Abstract
The time-course of responses to repeated presentations of affective stimuli is well characterized in healthy individuals but remains to be characterized in patients with bipolar disorder. Using functional magnetic resonance imaging (fMRI), we compared early- and late-stage brain activation during a two-block fearful face perception task in 14 adult bipolar patients to that of 13 healthy controls. Whereas control subjects showed increased orbitofrontal, anterior cingulate, and striatum activity during the late (versus early) stage of the task, bipolar patients failed to show normal task-related activity in these regions. Results suggest that bipolar disorder may involve cortico-striatal dysfunction.
Keywords: FMRI, Neuroimaging, Bipolar Disorder, Mania, Mood, Prefrontal Cortex, Limbic System, Striatum, Basal Ganglia
Bipolar Disorder is a severe psychiatric illness characterized by affective instability, extreme mood fluctuations [1], and neurocognitive deficits that implicate dysfunction of frontal, striatal, and limbic networks [2-4]. Bipolar patients may also have deficits in affective perception and emotional labeling [5,6], which may be present early in the course of the illness [7]. Functional neuroimaging studies have shown that during the perception of facial affect, patients with bipolar disorder have reduced prefrontal activity and elevated amygdala responses relative to healthy controls [6,8], suggesting prefrontal disinhibition of limbic circuits during the processing of facial affect.
Affective processing is not a static phenomenon, as healthy individuals show changes in activity within specific neural structures during repeated presentations of emotional stimuli [9]. In healthy volunteers, there is habituation of the amygdala and prefrontal cortex during very rapid and repetitive presentation of affective faces [10]. Interestingly, repetition of such stimuli also leads to response-facilitation in the ability to make gender discriminations of previously seen faces, which correlates with increased activation of the caudate nucleus in healthy individuals. Thus, striatal activity in response to repeated stimuli may reflect normal response learning [11]. Although well delineated in healthy subjects, the effects of repeated affective stimulation have not been examined in patients with bipolar disorder. In the present study, we attempted to characterize how these patients process emotional stimuli over time. We directly compared early versus late-stage functional activity during an emotional face perception task in a sample of bipolar patients during their first hospitalization to an age matched healthy control sample. It was hypothesized that patients would show differences in early versus late stage blood oxygen level dependent (BOLD) signal changes within the prefrontal cortex, amygdala, and striatum relative to controls during affect perception.
Methods
Subjects
Fourteen patients (Mage = 28.1; SD = 11.2) meeting criteria for bipolar disorder (11 male; 3 female) during their first inpatient psychiatric admission underwent scanning very early in their hospitalization (average of 9 days following admission). Diagnoses were based on the Structured Clinical Interview for DSM-III-R — Patient Version (SCID-P) [12]. Patients were clinically stable on the day of scanning, with 85.7% of the sample taking an atypical antipsychotic agent (olanzapine or risperidone); 35.7% taking a mood stabilizer (lithium or depakote); 28.6% taking a benzodiazepine (ativan or klonopin); 7.1% taking an antidepressant (sertraline); and 7.1% taking a beta blocker (atenolol). The mean score of patients on the Hamilton Rating Scale for Depression was 15.6 (SD = 9.9) and was 14.3 (SD = 8.9) on the Young Mania Rating Scale. Patients had a mean of 14.3 years of education. Patients were excluded if they had previous psychiatric hospitalizations, duration of illness exceeding one year, or treatment with mood stabilizers or antipsychotics exceeding a total of 3 months. Thirteen healthy (Mage = 25.5; SD = 4.7) age-matched control participants (12 male, 1 female) with no psychiatric history and a mean of 15.6 years of education were recruited. Participants provided written consent after a thorough explanation of the procedures. A nominal financial compensation was provided for participation.
FMRI Stimulation Paradigms
Participants completed a fearful face perception task while undergoing fMRI. These tasks and procedures have been described in detail elsewhere [13-15]. Briefly, the task involved passive viewing of a series of black and white photographs of faces expressing fear [16]. The study followed an alternating block design, beginning with 30 seconds of resting fixation that alternated with subsequent 30-second blocks of faces and fixation rest conditions. Each face was presented for 9.5 seconds, separated by a 0.5 second inter-stimulus interval. Blocks alternated five times for a total of 150 seconds. Participants were reminded that they would be asked questions about the photographs at the end of the scan. Face stimuli were projected by LCD video and viewed on a translucent screen at the end of the scanning bed via a mirror mounted on the head coil.
Neuroimaging Methods
Subjects were scanned on a 1.5 Tesla GE LX MRI scanner equipped with a quadrature RF head coil (TR = 3 sec, TE = 40 msec, flip angle = 90 degrees). Fifty echoplanar functional images were acquired for each subject across 21 coronal slices (7mm, 1mm gap), with a 20 cm field of view and an acquisition matrix of 64 × 64. This provided an in-plane resolution of 3.125 × 7 × 3.125 mm. Matched T1-weighted high-resolution images were also acquired for each subject.
Image Processing
Using standard realignment algorithms implemented in SPM99 [17], the images were motion corrected, convolved into the three-dimensional space of the Montreal Neurological Institute (MNI), spatially smoothed using a non-isotropic Gaussian kernel (full width half maximum [FWHM] = 9 mm), and resliced to 2×2×2 mm using sinc interpolation.
Statistical Analysis
Initially, fixed effects statistical contrasts were created between the baseline resting conditions and the active face perception conditions. These contrast images served as the input data for a series of second level random effects analyses to compare activity within the first and second active blocks within each diagnostic group separately (paired t-tests) and between diagnostic groups for each block separately (between groups t-tests). For whole brain analyses, activation was evaluated at p < .001 (uncorrected), k (extent) = 20 contiguous voxels. The resulting SPM99 [18] maps were displayed as maximum intensity projections in three dimensions. To reduce Type I error, the data were also examined at a whole brain False Discovery Rate correction of p < .05, as shown in Table 1. Additionally, mean fMRI activity during the entire task was extracted from two regions of interest (ROIs) and plotted separately for each group. The first ROI comprised the averaged activation across the basal gangila (i.e., caudate, putamen, pallidum), while the second included both amygdala, as defined by a published neuroanatomical atlas [19]. The mean parameter estimates representing signal covariation with Block A (controlling for Block B) and Block B (controlling for Block A) were extracted for each subject’s anatomical ROI and compared using mixed model analysis of variance and Bonferroni protected post-hoc comparisons (p < .05).
Table 1.
Local Maxima for Contrasts Between Block A and B in 13 Healthy Controls and 14 1st Episode Bipolar Patients.
| Analysis Condition Region |
Number of Voxels |
x | y | z | SPM {t} |
|---|---|---|---|---|---|
| Early vs Late Block Comparisons | |||||
| Control: A > B | |||||
| No Active Voxels | -- | -- | -- | -- | -- |
| Control: B > A | |||||
| L Inferior Orbital Frontal Gyrus | 182 | -22 | 14 | -18 | 6.18 |
| L Caudate | 146 | -10 | -2 | 22 | 6.12 |
| L Inferior Orbital Frontal Gyrus | 86 | -34 | 42 | -4 | 6.04 |
| L Posterior Cingulate Gyrus | 115 | -4 | -30 | 24 | 5.65 |
| R Pallidum | 122 | 16 | -4 | -4 | 5.00 |
| R Parahippocampal Gyrus | 65 | 16 | -6 | -30 | 4.79 |
| R Superior Orbital Frontal Gyrus | 29 | 20 | 42 | -12 | 4.71 |
| Bipolar: A > B | |||||
| No Active Voxels | -- | -- | -- | -- | -- |
| Bipolar: B > A | |||||
| R Hippocampus | 579 | 40 | -18 | -8 | 9.93* |
| L Cerebellum | 240 | -14 | -62 | -24 | 7.17* |
| L Postcentral Gyrus | 208 | -52 | -8 | 22 | 6.69* |
| L Cerebellum | 42 | -8 | -46 | -6 | 6.56* |
| L Superior Parietal Lobe | 170 | -14 | -64 | 44 | 6.06* |
| R Middle Frontal Gyrus | 81 | 34 | 28 | 46 | 5.97* |
| R Precu neus | 211 | 20 | -52 | 18 | 5.62* |
| L Parahippocampal Gyrus | 36 | -18 | -30 | -18 | 5.53* |
| L Fusiform Gyrus | 36 | -40 | -8 | -22 | 5.31* |
| L Hippocampus | 186 | -34 | -36 | -6 | 5.29* |
| R Insula | 34 | 36 | 32 | 8 | 5.22 |
| R Cerebellum | 147 | 10 | -60 | -18 | 5.12 |
| L Calcarine Cortex | 28 | -28 | -66 | 4 | 5.07 |
| L Middle Occipital Gyrus | 85 | -28 | -88 | 14 | 5.05 |
| R Precuneus | 40 | 14 | -58 | 44 | 4.87 |
| L Anterior Cingulate Gyrus | 22 | -14 | 22 | 22 | 4.26 |
| Diagnostic Group Comparisons | |||||
| Block A: Bipolar > Control | |||||
| No Active Voxels | -- | -- | -- | -- | -- |
| Block A: Control > Bipolar | |||||
| L Calcarine Cortex | 53 | -6 | -70 | 20 | 3.81 |
| Bloc k B: Bipolar > Control | |||||
| R Superior Temporal Pole | 34 | 54 | 14 | -18 | 3.87 |
| Block B: Control > Bipolar | |||||
| L Putamen | 693 | -16 | 12 | -4 | 4.82 |
| L Caudate | 242 | -20 | -6 | 22 | 4.60 |
| L Anterior Cingulate Gyrus | 106 | -8 | 34 | 12 | 4.35 |
| R Middle Frontal Gyrus | 40 | 42 | 24 | 36 | 4.33 |
| R Superior Temporal Pole | 133 | 34 | 14 | -32 | 4.21 |
| R Inferior Orbital Frontal Gyrus | 21 | 26 | 28 | -6 | 3.86 |
| L Medial Orbital Frontal Gyrus | 25 | -12 | 48 | -6 | 3.76 |
Note. L = left hemisphere; R = right hemisphere. Atlas coordinates are from the MNI standard atlas, such that x reflects the distance (mm) to the right or left of midline, y reflects the distance anterior or posterior to the anterior commissure, and z reflects the distance superior or inferior to the horizontal plane through the AC-PC line. All activated voxels are significant at p < .001 (uncorrected), with a spatial extent of 20 contiguous voxels.
Survives False Discovery Rate (FDR) correction for whole brain volume at p < .05, with a spatial extent of at least 20 contiguous voxels.
Results
Within Group Block Contrasts
For healthy control subjects viewing fearful faces, Table 1 shows that there were no regions that were more active during the first block of fearful faces (Block A) relative to the second block (Block B). In contrast, there was significantly greater activation within Block B relative to Block A. This activation included medially located regions such as the caudate, pallidum, posterior cingulate gyrus, and parahippocampal gyrus as well as a region of the lateral orbitofrontal cortex, (see Figure 1). For bipolar patients, there also were no regions that were more active during Block A than Block B (see Table 1 and Figure 1). In the patient group, however, Block B was associated with significantly greater activation distributed across a broad range of generally non-medial extra-striatal regions, including the hippocampus, parahippocampal gyrus, and cerebellum, as well as a several cortical regions including prefrontal, insular, parietal, and occipital lobes (see Table 1).
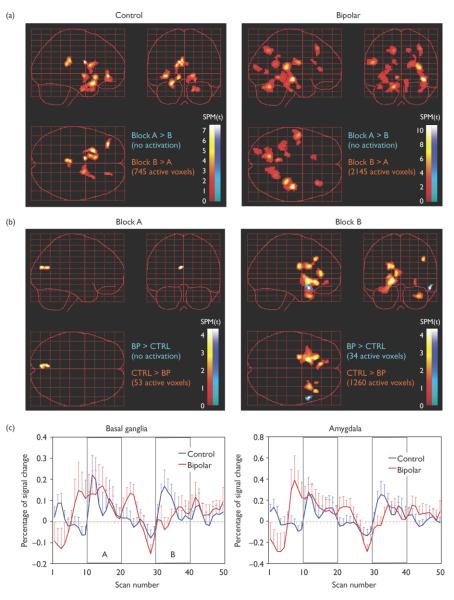
Figure 1.
Bipolar patients differed significantly in the pattern of early-versus late-stage activation in response to fearful faces. a) Control: B > A = basal ganglia, orbitofrontal cortex, and posterior cingulate; Bipolar: B > A = widespread posterior cortical regions. b) Block A: Controls > Bipolar Patients = left calcarine cortex; Block B: Control > Bipolar = striatum, anterior cingulate gyrus, and orbitofrontal cortex. Bipolar > Control = superior anterior temporal pole during Block B. c) Time-course plots showing the mean activation within the basal ganglia and amygdala during the task show clear and reliable task-related activation in healthy control subjects, whereas bipolar patients tended to show premature onset and delayed offset during the early block (block A) and minimal task-related activity during the late block (Block B).
Between Group Contrasts
Activation was contrasted between bipolar patients and healthy control subjects for each block separately. At Block A, there were no regions where bipolar patients showed greater activation than controls. Conversely, controls showed a small region of greater activation than the patients, which was localized in the left calcarine cortex (see Figure 1). For Block B, bipolar patients showed significantly greater activation than controls in one small region of the right superior temporal pole. In contrast, controls showed significantly greater activation than patients within a large region of left striatal regions including the left putamen and caudate nucleus, as well as cortical regions including the left anterior cingulate gyrus, medial orbital frontal gyrus and the right middle frontal gyrus, superior temporal pole, and inferior orbital frontal gyrus. None of these differences survived whole brain correction for multiple comparisons, however.
Regions of Interest
Figure 1 displays the extracted fMRI signal time course (percent signal change) across all conditions of the scan for the basal ganglia and amygdala separately in each group. Whereas a clear task-related “on-off” pattern can be observed in the time-courses of the healthy controls, bipolar patients showed premature onset of activation in both regions prior to Block A and delayed offset following discontinuation of the stimuli. Mixed model analysis of variance for the extracted signal from the basal ganglia showed a main effect of Block, F(1,25) = 8.43, p = .008, with greater activation during Block B, and a main effect of diagnosis, F(1,25) = 4.48, p = .045, with greater activation in the healthy controls relative to the patients. There was no significant interaction between Block and Diagnosis. For the amygdala, there was a main effect of Block, F(1,25) = 4.61, p = .042, a main effect of diagnosis, F(1,25) = 4.90, p = .036, and a significant Block × Diagnosis interaction, F(1,25) = 4.26, p = .049. Post-hoc comparisons showed no significant amygdala activation or change between Block A and B in patients, whereas controls showed a significant increase in amygdala activation from Block A to B (p = .007). Amygdala activation during Block B was greater in controls relative to patients (p = .006).
Discussion
During a fearful face perception task, patients with bipolar disorder showed a significantly different pattern of early-versus late-stage changes in BOLD signal relative to healthy controls. These differences suggest that in the early stage of the task, there were minimal group differences in whole brain activation, but over the course of the task, normal control subjects showed significant late-stage increases within the orbitofrontal cortex and striatum. Bipolar patients did not show this pattern. Instead they showed greater late-stage activation across a distributed network of posterior cortical and subcortical brain regions typically associated with perceptual and memory processes. Statistical comparison between the groups at this latter stage of the task suggest that bipolar patients showed significantly less task-related activation than controls within the putamen, caudate, anterior cingulate gyrus, orbitofrontal cortex, and superior temporal pole, regions that have been implicated in affective processing and its modulation [20,21], although these were not evident at a more stringent corrected threshold. Together, these findings suggest that during multiple presentations of faces expressing fear, healthy subjects show progressive activation of striatal regions involved in emotion-based learning and motivational control [22], possibly reflecting a process of affective-motivational-behavioral adaptation whereby affective information is learned and integrated with ongoing motivational routines to prepare the individual for appropriate action [11]. Conversely, bipolar patients failed to show this pattern, instead activating diffuse cortical areas involved in visual perception, memory encoding and recall, facial perception, and visceral sensation. Thus, these findings provide further evidence for a dysfunction within the fronto-striatal circuitry in patients with bipolar disorder [2,4,23].
Inspection of the ROI time-course plots clearly shows abnormal BOLD signal responses in the basal ganglia and amygdala in the bipolar group relative to the healthy controls. Whereas controls showed notable changes in signal intensity in both ROIs that coincided with the onset and offset of the stimuli, particularly during the second block, bipolar patients failed to show these task related increases in either ROI. The interaction between diagnostic group and stimulus Block was significant for the amygdala, suggesting that bipolar patients failed to show the increase in responsiveness upon later exposure to the emotional stimuli exhibited by the controls. Other possible differences between diagnostic groups, such as anticipatory activation and delayed offset of initial responses in the patient group were visually apparent, but these will require further research to substantiate. It remains unknown whether such response patterns are correlated with behavioral and cognitive features of bipolar disorder.
As with any study of actively medicated patients, these data are limited by potential differences in medication type, dosage, and current clinical state among the patients. Attempts were made to control for these factors at intake and by selecting only patients during their first psychiatric admission. Furthermore, due to the selection of only first admission patients, it is possible that there was some diagnostic heterogeneity in the sample that will only be evident longitudinally. It is also important to note that these findings should be considered as preliminary, as the whole brain activation was evaluated at an uncorrected height threshold of p < .001 and none of the whole brain imaging comparisons between diagnostic groups survived correction for multiple comparisons. Finally, this sample was small and will require replication. With due consideration given to these limitations, however, the present findings provide compelling preliminary evidence that first episode bipolar patients differ from healthy controls in the time-course of responsiveness of key affect processing regions to facial expressions of fear.
Conclusion
Compared to healthy controls, patients with bipolar disorder showed impaired late stage responsiveness of cortico-striatal regions to repeated visual presentations of fearful faces. Findings are consistent with previous evidence suggesting that bipolar disorder may involve cortico-striatal dysfunction and further suggest a potential neurobiological mechanism whereby patients may show anticipatory affective responses with slow habituation.
Acknowledgments
This project was supported by NIMH Grant # R01 MH069840 to Deborah A. Yurgelun-Todd
References
- [1].Phillips ML. The neural basis of mood dysregulation in bipolar disorder. Cognit Neuropsychiatry. 2006;11:233–249. doi: 10.1080/13546800444000290. [DOI] [PubMed] [Google Scholar]
- [2].Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151-103. [DOI] [PubMed] [Google Scholar]
- [3].Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [4].Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- [5].Getz GE, Shear PK, Strakowski SM. Facial affect recognition deficits in bipolar disorder. J Int Neuropsychol Soc. 2003;9:623–632. doi: 10.1017/S1355617703940021. [DOI] [PubMed] [Google Scholar]
- [6].Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- [7].Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, et al. Facial Emotion Labeling Deficits in Children and Adolescents at Risk for Bipolar Disorder. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- [8].Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- [9].Straube T, Weiss T, Mentzel HJ, Miltner WH. Time course of amygdala activation during aversive conditioning depends on attention. Neuroimage. 2007;34:462–469. doi: 10.1016/j.neuroimage.2006.08.021. [DOI] [PubMed] [Google Scholar]
- [10].Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- [11].Bunzeck N, Schutze H, Duzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- [12].Spitzer RL. Structured Clinical Interview for DSM-III-R -- Patient Version (SCID-P) New York State Psychiatric Institute, Biometrics Research; New York: 1988. [Google Scholar]
- [13].Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- [14].Killgore WDS, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- [15].Killgore WDS, Yurgelun-Todd DA. Neural correlates of emotional intelligence in adolescent children. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:140–151. doi: 10.3758/cabn.7.2.140. [DOI] [PubMed] [Google Scholar]
- [16].Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- [17].Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;5:189–201. [Google Scholar]
- [18].Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- [19].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- [20].Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- [21].Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, Smolka MN, et al. Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur J Neurosci. 2006;24:305–313. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- [22].Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- [23].Caetano SC, Olvera RL, Glahn D, Fonseca M, Pliszka S, Soares JC. Fronto-limbic brain abnormalities in juvenile onset bipolar disorder. Biol Psychiatry. 2005;58:525–531. doi: 10.1016/j.biopsych.2005.04.027. [DOI] [PubMed] [Google Scholar]