LF slope | −0.06 | −0.08 | −0.31 | |
| Q | 0.02 | 0.14 | −0.21 | |
| Tip level | −0.23 | −0.24 | 0.10 | |
| Reflectance phase (RP) | Tip-to-tail | 0.06 | −0.02 | 0.12 |
| LF slope | −0.17 | −0.24 | −0.21 | |
| Q | 0.09 | −0.01 | 0.12 | |
| Tip level | −0.04 | −0.04 | −0.04 | |
| Ear canal area (EC) | Tip-to-tail | 0.22 | … | … |
| LF slope | −0.14 | … | … | |
| Q | 0.25 | … | … | |
| Tip level | −0.25 | … | … | |
p<0.01.
III. RESULTS
A. DPOAE suppression
1. Newborn period
Figure 1 shows the mean DPOAE STCs recorded with both 6- and 2-dB suppression criteria from adults and infants at birth and through 6 months of age. Because STCs generated with 6- and 2-dB suppression criteria generally showed the same age-related trends, only the data from the 6-dB STCs are presented unless diverging results were observed. There was no discernible, consistent pattern that was unique to infants that were born prematurely (but tested at a corrected age equivalent to term birth) vs infants that were born following full gestation. A one-way ANOVA (collapsed across session) showed no significant difference between the two infant groups for any DPOAE variable measured; therefore, their data were combined into one infant group for all analyses. This result indicates that there is no obvious delay or difference in the maturation of DPOAE suppression for babies born prematurely, once their age is corrected for premature birth.
FIG. 1.
Mean infant DPOAE suppression tuning curves (f2=6000 Hz) around birth and at four additional ages (birth: n=20, 3, and 4 months: n =17, 5, and 6 months: n=18). The mean adult STC is included for comparison (n=10). Tuning curves are presented for two suppression criteria.
Four DPOAE STC variables showed infant-adult differences at birth: Q10, tip-to-tail level, slope on the low-frequency flank, and tip level. These four variables are shown in Fig. 2 as a function of age. Mean adult values are included at the far right of each graph (the asterisk represents a mean of values from a group of normal hearing children to be considered in the Discussion section). As is evident, at birth, STC width was significantly narrower in the combined group of infants (p=0.0001), the tip-to-tail value was larger (p=0.0001), the low-frequency flank was steeper (p =0.0001), and the tip level was lower in infants (p =0.0002). The slope on the high-frequency flank of the STC and the tuning-curve tip frequency (not shown) were similar in adults and infants at birth. These trends are consistent with previously reported DPOAE suppression data in newborns.
FIG. 2.
The four DPOAE STC features showing newborn-adult age differences (Q10, tip-to-tail level, slope on the low-frequency flank, and tip level) as a function of age. Each mean represents between 17–20 infant subjects and 10 adult subjects. Error bars= +/− 1 s.d. The asterisk represents mean values from a group of 15 normal-hearing children with an average age of 10.5 years.
The slope or “rate” of suppression growth was analyzed only for the four lowest suppressor tones because age differences were not present on the high-frequency flank of the STC (Abdala, 1998, 2001). As previously reported, suppression growth was shallower in newborns than adults for suppressor tones at 3047, 3621, and 4090 Hz (p=0.04, p=0.01, and p=0.02, respectively) (Fig. 3). The suppressor frequency of 4559 Hz did not show any infant-adult differences in the growth of suppression at birth.
FIG. 3.
Slope of suppression growth for the three lowest frequency suppressor tones (3047, 3621, and 4090 Hz) as a function of age. Each mean represents between 16–20 infants and 10 adult subjects. Error bars= +/− 1 s.d.
2. Birth through 6 months
Age comparisons between adult data and infant data at 6 months showed persistent age differences for the four DPOAE STC variables that were immature during the newborn period: Q10, tip-to-tail level, slope on low-frequency STC flank, and tip level (6 month vs adult: p=0.0001 for all four variables). It is clear from Figs. 1 and 2 that these four features of the DPOAE STC remained immature at 6 months of age. The only exception to this result was observed for STCs recorded with 2-dB suppression criteria, in which the infant tip level was lower than adults at birth (p=0.02), but by 6 months of age the tip level had increased to within adult values. Additionally, slope of suppression growth for suppressor tone 3047 Hz was adultlike by 6 months of age, although it had been excessively shallow at birth. The two other low-frequency suppressor tones showing non-adultlike suppression growth at birth (3621 and 4090 Hz) remained immature at 6 months as well (p=0.008 and p=0.01, respectively).
The repeated measures ANOVAs, conducted to directly assess change across session/infant age, included a somewhat reduced data set because each infant subject did not have measurements at each of the five test sessions. Because five ANOVAs were conducted on the same data set, the alpha level was adjusted using the Bonferonni correction factor (0.05/5=0.01). The five variables tested were Q10 (n=12), slope on the low-frequency flank (n=13), tip-to-tail (n=7), slope of suppression growth for the lowest suppressor tone (n=9), and tip level (n=13). There was no significant effect of age on any of these five DPOAE suppression features.
3. DPOAE suppression at f2 =2000 Hz
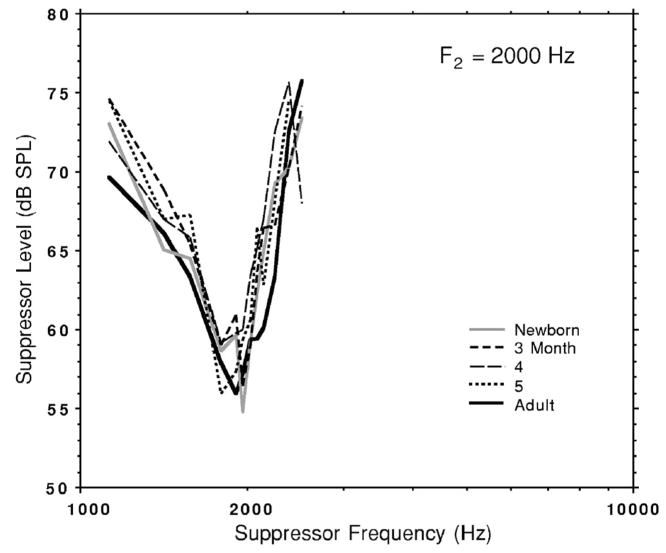
Three infants were tested successfully at this frequency and followed from birth through 5 months of age. In past work, low-frequency DPOAE STCs were reported to be generally adultlike or nearly adultlike by term birth and did not show robust newborn-adult age differences. As seen in Fig. 4, infant DPOAEs for f2 =2000 Hz were also generally adult-like in morphology and width and did not show systematic changes in STC width with age.
FIG. 4.
Mean infant DPOAE suppression tuning curves (f2=2000 Hz) for three infants tested around birth and at three additional ages. The mean adult STC is included for comparison (n=7). The suppression criterion was 6 dB.
B. Acoustic admittance and reflectance
It was not the objective of the present study to provide an overall description of YR response maturation in humans. Several publications are available in the literature to describe this maturational time course in human infants, and the reader is referred to these for an overview (Keefe et al., 1993, 1994; Keefe and Levi, 1996; Keefe et al., 2000; Keefe and Abdala, 2007). In the present study, our interest was limited to how these middle-ear variables relate to and possibly account for changes in DPOAE suppression tuning. Although YR responses were measured in the ear canal over a wide frequency range from 250 to 8000 Hz, the DPOAE, stimuli used to evoke the DPOAE, and the suppressor tones were centered within a more narrow frequency range from 3000–6000 Hz. Thus, our analysis of YR responses was limited to this same frequency range (see Data Analysis section). The admittance is plotted as a function of age in Fig. 5 for 2800, 4000, and 5700 Hz. The entire wideband response for energy reflectance was plotted as a function of age in a companion report (Keefe and Abdala, 2007) and is not repeated here, because admittance was the more salient feature and the strongest predictor of DPOAE suppression.
FIG. 5.
The admittance magnitude (YM) at 2800, 4000, and 5700 Hz plotted as a function of age in the upper panel. YM is expressed as a level (in dB) defined by 20 log10 YM, with YM measured in mmhos (i.e., YM level is 0 dB when YM=1 mmho). The admittance phase (YP), in degrees plotted as a function of age in the lower panel. Each symbol shows the mean YM or YP at each age. Error bars= +/−1 s.e.
Generally, the maturation of reflectance and admittance observed here was consistent with what has been reported previously for corresponding frequencies (Keefe et al., 1993). The admittance magnitude (YM) increased with increasing age at each frequency (2800, 4000, 5700) but was not yet adultlike at age 6 months. The typical pattern was that YM was largest in infant ears at 5700 Hz, next largest at 4000 Hz, and smallest at 2800 Hz, although the newborn response deviated slightly from this pattern. The YM in adult ears did not conform to this pattern either, which was likely due to the effects of standing waves in the longer adult ear canals at higher frequencies.
The mean admittance phase (YP) increased systematically with increasing age at 2800 Hz. The admittance phase was approximately independent of infant age at 4000 Hz with a slight decrease in the adult ear. Admittance phase at 5700 is described later as having the strongest correlation with one DPOAE STC parameter (for an f2 =6000 Hz), and so is of particular interest. It shows a marked change between birth and 3 months and little change beyond this age. The mean YP at 5700 Hz was close to −10 deg in newborns, approximately constant at −26 deg for ages 3–6 months, and −63 deg in adults.
C. Relationship between DPOAE suppression and acoustic admittance
We calculated 76 individual correlations (4 DPOAE variables × 6 YR variables × 3 frequencies, plus 4 DPOAE variables × ear-canal area), each including approximately 55 observations. Individual correlation coefficients generated between YR variables measured at the three relevant input frequencies and DPOAE STC features are shown in Table I. Only two correlations were significant at the 0.01 level and those produced R2 values of 0.18 and 0.25 (Table I).
As seen in Table I, the strongest relationship was noted between STC tip-to-tail and admittance and one significant correlation, YP at 5700 Hz, accounted for as much as 25% of the variance in STC tip-to-tail (Fig. 6). The other significant correlation, susceptance at 5700 Hz, accounted for 18%. Because STC tip-to-tail ratio showed the highest correlations with YR variables, a multiple regression was conducted with significant YR variables and ear-canal area to see whether the variance of the tip-to-tail value could be better explained using two or more features combined. Ear-canal area was included as an input because of its importance in describing DPOAE reverse transmission (Keefe and Abdala, 2007). A forward stepwise procedure was performed to calculate the multiple linear regression. Results showed that only YP at 5700 Hz was included in the final model. Thus, the regression model was not significantly better with the addition of any other variables. Cumulatively, these analyses indicate that acoustic admittance variables are not strong predictors of DPOAE suppression tuning during the first 6 months of life; that is, the most variance accounted for by any particular admittance variable was 25%.
FIG. 6.
The strongest correlation was observed between admittance phase (YP) at 5700 Hz and DPOAE STC tip-to-tail value. R=0.49.
IV. DISCUSSION
A. Maturational time course for DPOAE suppression
Because the DPOAE is a cochlear assay, we originally hypothesized that DPOAE suppression would be adultlike by 6 months of age, when the cochlea is expected to be functionally mature in humans based on anatomical, electro-physiological, and psychoacoustic data (Abdala and Folsom, 1995; Lavigne-Rebillard and Pujol, 1987, 1988; Spetner and Olsho, 1990). However, results from the present experiment did not support this hypothesis. DPOAE STCs at f2 =6000 Hz were not adultlike at 6 months of age and probably remain immature well beyond this age because there was no significant shift toward adult values in these data (see Figs. 1 and 2).
Although our primary research objective was to define the maturational time course for DPOAE suppression at f2 =6000 Hz, we were unable to do so based on the age range selected for study. Clearly, maturation of DPOAE suppression tuning occurs some time between 6 months of age and adulthood. By considering DPOAE STC results collected from school-aged children in a previous experiment (Abdala and Fitzgerald, 2003), it is possible to further narrow the time frame. Abdala and Fitzgerald reported that normal-hearing children with a mean age of 10.5 years (range=6.5 to 13 years) had generally adultlike DPOAE STC features at f2 =6000 Hz. In Fig. 2, the asterisk to the right of the adult data represents the mean value from this group of 15 normal-hearing children for the four DPOAE suppression features studied here. Clearly, DPOAE suppression is adultlike in these children. Therefore, we can assume that DPOAE suppression tuning at f2 =6000 Hz becomes mature sometime between 6 months and approximately 10 years of age. Limited data from the present experiment at f2 =2000 Hz, and data from previously published experiments at f2 =1500 Hz, suggest that low-mid-frequency DPOAE suppression is adultlike at birth or soon thereafter. Thus, we are observing an immaturity in auditory peripheral function that is present only in the high-frequency range.
B. Relationship between DPOAE suppression and acoustic admittance/reflectance
The second objective of this study was to assess whether changes in DPOAE suppression during maturation could be explained by changes in YR responses. To answer this question, the maturation of both relevant components, DPOAE suppression and admittance/reflectance, was examined for significant associations. A strong association would support the hypothesis that changes in admittance over time might account for changes in DPOAE suppression over time. It would then be reasonable to speculate that the oft-reported immaturities in DPOAE suppression are explained by changes of admittance, indicative of middle-ear rather than cochlear immaturity.
None of the DPOAE variables measured showed significant change across session, and any change that is evident from data presented in Fig. 2 clearly occurred from birth to 3 months of age, with little movement beyond this age. At the completion of this test protocol, 6 months of age, DPOAE suppression features remained non-adultlike; thus, we measured suppression during a fairly static period in development that was too short to capture the transition to adultlike functioning.
Data presented in Fig. 2 showing DPOAE suppression features, and in Fig. 5 showing admittance features as a function of age, suggest a complex relationship between these two sets of variables. Admittance data measured in the same frequency range, for the same infants, shows systematic, incremental growth from birth through 6 months of age for YM at all three frequencies shown and for YP, at 2800 Hz. This steady shift with age is in contrast to the lack of change in DPOAE suppression with age and appears to imply a dissociation between the maturational time course of these two indices over the first 6 months of life. At the same time, YP at 5700 Hz shows a pattern of change similar to what is evident for some DPOAE suppression features as seen in Fig. 2. The greatest shift is noted from birth through 3 months of age, and values remain static values beyond this age. Neither DPOAE suppression nor admittance features are mature by 6 months. Clearly, whatever underlying maturational processes ultimately produce adultlike DPOAE suppression tuning and adultlike admittance in infants occur some time after the first half-year of postnatal life.
The linear correlations conducted between suppression and admittance data from birth through 6 months of age were not strong. Only 2 of 76 correlations were significant and their YR features accounted for 18% and 25% of the variance in DPOAE suppression. The stronger correlation between YP at 5700 Hz and tip-to-tail value was intriguing. It suggests that YP may encode information on forward middle-ear transmission near the f2 frequency (5700 Hz), and that the variance in this transmission across subjects explains some of the variance observed in the STC tip-to-tail ratio. This DPOAE STC feature has been hypothesized to reflect cochlear amplifier gain (Mills, 1998; Gorga et al., 2002; Pienkowski and Kunov, 2001). If DPOAE STC tip-to-tail values are related to cochlear amplifier gain, and correlated with middle-ear admittance, it follows that middle-ear function must be related to cochlear amplifier gain as well. This is logical given that the cochlear amplifier functions in a level-dependent manner and the middle ear greatly influences input level (forward transmission) to the cochlea (Abdala and Keefe, 2006).
Mills (1998) suggests a strong link between DPOAE STC tip-to-tail in gerbils and estimates of cochlear amplifier gain. Through a series of model calculations and subsequent application to actual measurements in gerbils, Mills showed that this STC index can provide a fairly accurate estimate of amplifier gain. In humans, results have not been as definitive (Gorga et al., 2002; Pienkowski and Kunov, 2001). Pienkowski and Kunov (2001) tested this hypothesis in humans by correlating the DPOAE STC tip-to tail values with audiometric hearing thresholds in normal-hearing individuals. They found low-to-moderate negative correlations, suggesting that the tip-to-tail index reflects cochlear amplifier gain to a limited extent in humans. Further study is needed to unravel the functional relationships underlying the link found in the present study between admittance phase around f2 and STC tip-to-tail values.
The generally modest correlations observed here suggest that YR variables did not explain most of the variance observed in DPOAE suppression during the first 6 months of life. One factor that has not been well defined, and may have contributed to the modest correlations, is repeatability of infant DPOAE suppression and YR measurements. Additionally, although YR responses as a whole were not greatly successful in predicting changes in DPOAE suppression tuning, they may explain maturation of DPOAE suppression later in life, once suppression begins to shift toward adultlike values. This is a possibility, although the final stage of maturation for DPOAE suppression and admittance does not appear to coincide closely. DPOAE suppression tuning is adult-like by at least 10 years of age as shown by the children’s mean data included in Fig. 2 (asterisk), whereas acoustic admittance is reported to remain immature beyond 11 years of age (Okabe et al., 1988). One later contributor to this immaturity is ear-canal growth, which likely continues through adolescence.
C. Source of immaturity
How can we explain immature DPOAE suppression tuning so late in development? Morphological and anatomical data from humans suggest that the cochlea is mature early in gestation (21–23-weeks gestational age) and the OHC completes its final maturation sometime late in the third trimester or possibly around the time of birth. This late maturation appears to involve OHC synaptic specializations and innervation by medial efferent fibers (Lavigne-Rebillard and Pujol, 1987, 1988) The early peripheral maturation that these studies describe does not provide support for the hypothesis that cochlear function remains immature into the sixth post-natal month.
It is perhaps more parsimonious to consider segments of the auditory system that show documented postnatal maturation, such as the middle ear or the medial olivocochlear (MOC) reflex. There is compelling evidence that middle-ear transmission properties remain immature at age 6 months and can partially account for the non-adultlike morphology of newborn STCs at f2 =6000 Hz (Abdala and Keefe, 2006; Keefe and Abdala, 2007). We have shown that once these transmission properties are compensated for, adult and infant STCs become very similar in morphology. Thus, it is clear that middle-ear transmission properties are contributing to STC morphology in infants up to age 6 months. More research is needed to better understand the influences of functional immaturity in older infants.
The MOC reflex exerts its influence on cochlear function via the OHCs (Kujawa et al., 1993; Kujawa and Liberman, 2001) and remains immature into the early postnatal period (Abdala et al., 1999; Morlet et al., 1993; Ryan and Piron, 1994). It is possible that immaturities in this feedback loop might produce immaturities in DPOAE suppression tuning. One possible argument against this, is the observation that the most robust MOC reflex is seen at low-to-mid frequencies and not in the frequency range where DPOAE suppression immaturities are strongest. Studies with laboratory animals have not reported this frequency effect (Puel and Rebillard, 1990), and some have observed the opposite trend, finding that high-frequency stimuli evoked the strongest MOC reflex (Varghese et al., 2005). At present, it is not certain whether the MOC reflex in humans shows a true frequency-dependent nature, or whether results are related to the stimulus used to elicit medial efferent activity. Contralateral broadband noise may not evoke equal firing in all efferent fibers (Moulin et al., 1993). There have been no studies examining maturation of the MOC reflex in human infants beyond the newborn period, so it is not known if its maturational course is similar to that of DPOAE suppression tuning. Because of its strong potential influence on cochlear mechanics and the possibility that it shows a postnatal maturational time course, the relationship between development of the MOC reflex and DPOAE suppression tuning should be further explored.
Finally, relatively recent research has confirmed that the DPOAE recorded in the ear canal is a vector sum of at least two components arising at the overlap region between traveling waves evoked by f1 and f2 and the DP-site (Dhar et al., 2002; Kim, 1980; Talmadge et al., 1999). The results of the constructive and destructive interference between these components can be observed in DPOAE fine structure measured with high-resolution recordings of DPOAE level. There have been no published studies of DPOAE fine structure in infants. It is possible that DPOAEs measured in the infant ear canal are comprised of an immature distribution of sources relative to the adult DPOAE. An immaturity in the relative contribution of sources to the ear canal DPOAE could account for or be associated with immaturities of DPOAE suppression tuning. Middle-ear functioning can also affect DPOAE fine structure if multiple internal reflections are present (Puria, 2003; Talmadge et al., 1998).
V. CONCLUSIONS
DPOAE suppression tuning at f2 =6000 Hz remains immature until at least 6 months of age, indicating a postnatal immaturity in peripheral auditory function. The source of this immaturity is not clear, although several indices of auditory function, such as DPOAE fine structure, middle-ear transmission, acoustic reflectance/admittance responses, and the MOC reflex, are also developing into the postnatal period and should be further explored. The present study also examined the relationship between maturation of DPOAE suppression and middle-ear function in a group of infants followed longitudinally from birth through 6 months of age. DPOAE suppression at f2 =6000 Hz showed little maturation during the test period studied, although admittance at corresponding frequencies changed systematically over time. Both sets of variables showed the greatest shift toward adult values from birth through 3 months. Linear correlations between suppression and admittance variables were not strong in infants: 74 of 76 YR variables were not correlated with DPOAE suppression features and could explain none of their variance over the first 6 months of life. Admittance phase near the f2 frequency, a frequency at which middle-ear forward transmission would be expected to be important, explained 25% of the variance in STC tip-to-tail value. It is not known from these data whether a stronger association exists between maturation of DPOAE suppression and middle-ear function later in life during a time period in development when either or both responses are shifting toward adult values.
Acknowledgments
This research was supported by the NIH (NIDCD Grants DC003552 and DC003784) and the House Ear Institute. The authors would like to thank Dr. Ellen Ma for collection and management of infant data, and Dr. Rangasamy Ramanathan, Chief of Neonatology at the University of Southern California, for continued support of infant research. The lead programmer in implementing the reflectance software at BT-NRH was Dr. Denis F. Fitzpatrick.
Contributor Information
Carolina Abdala, House Ear Institute, 2100 West Third Street, Children’s Auditory Research and Evaluation Center, Los Angeles, California 90057.
Douglas H. Keefe, Boys Town National Research Hospital, Omaha, Nebraska 68131
Sandra I. Oba, House Ear Institute, 2100 West Third Street, Children’s Auditory Research and Evaluation Center, Los Angeles, California 90057
References
- Abdala C. A developmental study of DPOAE (2f1-f2) suppression in humans. Hear Res. 1998;121:125–138. doi: 10.1016/s0378-5955(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Abdala C. Maturation of human cochlear amplifier function: DPOAE (2f1-f2) ipsilateral suppression at low- and high-levels in human adults and neonates. J Acoust Soc Am. 2001;110:1465–1476. doi: 10.1121/1.1388018. [DOI] [PubMed] [Google Scholar]
- Abdala C. Distortion product otoacoustic emission (2f1-f2) suppression in 3-month-old infants: Evidence for postnatal maturation of human cochlear function? J Acoust Soc Am. 2004;116(6):3572–3580. doi: 10.1121/1.1811472. [DOI] [PubMed] [Google Scholar]
- Abdala C. Effects of aspirin on distortion product otoacoustic emissions suppression in adults: A comparison with neonatal data. J Acoust Soc Am. 2005;118:1566–1575. doi: 10.1121/1.1985043. [DOI] [PubMed] [Google Scholar]
- Abdala C, Fitzgerald T. Ipsilateral distortion product otoacoustic emission (2f1-f2) suppression in children with sensorineural hearing loss. J Acoust Soc Am. 2003;114:919–931. doi: 10.1121/1.1587147. [DOI] [PubMed] [Google Scholar]
- Abdala C, Folsom R. Frequency contribution to the click-evoked auditory brainstem response in human adults and infants. J Acoust Soc Am. 1995;97:2394–2404. doi: 10.1121/1.411961. [DOI] [PubMed] [Google Scholar]
- Abdala C, Keefe DH. Effects of middle-ear immaturity on distortion-product otoacoustic emission suppression tuning in infant ears. J Acoust Soc Am. 2006;120:3832–3842. doi: 10.1121/1.2359237. [DOI] [PubMed] [Google Scholar]
- Abdala C, Ma E, Sininger Y. Maturation of medial efferent system function in human. J Acoust Soc Am. 1999;105:2392–2402. doi: 10.1121/1.426844. [DOI] [PubMed] [Google Scholar]
- Abdala C, Sininger Y, Eyelid M, Zeng FG. Distortion product otoacoustic emission suppression tuning curves in human adults and neonates. Hear Res. 1996;98:38–53. doi: 10.1016/0378-5955(96)00056-1. [DOI] [PubMed] [Google Scholar]
- Dhar S, Talmadge C, Long G, Tubis A. Multiple internal reflections in the cochlea and their effect on DPOAE fine structure. J Acoust Soc Am. 2002;112:2882–2897. doi: 10.1121/1.1516757. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Dorn PA, Konrad-Martin D. The use of distortion product otoacoustic emission suppression as an estimate of response growth. J Acoust Soc Am. 2002;111:271–284. doi: 10.1121/1.1426372. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing. Joint Committee on Infant Hearing Year 2000 Position Statement. Principles and guidelines for early hearing detection and intervention programs. Audiology Today. 2000;12:6–27. [PubMed] [Google Scholar]
- Keefe DH. Influence of Middle-Ear Function and Pathology on Otoacoustic Emissions. In: Robinette MR, Glattke TJ, editors. Otoacoustic Emissions: Clinical Applications. 3. Chap. 7 Thieme; New York: 2007. [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am. 2007;121(2):978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Levi E. Maturation of the middle and external ears: Acoustic power-based responses and reflectance tympanometry. Ear Hear. 1996;17:361–373. doi: 10.1097/00003446-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Hoberg Arehart K, Burns EM. Ear-canal impedance and reflection coefficient of human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Campbell S, Burns EM. Pressure transfer function and absorption cross-section from the diffuse field to the human infant ear canal. J Acoust Soc Am. 1994;95:355–371. doi: 10.1121/1.408380. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Folsom RC, Gorga MP, Vohr BR, Bulen JC, Norton SJ. Identification of neonatal hearing impairment: Ear-canal measurements of acoustic admittance and reflectance in neonates. Ear Hear. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Simmons JL. Energy transmittance predicts conductive hearing loss in older children and adults. J Acoust Soc Am. 2003;114(6):3217–3238. doi: 10.1121/1.1625931. [DOI] [PubMed] [Google Scholar]
- Kim D. Cochlear mechanics: Implications of electrophysiological and acoustical observations. Hear Res. 1980;22:95–104. doi: 10.1016/0378-5955(80)90064-7. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. Contralateral sound suppresses distortion product otoacoustic emissions through cholinergic mechanisms. Hear Res. 1993;68:97–106. doi: 10.1016/0378-5955(93)90068-c. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Effects of olivocochlear feedback on distortion product otoacoustic emissions in guinea pig. J Assoc Res Otolaryngol. 2001;2:268–278. doi: 10.1007/s101620010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Surface aspects of the developing human organ of Corti. Acta Oto-Laryngol, Suppl. 1987;436:43–50. doi: 10.3109/00016488709124975. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M, Pujol R. Hair cell innervation in the fetal human cochlea. Acta Oto-Laryngol. 1988;105:398–402. doi: 10.3109/00016488809119492. [DOI] [PubMed] [Google Scholar]
- Mills D. Interpretation of distortion product otoacoustic emission measurements. II. Estimating tuning characteristics using three stimulus tones. J Acoust Soc Am. 1998;103:507–523. doi: 10.1121/1.421101. [DOI] [PubMed] [Google Scholar]
- Morlet T, Collet L, Salle B, Morgon A. Functional maturation of cochlear active mechanisms and of the medial olivocochlear system in humans. Acta Oto-Laryngol. 1993;113:271–277. doi: 10.3109/00016489309135808. [DOI] [PubMed] [Google Scholar]
- Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters acoustic distortion products in humans. Hear Res. 1993;65:193–210. doi: 10.1016/0378-5955(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Okabe K, Tanaka S, Hamada H, Miura T, Funai H. Acoustic impedance measurement on normal ears of children. J Acoust Soc Jpn. 1988;9:287–294. [Google Scholar]
- Pienkowski M, Kunov H. Suppression of distortion product otoacoustic emissions and hearing threshold. J Acoust Soc Am. 2001;109:1496–1502. doi: 10.1121/1.1354202. [DOI] [PubMed] [Google Scholar]
- Puel JL, Rebillard G. Effect of contralateral sound stimulation on the distortion product 2F1-F2: Evidence that the medial efferent system is involved. J Acoust Soc Am. 1990;87:1630–1635. doi: 10.1121/1.399410. [DOI] [PubMed] [Google Scholar]
- Puria S. Measurements of human middle ear forward and reverse acoustics: Implications for otoacoustic emission. J Acoust Soc Am. 2003;113:2773–2789. doi: 10.1121/1.1564018. [DOI] [PubMed] [Google Scholar]
- Ryan S, Piron J. Functional maturation of the medial efferent olivocochlear system in human neonates. Acta Oto-Laryngol. 1994;114:485–489. doi: 10.3109/00016489409126091. [DOI] [PubMed] [Google Scholar]
- Spetner N, Olsho L. Auditory frequency resolution in human infancy. Child Dev. 1990;63:632–652. [PubMed] [Google Scholar]
- Talmadge C, Tubis A, Long G, Piskorski P. Modeling otoacoustic emission and hearing threshold fine structures. J Acoust Soc Am. 1998;104:1517–1543. doi: 10.1121/1.424364. [DOI] [PubMed] [Google Scholar]
- Talmadge C, Tubis A, Long G, Piskorski P. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J Acoust Soc Am. 1999;105:275–292. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Zhu X, Frisina RD. Age-related declines in distort product otoacoustic emissions utilizing pure tone contralateral stimulation in CBA/CaJ mice. Hear Res. 2005;209:60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]