Abstract
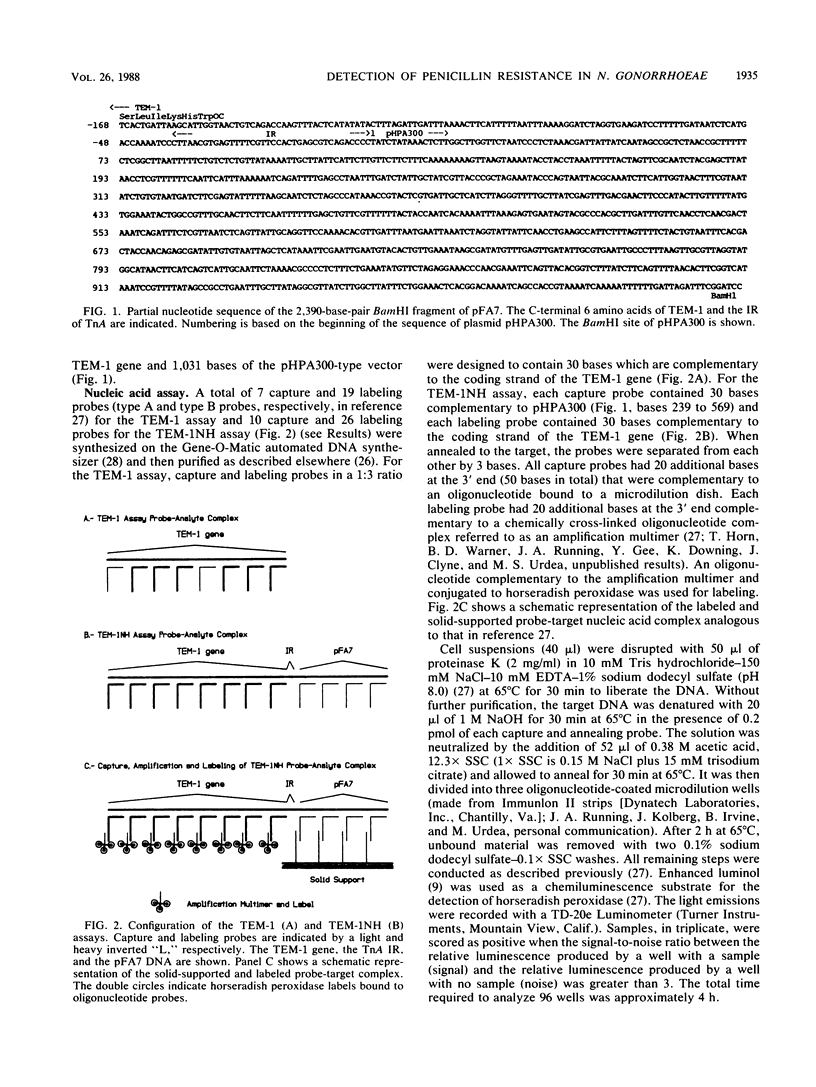
Two new assays for the detection of TEM-1 beta-lactamase-mediated bacterial penicillin resistance were developed that involve the use of specific nucleic acid hybridization. Both techniques are based on a solution-phase hybridization of oligonucleotide probes to the target DNA sequence, solid-phase capture of the probe-target complex, and an amplified chemiluminescent labeling method. One configuration of hybridization probes detected the presence of TEM-1 in Neisseria gonorrhoeae (45 strains), Haemophilus spp., Escherichia coli, Shigella sonnei and Salmonella typhi. A second configuration (TEM-1NH) detected TEM-1 beta-lactamase-mediated penicillin resistance only in N. gonorrhoeae (97 strains) and Haemophilus (6 strains) isolates in which TEM-1 is inserted in a pFA7-type plasmid. Both methods were 100 times more sensitive than a commercially available colorimetric beta-lactamase activity test and approximately 5 times more sensitive than radioisotopic dot blot screening for the gene. The assays are particularly well suited to the analysis of large numbers of samples, can be performed in a total of 4 h, and are sensitive to 10(4) to 10(5) CFU.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalen R. B., Gundersen W. B. Molecular characterization and comparison of plasmid content in seven different strains of Neisseria gonorrhoeae. Acta Pathol Microbiol Immunol Scand B. 1987 Feb;95(1):13–21. doi: 10.1111/j.1699-0463.1987.tb03081.x. [DOI] [PubMed] [Google Scholar]
- Brunton J., Clare D., Meier M. A. Molecular epidemiology of antibiotic resistance plasmids of Haemophilus species and Neisseria gonorrhoeae. Rev Infect Dis. 1986 Sep-Oct;8(5):713–724. doi: 10.1093/clinids/8.5.713. [DOI] [PubMed] [Google Scholar]
- Brunton J., Meier M., Erhman N., Clare D., Almawy R. Origin of small beta-lactamase-specifying plasmids in Haemophilus species and Neisseria gonorrhoeae. J Bacteriol. 1986 Oct;168(1):374–379. doi: 10.1128/jb.168.1.374-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón E., Conde C. J., de la Cruz R., Narcio L., Hirata C. Treatment of ordinary and penicillinase-producing strains of Neisseria gonorrhoeae in Mexico City. Diagn Microbiol Infect Dis. 1987 Sep;8(1):13–18. doi: 10.1016/0732-8893(87)90041-1. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Nucleotide sequence comparisons of plasmids pHD131, pJB1, pFA3, and pFA7 and beta-lactamase expression in Escherichia coli, Haemophilus influenzae, and Neisseria gonorrhoeae. J Bacteriol. 1987 Jul;169(7):3124–3130. doi: 10.1128/jb.169.7.3124-3130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Cooney M. H., Blackman E., Sparling P. F. In vitro antimicrobial susceptibility of penicillinase-producing and intrinsically resistant Neisseria gonorrhoeae strains. Antimicrob Agents Chemother. 1983 Oct;24(4):597–599. doi: 10.1128/aac.24.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Genetic analysis and penicillin-binding protein alterations in Neisseria gonorrhoeae with chromosomally mediated resistance. Antimicrob Agents Chemother. 1986 Nov;30(5):649–652. doi: 10.1128/aac.30.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T. L., Kraus S. J. Quantitation of Neisseria gonorrhoeae from women with gonorrhea. J Infect Dis. 1976 Jun;133(6):621–626. doi: 10.1093/infdis/133.6.621. [DOI] [PubMed] [Google Scholar]
- Martel A. Y., Gosselin P., Ouellette M., Roy P. H., Bergeron M. G. Isolation and molecular characterization of beta-lactamase-producing Haemophilus parainfluenzae from the genital tract. Antimicrob Agents Chemother. 1987 Jun;31(6):966–968. doi: 10.1128/aac.31.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Transfer of plasmid-mediated ampicillin resistance from Haemophilus to Neisseria gonorrhoeae requires an intervening organism. Sex Transm Dis. 1986 Jul-Sep;13(3):145–150. doi: 10.1097/00007435-198607000-00006. [DOI] [PubMed] [Google Scholar]
- Rice R. J., Thompson S. E. Treatment of uncomplicated infections due to Neisseria gonorrhoeae. A review of clinical efficacy and in vitro susceptibility studies from 1982 through 1985. JAMA. 1986 Apr 4;255(13):1739–1746. [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Urdea M. S. Use of unpurified synthetic deoxynucleotide primers for rapid dideoxynucleotide chain termination sequencing. DNA. 1984 Aug;3(4):339–343. doi: 10.1089/dna.1.1984.3.339. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfass D. A., Mendelman P. M., Chaffin D. O., Needham C. A. Ampicillin resistance and penicillin-binding proteins of Haemophilus influenzae. J Gen Microbiol. 1986 Oct;132(10):2855–2861. doi: 10.1099/00221287-132-10-2855. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Urdea M. S. Design, chemical synthesis, and molecular cloning of a gene for human epidermal growth factor. Methods Enzymol. 1987;146:22–41. doi: 10.1016/s0076-6879(87)46006-0. [DOI] [PubMed] [Google Scholar]
- Urdea M. S., Running J. A., Horn T., Clyne J., Ku L. L., Warner B. D. A novel method for the rapid detection of specific nucleotide sequences in crude biological samples without blotting or radioactivity; application to the analysis of hepatitis B virus in human serum. Gene. 1987;61(3):253–264. doi: 10.1016/0378-1119(87)90189-2. [DOI] [PubMed] [Google Scholar]
- Warner B. D., Warner M. E., Karns G. A., Ku L., Brown-Shimer S., Urdea M. S. Construction and evaluation of an instrument for the automated synthesis of oligodeoxyribonucleotides. DNA. 1984 Oct;3(5):401–411. doi: 10.1089/dna.1984.3.401. [DOI] [PubMed] [Google Scholar]
- Yeung K. H., Dillon J. R., Pauzé M., Wallace E. A novel 4.9-kilobase plasmid associated with an outbreak of penicillinase-producing Neisseria gonorrhoeae. J Infect Dis. 1986 Jun;153(6):1162–1165. doi: 10.1093/infdis/153.6.1162. [DOI] [PubMed] [Google Scholar]
- Zyzik E., Gerlich W. H., Uy A., Köchel H., Thomssen R. Assay of hepatitis B virus genome titers in sera of infected subjects. Eur J Clin Microbiol. 1986 Jun;5(3):330–335. doi: 10.1007/BF02017791. [DOI] [PubMed] [Google Scholar]