Abstract
The roles of astrocytes in the CNS have been expanding beyond the long held view of providing passive, supportive functions. Recent evidence has identified roles in neuronal development, extracellular matrix maintenance, and response to inflammatory challenges. Therefore, insights into astrocyte secretion are critically important for understanding physiological responses and pathological mechanisms in CNS diseases. Primary astrocyte cultures were treated with inflammatory cytokines for either a short (1 day) or sustained (7 days) exposure. Increased interleukin-6 secretion, nitric oxide production, cyclooxygenase-2 activation, and nerve growth factor (NGF) secretion confirmed the astrocytic response to cytokine treatment. MS/MS analysis, computational prediction algorithms, and functional classification were used to compare the astrocyte protein secretome from control and cytokine-exposed cultures. In total, 169 secreted proteins were identified, including both classically and nonconventionally secreted proteins that comprised components of the extracellular matrix and enzymes involved in processing of glycoproteins and glycosaminoglycans. Twelve proteins were detected exclusively in the secretome from cytokine-treated astrocytes, including matrix metalloproteinase-3 (MMP-3) and members of the chemokine ligand family. This compilation of secreted proteins provides a framework for identifying factors that influence the biochemical environment of the nervous system, regulate development, construct extracellular matrices, and coordinate the nervous system response to inflammation.
Keywords: Astrocytes, Comparative proteomics, Cytokines, Development, Protein secretion
1 Introduction
Appreciation for the function of astrocytes in the CNS has been growing with the identification of integral roles in neurogenesis and synaptogenesis [1, 2]. Specifically, astrocytic secretion of glutamate, ATP, and D-serine serve as paracrine and autocrine factors regulating synaptic plasticity and the coordination of synaptic networks [3, 4]. Astrocytes are also important components of the blood brain barrier, providing dynamic regulation of the microvasculature through the release of nitric oxide and lipid metabolites [5, 6], as well as modulating brain energy metabolism through the coordination of glutamate homeostasis between neurons and astrocytes [7, 8].
In contrast, the release of proteins by astrocytes under nondisease states has not been extensively explored. Proteins released by astrocytes include thrombospondin-1 and apolipoprotein E, which were found to mediate synaptogenesis and processing of amyloid-β peptides, respectively [9, 10]. The advent of MS-based proteomics has allowed for the global interrogation of astrocyte proteomes, including intra-cellularly expressed proteins as well as secreted proteins [11–14]. However, only a limited number of proteins have been detected in the astrocyte secretome. Therefore, an expanded proteome of astrocyte-secreted proteins employing recent advances in proteomic methodology, instrumentation, and computational analyses is warranted. A more comprehensive astrocyte secretome would provide new insights into astrocyte function and uncover novel mediators that can influence the extracellular biochemical environment in the CNS.
Astrocytes play a critical role in regulating the type and extent of CNS immune response by responding to inflammatory mediators such as interferon (IFN)-γ and TNF-α and by producing additional cytokines and chemokines [15]. While an inflammatory response is necessary following tissue and cellular injury, it is also seen as a central process in the development and progression of disease states. Under certain pathological conditions, recent studies provide evidence that astrocytes secrete factors that are toxic to other cells in the CNS. For example, in patients with multiple sclerosis, astrocytes expressing syncytin released factors that are toxic to oligodendrocytes [16]. Recently, soluble factors released from astrocytes that expressed familial amyotrophic lateral sclerosis (ALS)-causing mutant forms of superoxide dismutase 1-induced motor neuron death [17–20]. Collectively, these data emphasize that astrocytes under pathological conditions are capable of unleashing toxic, but in many cases unidentified factors. Insights into the factors secreted by astrocytes after treatment with inflammatory mediators may identify disease mediators and reveal targets for the therapy.
2 Materials and methods
2.1 Astrocyte culture and cytokine treatment
All mouse studies were reviewed and approved by the Institutional Animal Care and Use Committee of the Stokes Research Institute, Children’s Hospital of Philadelphia. Cortical astrocyte cultures were prepared from neonatal CD-1 mice (Charles River, Wilmington, MA) on DOL 1–2. Briefly, the animals were anesthetized, the brain was removed and cortex was dissected free. Cortices were washed twice with Earle’s Balanced Salt Solution (EBSS; Invitrogen, Carlsbad, CA) and trypsinized (0.05%) for 12 min at 37°C. Cortices were then titruated in Minimal Essential Media (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone), sodium pyruvate (1 mM), L-glutamine (2 mM), D-glucose (42 mM), sodium bicarbonate (14 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), fungizone (2.5 μg/mL) and plated at one cortex per T-25 vent-cap flask (Corning, Corning, NY). Mixed cortical cultures were raised for 14 days in 37°C and 5% CO2 with media change every 3–4 days. Cultures were then washed with cold EBSS and separated from neurons and microglia by shaking overnight at 37°C. Adherent cells were trypsinized (0.25%) and seeded in 100 mm Petri dishes (Corning) at 5×106 cells/plate (6 mL). Forty-eight hours after plating, cells were washed with EBSS, and serum-free media was added containing IL-1β (0.2 ng/mL), IFN-γ (1 ng/mL), and TNF-α (10 ng/mL) (Roche Pharmaceuticals, Switzerland). Control astrocytes were cultured in serum-free media alone. Astrocyte-conditioned media (ACM) was either collected after 24 h (1D ACM) or were either left untreated or treated with the cytokine cocktail (see above) every 48 h for an additional 6 days (three total exposures) with no media change. ACM was again collected (7 days ACM). For each collection, ACM was combined from two plates and centrifuged at 200×g for 5 min to remove cell debris. The protein fraction (>3 kDa) was obtained by ultra-filtration of ACM using CentriPrep Ultracel YM-3 filters (Millipore, Billerica, MA). The protein retentate (final vol. = 0.8–1.0 mL) was aliquoted and stored at −80°C for future use. Filtrates were reserved for lipid analyses. To assess cell viability, astrocytes were trypsinized and combined with cell pellets obtained from the low speed centrifugation of ACM. Cell death was quantified either by flow cytometry using Vybrant Apoptosis Assay Kit #3 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) or by trypan blue exclusion. For flow cytometry, a minimum of 20 000 events was required for each analysis performed in quadruplicate.
2.2 Gel/LC-MS/MS analysis of conditioned media
The protein fraction obtained from ACM was analyzed by Gel/LC-MS/MS as described previously [21]. For each treatment condition described above, the concentrated protein fraction was mixed with 6X Laemmli sample buffer and equal volumes (30 μL) were loaded on NuPAGE 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) and electrophoresed in MOPS running buffer for approximately 2 cm. For experiments that assessed reproducibility, ACM was collected and proteins were separated by electrophoresis from independent astrocyte cultures (biological duplicates) treated for 7 days with and without cytokines. Proteins were visualized by Colloidal Blue (Invitrogen, Carlsbad, CA) and each lane was cut into uniform slices using a MEF-1.5 Gel Cutter (The Gel Company, San Francisco, CA). Gel slices were digested in-gel with trypsin as previously described [22]. Tryptic digests were analyzed on an LTQ linear IT mass spectrometer (Thermo Electron, San Jose, CA) coupled with a NanoLC pump (Eksigent Technologies, Livermore, CA) and auto-sampler. Tryptic peptides were separated by RP-HPLC on a nanocapillary column, 75 μm id × 20 cm PicoFrit (New Objective, Woburn, MA, USA), packed with MAGIC C18 resin, 5 μm particle size (Michrom BioResources, Auburn, CA). Solvent A was 0.58% acetic acid in Milli-Q water, and solvent B was 0.58% acetic acid in ACN. Peptides were eluted into the mass spectrometer at 200 nL/min using an ACN gradient. Each RP-LC run consisted of a 10 min sample load at 1% B; a 75 min total gradient consisting of 1–28% B over 50 min, 28–50% B over 14 min, 50–80% B over 5 min, 80% B for 5 min before returning to 1% B in 1 min. To minimize carryover, a 36 min blank cycle was run between each sample. Hence, the total sample-to-sample cycle time was 121 min. The mass spectrometer was set to repetitively scan m/z from 375 to 1600 followed by data-dependent MS/MS scans on the ten most abundant ions with dynamic exclusion enabled.
2.3 Protein identification and validation
DTA files were generated from MS/MS spectra extracted from the RAW data file (intensity threshold of 5 000; minimum ion count of 30) and processed by the ZSA, CorrectIon, and IonQuest algorithms of the SEQUEST Browser program. Database searching was performed by TurboSE-QUEST v.27 (rev. 14) against the NCBI nonredundant protein database (4 379 558 proteins; 1/2007), which had been indexed with the following parameters: average mass range of 500–3500, length of 6–100, tryptic cleavages with one internal missed cleavage sites, static modification of Cys by carboxyamidomethylation (+57 amu), and variable modification of methionine (+16 amu). The DTA files were searched with a 2.5 amu peptide mass tolerance and 1.0 amu fragment ion mass tolerance. Potential sequence-to-spectrum peptide assignments generated by SEQUEST were loaded into Scaffold (version Scaffold-01_06_17, Proteome Software, Portland, OR) to validate MS/MS peptide and protein identifications as well as to compare protein identifications across experimental conditions. Peptide and protein probabilities were calculated by the Peptide and Protein Prophet algorithms [23, 24], respectively. A protein was identified if it received ≥99.0% protein confidence with ≥3 unique peptides at ≥95% confidence. A protein that received ≥99.0% protein confidence with two peptides at ≥50% probability was considered identified only if the same protein had been identified by the above criteria in another treatment group. If either of these criteria were not satisfied, the protein was considered to be low confidence and was scored as not detected. Proteins identifications not assigned to the Mus musculus taxonomy were manually inspected. These proteins were either contaminants and were removed in the final analysis, or contained identified peptides identical to the mouse sequence and therefore, based on rules of parsimony, were considered to be of mouse origin.
2.4 Computational and functional gene ontology analysis
NCBI database protein identifiers (gi) were matched to equivalent entries (accession) in the Uniprot database (www.uniprot.org), and if known, were reported as unprocessed precursors. Protein Prowler (http://pprowler.imb.uq.edu.au/) was used to identify proteins that possess a secretory pathway (SP) signal peptide (SP score>mTP/Other). Cytoscape/BiNGO was used to perform gene ontology (GO) assignments and determine significantly under-and over-represented functional GO categories. Cytoscape network visualization platform (ver 2.5; 7/23/2007; http://www.cytoscape.org/) implementing the latest release of the BiNGO plugin (ver 2.0; 1/17/2007;http://www.psb.ugent.be/cbd/papers/BiNGO/) [25] was used to identify proteins that were annotated to the extracellular space (GO: 5 576) and cell surface (GO: 9 986). Analyses were performed using the default BiNGO mouse annotation containing 19 224 members (1/12/2007; ftp://ftp.ncbi.nlm.nih.gov/gene/DATA/) and either the GOSlim_GOA or GO_Full ontology (12/18/2006; http://www.geneontology.org). Statistical significance was determined by hypergeomtric analysis followed by Benjamini and Hochberg false discovery rate (FDR) correction (p<0.001). SecretomeP 2.0 (http://www.cbs.dtu.dk/services/SecretomeP/) was used to evaluate proteins that may be nonclassically secreted (p>0.5) in conjunction with prior experimental evidence. The proteins designated as secretory/extracellular were assigned to broad functional categories relevant to extracellular functions. SledgeHMMER [26] was used to perform batch searching of the Pfam database, followed by conversion of Pfam entries to their equivalent InterPro domain (release 14.1; http://www.ebi.ac.uk/inter-pro/).
3 Results
3.1 Cytokine-induced responses of murine astrocytes
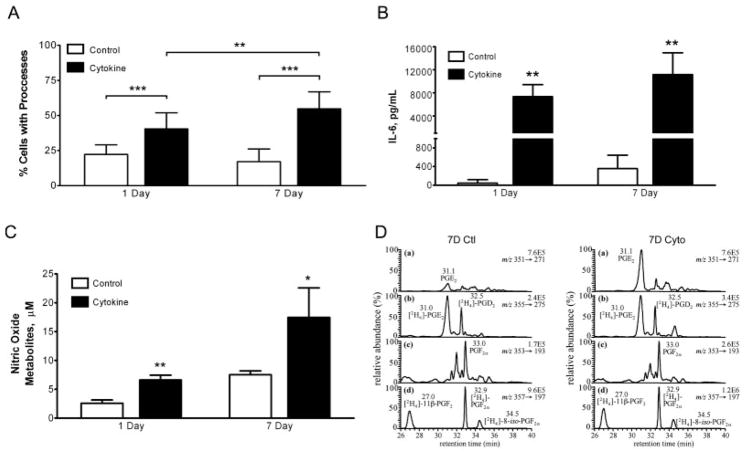
Enriched neonatal cortical astrocyte cultures were prepared as described in the Materials and Methods and were treated under serum-free conditions with either vehicle (control) or TNF-α (10 ng/mL), interleukin (IL)-1β (0.2 ng/mL), and IFN-γ (1 ng/mL) for either an acute (1 day) or sustained (7 days) exposure interval. Cell viability evaluated by flow cytometry after 1 day or 7 days did not significantly differ between control and cytokine-exposed cells (Fig. 1B of Supporting Information). Astrocyte activation by inflammatory mediators induced stereotypical morphological changes such as process formation and elongation (Fig. 1A of Supporting Information). These changes were quantified by counting the number of cells with processes, which revealed that 1 day and 7 days cytokine treatment significantly increased the percent of cells with processes compared to untreated cultures (Fig. 1A). In addition, the percent of cells with processes was also significantly increased from 1 day to 7 days of cytokine treatment (Fig. 1A).
Figure 1.
Morphological and biochemical responses of murine astrocytes to cytokine exposure. (A) Quantification of percent GFAP-positive cells with processes. The percent of cells with processes were calculated from 3–6 fields/condition taken from three independent experiments. **p<0.01, ***p<0.001 by unpaired, two-tailed t-test. (B) IL-6 production measured by ELISA in ACM. Data are reported as the mean±SD. **p<0.01 by unpaired, two-tailed t-test (n = 3–6). (C) Nitric oxide synthase (NOS) activity measured by nitric oxide metabolite accumulation in ACM. Metabolites were measured by reductive chemistries coupled to chemiluminescence. Data are reported as the mean±SD. *p<0.05, **p<0.01 by unpaired, two-tailed t-test (n = 3–6). (D) LC-electron capture APCI/MRM/MS analysis of PGE2 (a) and PGF2α (c) in ACM from 7 days control-treated (left) and 7 days cytokine-treated (right) astrocytes. Concentrations of PGE2 (retention time (rt) = 31.0 min) and PGF2α(rt = 33.0 min) were calculated by interpolation of linear regression curves constructed from authentic lipid standards. Variation due to sample processing and MS analysis was accounted for by addition of [2H4]-PGE2 (b) and [2H4]-PGF2α (d) internal standards.
Astrocytic responses to cytokine treatment were further characterized for each exposure interval by quantifying well-characterized markers of inflammation, namely IL-6, nitric oxide, and prostaglandin E2 (PGE2) in ACM (See Materials and Methods of Supporting Information). After 1 day and 7 days treatment with inflammatory mediators, robust production of IL-6 was detected in ACM compared to the control conditions (Fig. 1B). The concentration of nitric oxide metabolites measured by reductive chemistries coupled to chemiluminescence detection was significantly increased in ACM compared to controls following 1 day and 7 days inflammatory mediator treatment (Fig. 1C). Astrocytic responses to inflammatory mediators are also characterized by increased production of prostaglandins, such as prostaglandin E2 (PGE2), which is the most abundant prostanoid in the CNS. A lipidomic profile of 24 lipids was carried out on ACM using LC-electron capture atmospheric pressure chemical ionization/multiple reaction monitoring (APCI/MRM) MS analysis. Absolute quantification was performed against standard curves constructed using authentic lipids and normalized to deuterated lipid internal standards (Fig. 1D). These analyses revealed a selective increase of PGE2 (retention time (rt) = 31.0 min) after 7 days cytokine treatment (0.49 ± 0.03 pmol/106 cells to 1.50 ± 0.12 pmol/106 cells; Fig. 1D). In contrast, there was no difference in the levels of PGF2α (rt = 33.0 min) detected in ACM from control (0.12 ± 0.01 pmol/106 cells) and cytokine-treated (0.13 ± 0.01 pmol/106 cells) astrocytes (Fig. 1D).
While controlled inflammation is critical for innate immune defense as well as cellular remodeling and tissue repair, unregulated inflammation would clearly be detrimental. Therefore, glia, one of the primary immune cells in the CNS, possesses compensatory, anti-inflammatory mechanisms to limit the scope of inflammation. In particular, trophic factors such as nerve growth factor (NGF) have been identified as initiators of signaling cascades that promote anti-inflammatory processes following proinflammatory events [27]. Consistent with this mechanism, we detected significantly elevated levels of NGF only after 7 days cytokine treatment compared to 7 days control (203.2 ± 158.4 pg/mL vs. 964.2 ± 433.7 pg/mL; P = 0.0025). Collectively, morphological evaluation, flow cytometry analysis, and quantification of IL-6, nitric oxide, and lipid markers of inflammatory responses established the secretory capacity, viability, and stereotypical responses to inflammatory mediators.
3.2 Proteomic evaluation of the astrocyte secretome
Extracellular and secretory proteins play a fundamental role in transforming the extracellular space and facilitating cell–cell contacts, such as during development or after synaptic remodeling following brain injury [28]. While neuron–neuron communication has been an area of intense study during synaptogenesis, the capacity of astrocytes to influence this process, specifically through secreted proteins, is not completely understood. Toward this goal, we employed a proteomic approach to identify soluble proteins secreted by murine astrocytes under control and cytokine-treated conditions. The protein fraction of ACM from control or cytokine-treated cultures was obtained by ultrafiltration and was subjected to Gel/LC-MS/MS analysis. Briefly, proteins were separated by 1-D PAGE for approximately 2 cm and visualized by Colloidal blue (Fig. 2A of Supporting Information). Each lane was cut into 12 equal slices and digested in-gel with trypsin [22]. Tryptic digests were then analyzed by nanocapillary RP-LC interfaced directly with a linear IT mass spectrometer (Thermo LTQ) operated in data dependent mode [21]. MS/MS sequence-to-spectrum assignments were generated using the SEQUEST algorithm searching against the NCBI nr database. SEQUEST search results from the 12 LC-MS/MS runs that comprised a complete proteome, i.e, a complete gel lane, were combined into a single biological sample within Scaffold (Proteome Software, Portland, OR). Scaffold served as a validation tool, employing the PeptideProphet and ProteinProphet algorithms, which provide statistical evaluation of the SEQUEST results by expressing potential sequence-to-spectrum assignments as confidence scores [23, 24]. A protein was identified if it received ≥99.0% protein confidence with ≥3 unique peptides at ≥95% confidence. A protein that received ≥99.0% protein confidence with two peptides at ≥50% probability was considered identified only if the same protein had been identified by the criteria listed above in another treatment group. If either of these criteria were not satisfied, the protein was considered to be low confidence and was scored as not detected. In total, 290 proteins were identified across all treatment groups (Table 1, and Table 1 of Supporting Information).
Table 1.
Basal and cytokine-induced astrocyte secretome
| Protein name (Synonym) | Accessiona) | MW (kDa) | 1 day control | 1 day cytokine | 7 days control | 7 days cytokine |
|---|---|---|---|---|---|---|
| Unique peptidesb) | ||||||
| Extracellular Matrix and Adhesion | ||||||
| Agrin | A2ASQ0 | 205.0 | 6 | 26 | 8 | 27 |
| Basement membrane-specific heparan sulfate proteoglycan core protein | Q05793 | 398.3 | 3 | 18 | 17 | 46 |
| Cadherin-2 precursor (Neural-cadherin) | P15116 | 99.8 | 7 | 6 | 11 | 11 |
| Calsyntenin 1 | Q9EPL2 | 108.9 | 7 | 6 | 10 | 6 |
| Collagen alpha-1(I) chain precursor | P11087 | 138.0 | 3 | 5 | 9 | 6 |
| Collagen alpha-1(IV) chain precursor | P02463 | 160.7 | - | - | 2 | 3 |
| c) Collagen alpha-1(VI) chain precursor | Q04857 | 108.5 | - | 2 | - | 3 |
| d) Collagen alpha-1(XI) chain precursor | Q61245 | 181.0 | - | - | 2 | - |
| Collagen alpha-1(XII) chain precursor | Q60847 | 340.2 | 3 | 13 | 41 | 27 |
| Collagen alpha-2(I) chain precursor | Q01149 | 129.6 | - | 2 | 6 | 4 |
| Collagen alpha-2(V) chain precursor | Q3U962 | 145.0 | 4 | 12 | 9 | 7 |
| EGF-containing fibulin-like extracellular matrix protein 2 | Q9WVJ9 | 49.4 | 3 | 4 | 11 | 4 |
| Extracellular matrix protein | Q61508 | 62.8 | - | - | 7 | 4 |
| Fat 1 cadherin | Q9QXA3 | 506.0 | - | - | 8 | 4 |
| Fibromodulin precursor (FM) | P50608 | 43.1 | 4 | 5 | 6 | 6 |
| Fibronectin 1 | Q3UGY5 | 262.8 | 54 | 59 | 65 | 65 |
| Fibulin-5 precursor (FIBL-5) | Q9WVH9 | 50.2 | 4 | 2 | 8 | 3 |
| Galectin-3 (Galactose-specific lectin 3) | P16110 | 27.5 | - | 2 | 3 | 2 |
| Glypican-4 precursor (K-glypican) | P51655 | 62.6 | - | 3 | 6 | 6 |
| c) Intercellular adhesion molecule (Icam1) | Q922B3 | 58.9 | - | - | - | 4 |
| c) Laminin subU alpha-4 precursor | P97927 | 201.8 | - | - | - | 7 |
| Laminin subunit alpha-5 precursor | Q61001 | 404.0 | - | 7 | 2 | 23 |
| c) Laminin subunit beta-2 precursor (S-laminin) | Q61292 | 196.4 | - | 8 | - | 19 |
| Laminin subunit gamma-1 precursor (Laminin B2 chain) | P02468 | 177.3 | - | 11 | 4 | 25 |
| Legumain | A2RTI3 | 49.4 | 2 | 6 | 6 | 6 |
| Lysyl oxidase-like 3 | Q91VN8 | 83.7 | - | - | 3 | 2 |
| Mammalian ependymin related protein-2 | Q99M71 | 25.5 | 7 | 7 | 8 | 7 |
| Mimecan precursor (Osteoglycin) | Q62000 | 34.0 | - | 4 | 6 | 3 |
| Mini-agrin | Q5EBX5 | 103.4 | 2 | 2 | 2 | 3 |
| Neurocan core protein precursor (Chondroitin sulfate proteoglycan 3) | P55066 | 137.2 | 7 | 8 | 6 | 5 |
| Nidogen 2 protein | Q8R5G0 | 153.9 | 3 | - | 7 | 2 |
| SPARC-like protein 1 precursor (Matrix glycoprotein Sc1) | P70663 | 72.3 | - | 4 | 6 | 5 |
| Tenascin precursor (TN-C) | Q80YX1 | 231.8 | 3 | 14 | 7 | 24 |
| Tenascin-N precursor (TN-N) | Q80Z71 | 173.1 | - | - | 5 | 14 |
| Thrombospondin 1 | P35441 | 129.7 | - | 2 | 5 | 14 |
| Vascular cell adhesion protein 1 precursor (CD106 antigen) | P29533 | 81.3 | 5 | 4 | 13 | 19 |
| Vitamin K-dependent protein S precursor | Q08761 | 74.9 | - | - | 4 | 5 |
| Protein Processing and Proteolysis | ||||||
| 78 kDa glucose-regulated protein precursor | P20029 | 72.4 | 8 | 8 | 9 | 5 |
| α-2-macroglobulin-P precursor (α-2-macroglobulin) | Q6GQT1 | 164.3 | 38 | 48 | 51 | 47 |
| Bone morphogenetic protein 1 | P98063 | 111.7 | 2 | 4 | 3 | 4 |
| Calreticulin precursor | P14211 | 48.0 | 3 | 2 | 3 | - |
| Carboxypeptidase E precursor | Q00493 | 53.3 | 11 | 10 | 14 | 14 |
| Cathepsin B precursor | P10605 | 37.3 | 13 | 11 | 13 | 13 |
| Cathepsin D precursor | P18242 | 45.0 | 7 | 8 | 13 | 12 |
| Cathepsin L1 precursor | P06797 | 37.5 | 7 | 3 | 7 | 7 |
| Cathepsin S precursor | O70370 | 38.4 | - | - | 7 | 6 |
| Cathepsin Z precursor | Q9WUU7 | 34.0 | 4 | 2 | 6 | 8 |
| Cystatin-C precursor (Cystatin-3) | P21460 | 15.5 | 9 | 10 | 9 | 12 |
| Dipeptidyl-peptidase 1 precursor (Cathepsin C) | P97821 | 52.4 | - | 2 | 2 | 3 |
| Dipeptidyl-peptidase 2 precursor | Q9ET22 | 56.3 | 2 | 3 | - | 2 |
| d) ER protein ERp29 precursor | P57759 | 28.8 | - | - | 3 | - |
| Endoplasmin precursor (GRP94) | P08113 | 92.5 | 2 | 2 | 3 | 2 |
| Glia-derived nexin precursor (Serpine 2) | Q07235 | 44.2 | - | 8 | 9 | 9 |
| d) Inter-alpha-trypsin inhibitor heavy chain H3 | Q61704 | 99.0 | - | - | 7 | - |
| Inter-alpha-trypsin inhibitor heavy chain H5 | Q8BJD1 | 106.7 | - | - | 3 | 6 |
| Lysosomal protective protein precursor | P16675 | 53.8 | - | 6 | 7 | 9 |
| Metalloproteinase inhibitor 2 precursor (TIMP-2) | P25785 | 24.3 | 4 | 5 | 8 | 7 |
| Peptidyl-prolyl cis-trans isomerase A | P17742 | 18.0 | 4 | 3 | 7 | 6 |
| Peptidyl-prolyl cis-trans isomerase B | P24369 | 22.7 | 5 | 6 | 7 | 4 |
| Plasma glutamate carboxypeptidase precursor | Q9WVJ3 | 51.8 | 6 | 6 | 7 | 5 |
| Plasma protease C1 inhibitor | P97290 | 55.6 | 2 | 3 | 12 | 14 |
| Plasminogen activator inhibitor 1 | P22777 | 45.0 | - | 7 | 3 | 9 |
| Pigment epithelium-derived factor (PEDF) | P97298 | 46.2 | 13 | 14 | 15 | 13 |
| Protein disulfide-isomerase A3 precursor | P27773 | 56.7 | 10 | 11 | 13 | 6 |
| Protein disulfide-isomerase A4 precursor | P08003 | 72.0 | - | 3 | 7 | 2 |
| d) Protein disulfide-isomerase A6 precursor | Q922R8 | 48.1 | - | - | 3 | - |
| Protein disulfide-isomerase precursor (PDI) | P09103 | 57.1 | 3 | 3 | 5 | - |
| Puromycin-sensitive aminopeptidase | Q11011 | 103.3 | 2 | 6 | 3 | - |
| Serine protease inhibitor A3N precursor (Serpin A3N) | Q91WP6 | 46.7 | 5 | 11 | 11 | 10 |
| c) Stromelysin-1 precursor (MMP-3) | P28862 | 53.8 | - | 3 | - | 8 |
| Sulfhydryl oxidase 1 precursor (Quiescin Q6) | Q8BND5 | 82.8 | - | - | 9 | 5 |
| Tripeptidyl-peptidase 1 precursor | O89023 | 61.3 | - | - | 2 | 4 |
| Metabolism | ||||||
| Acid ceramidase precursor (Acylsphingosine deacylase) | Q9WV54 | 44.7 | - | - | 4 | 5 |
| α-N-acetylglucosaminidase | O88325 | 82.6 | - | - | 3 | 3 |
| Aspartate aminotransferase | P05202 | 47.4 | 3 | 4 | 4 | 2 |
| d)β-1,3-N-acetylglucosaminyltransferase lunatic fringe | O09010 | 42.0 | - | - | 3 | - |
| d)β-glucuronidase precursor | P12265 | 74.2 | - | - | 5 | - |
| β-hexosaminidase alpha chain precursor (Hexosaminidase A) | P29416 | 60.6 | - | - | 4 | 2 |
| β-hexosaminidase beta chain precursor (Hexosaminidase B) | P20060 | 61.1 | 4 | 3 | 13 | 4 |
| d) Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 1 | Q3UHN9 | 100.7 | - | - | 3 | - |
| Chitinase-3-like protein 1 precursor (Cartilage glycoprotein 39) | Q61362 | 43.0 | 5 | 16 | 14 | 15 |
| Epididymis-specific alpha-mannosidase precursor | O54782 | 115.6 | 2 | 10 | 7 | 6 |
| Exostosin-2 | P70428 | 82.1 | - | - | 5 | 2 |
| Galactocerebrosidase precursor (GALCERase) | P54818 | 75.5 | - | - | 3 | 2 |
| Gamma-glutamyl hydrolase precursor | Q9Z0L8 | 35.4 | 2 | 2 | 5 | 4 |
| Ganglioside GM2 activator precursor | Q60648 | 20.8 | 3 | 3 | 3 | 2 |
| Glucose-6-phosphate isomerase (GPI) | P06745 | 62.8 | 7 | 5 | 7 | 4 |
| c) Lysosomal alpha-glucosidase precursor (Acid maltase) | P70699 | 106.2 | - | - | - | 4 |
| N-acetylglucosamine-6-sulfatase precursor (G6S) | Q8BFR4 | 61.2 | - | 3 | 4 | 4 |
| d) N-acetyllactosaminide beta-1,3-N-acetyl-glucosaminyltransferase | Q8BWP8 | 47.4 | - | - | 4 | - |
| N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase precursor | Q64191 | 37.0 | - | 3 | 3 | 4 |
| Palmitoyl-protein thioesterase 1 precursor (PPT-1) | O88531 | 34.5 | - | - | 5 | 5 |
| Platelet-activating factor acetylhydrolase | Q60963 | 49.2 | - | 2 | 8 | 2 |
| Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 (Lysyl hydroxylase 1) | Q9R0E2 | 83.6 | 3 | 4 | 14 | 7 |
| Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (Lysyl hydroxylase 2) | Q9R0B9 | 84.5 | - | 3 | 5 | 3 |
| Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 3 (Lysyl hydroxylase 3) | Q9R0E1 | 84.9 | - | - | 7 | 6 |
| Putative phospholipase B-like 2 precursor | Q3TCN2 | 66.7 | - | 2 | 2 | 5 |
| Ribonuclease T2 isoform 1 | Q9CQ01 | 29.6 | 2 | - | 3 | 5 |
| Immune | ||||||
| β-2-microglobulin precursor | P01887 | 13.8 | 2 | 2 | 3 | 5 |
| d) Cell adhesion molecule four precursor | Q8R464 | 42.7 | - | - | 4 | - |
| c) Chemokine (C-C motif) ligand 7 | Q03366 | 11.0 | - | - | - | 3 |
| c) Chemokine (C-C motif) ligand 8 | Q9Z121 | 11.0 | - | - | - | 3 |
| c) Chemokine (C-X3-C motif) ligand 1 (Fractalkine) | O35188 | 42.1 | - | - | - | 4 |
| c) Chemokine (C-X-C motif) ligand 1 (Growth-regulated alpha protein) | P12850 | 10.3 | - | 3 | - | 3 |
| Complement C1q subcomponent subunit B precursor | P14106 | 26.7 | - | - | 2 | 3 |
| Complement C1q tumor necrosis factor-related protein 5 precursor | Q8K479 | 25.4 | 3 | 5 | 3 | 4 |
| Complement C3 precursor | P01027 | 186.5 | 33 | 72 | 66 | 89 |
| Complement C4-B precursor | P01029 | 192.9 | 12 | 7 | 40 | 39 |
| Complement C1q subcomponent subunit C precursor | Q02105 | 26.0 | - | - | 3 | 3 |
| Complement C1r-A subcomponent | Q8CG16 | 80.1 | - | 4 | 9 | 15 |
| Complement C1s-A subcomponent | Q8CG14 | 77.4 | - | - | 7 | 19 |
| Complement factor B precursor | P04186 | 85.0 | - | - | 4 | 18 |
| Cyclophilin C-associated protein | O35649 | 64.1 | 4 | - | 8 | 12 |
| H-2 class I histocompatibility antigen, D-B alpha chain precursor | P01899 | 40.8 | - | 2 | 4 | 4 |
| H-2 class I histocompatibility antigen, Q8 alpha chain | P14430 | 37.5 | - | 5 | 3 | 4 |
| Ig superfamily containing leucine-rich repeat | Q6GU68 | 45.6 | 2 | 4 | 3 | 2 |
| Lysozyme C type M precursor | P08905 | 16.7 | - | - | 4 | 3 |
| Macrophage colony-stimulating factor 1 precursor (CSF-1) | P07141 | 60.6 | 2 | 5 | 4 | 8 |
| d) Macrophage colony-stimulating factor 1 receptor | P09581 | 109.3 | - | - | 3 | - |
| Macrophage migration inhibitory factor (MIF) | P34884 | 12.5 | 3 | 2 | 2 | 3 |
| Monocyte differentiation antigen CD14 precursor | P10810 | 39.2 | - | 2 | 9 | 6 |
| Pentraxin-related protein PTX3 precursor | P48759 | 41.8 | 5 | 10 | 3 | 11 |
| Platelet-derived growth factor receptor-like protein precursor | Q6PE55 | 41.9 | - | 2 | 2 | 3 |
| Binding and Transport | ||||||
| 45 kDa calcium-binding protein precursor (SDF-4) | Q61112 | 42.1 | 2 | 2 | 4 | 5 |
| d) Acyl-CoA-binding protein (ACBP) | P31786 | 10.0 | - | - | 3 | - |
| c) Adipocyte enhancer-binding protein | Q640N1 | 128.4 | - | - | - | 6 |
| Annexin A2 | P07356 | 38.7 | 6 | 2 | 8 | - |
| Apolipoprotein E precursor (Apo-E) | P08226 | 35.9 | 10 | 3 | 14 | 13 |
| d) Biotinidase precursor | Q8CIF4 | 58.6 | - | - | 3 | - |
| Ceruloplasmin | Q61147 | 121.2 | 12 | 34 | 24 | 47 |
| Dystroglycan precursor (Dystrophin-associated glycoprotein 1) | Q62165 | 96.9 | 5 | 4 | 10 | 7 |
| Follistatin-related protein 1 precursor | Q62356 | 34.6 | 5 | 9 | 7 | 12 |
| Gelsolin precursor (Actin-depolymerizing factor) | P13020 | 85.9 | 2 | - | 8 | 3 |
| Lipopolysaccharide binding protein | Q61805 | 53.1 | - | 5 | 5 | 6 |
| c) Lysosomal-associated membrane glycoprotein 1 (LAMP1) | P11438 | 43.9 | - | - | - | 3 |
| Neutrophil gelatinase-associated lipocalin precursor (Lipocalin 2) | P11672 | 22.9 | - | 6 | 5 | 7 |
| Nucleobindin-1 precursor (CALNUC) | Q02819 | 53.4 | 2 | 9 | 7 | 7 |
| Phosphatidylethanolamine-binding protein 1 (PEBP-1) | P70296 | 20.8 | 4 | 5 | 6 | 4 |
| Phospholipid transfer protein precursor (Lipid transfer protein II) | P55065 | 54.5 | 3 | - | 16 | 13 |
| Rab GDP dissociation inhibitor beta | Q61598 | 50.5 | 4 | 5 | 9 | 3 |
| Renin receptor precursor | Q9CYN9 | 39.1 | - | - | 2 | 3 |
| SPARC precursor | P07214 | 34.3 | 11 | 11 | 12 | 11 |
| Serotransferrin precursor (Transferrin) | Q921I1 | 76.7 | - | - | 12 | 3 |
| d) Sortilin-related receptor, LDLR class A repeats- containing | O88307 | 247.1 | - | - | 3 | - |
| Sulfated glycoprotein 1 precursor (Prosaposin) | Q61207 | 61.4 | 2 | 4 | 12 | 13 |
| Superoxide dismutase, extracellular | O09164 | 27.4 | - | - | 5 | 6 |
| Transcobalamin-2 precursor | O88968 | 47.6 | 7 | 7 | 8 | 9 |
| Vacuolar ATP synthase subunit S1 precursor | Q9R1Q9 | 51.0 | - | 4 | 5 | 5 |
| Vesicular integral-membrane protein VIP36 precursor | Q9DBH5 | 40.4 | - | - | 3 | 4 |
| Cell Growth & Maintenance | ||||||
| Epididymal secretory protein E1 precursor | Q9Z0J0 | 16.4 | 4 | 5 | 5 | 5 |
| Growth-arrest-specific protein 6 precursor (GAS-6) | Q61592 | 74.6 | - | - | 6 | 4 |
| Insulin-like growth factor-binding protein 2 | P47877 | 32.8 | 8 | 4 | 13 | 13 |
| Insulin-like growth factor-binding protein 3 | P47878 | 31.7 | - | 2 | 3 | 3 |
| Insulin-like growth factor-binding protein 5 | Q07079 | 30.4 | 3 | 4 | 4 | 6 |
| Insulin-like growth factor-binding protein 7 | Q61581 | 28.9 | - | 2 | 5 | 12 |
| d) Plexin domain-containing protein 2 precursor | Q9DC11 | 59.6 | - | - | 3 | - |
| Prolow-density lipoprotein receptor-related protein 1 precursor (A2MR) | Q91ZX7 | 504.7 | - | - | 4 | 5 |
| Ptprz1 protein (DSD-1 Proteoglycan) | B2RXS8 | 164.4 | 3 | 3 | 6 | 5 |
| Other | ||||||
| 4632419I22Rik protein | Q6NXM5 | 31.9 | - | - | 4 | 3 |
| Acid sphingomyelinase-like phosphodiesterase 3a precursor | P70158 | 49.9 | - | - | 3 | 2 |
| Amyloid beta A4 protein precursor (APP) | P12023 | 86.7 | 2 | 5 | 8 | 8 |
| CD109 antigen precursor | Q8R422 | 161.7 | - | 5 | 10 | 3 |
| Clusterin precursor (Apolipoprotein J) | Q06890 | 51.7 | 10 | 4 | 15 | 18 |
| d) Meteorin-like protein | Q8VE43 | 34.5 | - | - | 3 | - |
| Profilin 1 | P62962 | 11.8 | 6 | 6 | 6 | 5 |
| Retinoic acid receptor responder protein 2 precursor | Q9DD06 | 18.4 | - | - | 3 | 4 |
| Secretogranin 3 | P47867 | 53.3 | 2 | 2 | 14 | 4 |
| Thioredoxin-dependent peroxide reductase | P20108 | 28.1 | - | - | 2 | 3 |
| Vimentin | P20152 | 53.7 | 18 | 20 | 23 | 20 |
Proteins are organized by functional category and reported with their corresponding Uniprot accession number, molecular weight (MW), and unique peptides identified for each treatment condition.
Accession numbers are reported from the Uniprot database (www.uniprot.org) and, when available, refer to the unprocessed precursor protein.
The average numbers of unique peptides identified are reported for protein identifications that passed the selection criteria as detailed in the Experimental Procedures. A null value indicates the protein did not meet the minimum criteria for identification.
Protein was exclusively detected under cytokine-treated conditions.
Protein was exclusively detected under control conditions.
The ability to compare and contrast protein identifications across several conditions is highly dependent upon the reproducibility of the treatment conditions and the proteomic approach. Gel/LC-MS/MS analysis of 7 days ACM biological duplicates from control and cytokine-treated conditions showed 96% protein identity. As an additional measure of technical reproducibility, frequency versus fold difference in spectral counts (redundant peptides) between biological duplicates for each confirmed protein was calculated (Fig. 2B of Supporting Information). Both 7 days control and cytokine-treated conditions showed similar reproducibility, with >85% of the confirmed proteins varying by ≤2.5 fold between biological duplicates. Importantly, slicing and analyzing the entire gel lane enabled identification of substantially more proteins than single protein band excision while not compromising depth of analysis or reproducibility as 90% of the proteins identified by a single band excision were confirmed by the Gel/LC-MS/MS method (data not shown). The high reproducibility of the experimental design and proteomic method paired with rigorous selection criteria permitted us to compare and contrast proteins identified between control and cytokine-treated conditions.
3.3 Computational and functional classification of secretory proteins
Previous studies have investigated mouse astrocyte intracellular proteomes [13, 14], but only two studies have explored the secreteome, identifying a total of 40 unique proteins by 2-D SDS-PAGE and MALDI-TOF-MS [11, 12]. The current study found 38 of these proteins while identifying an additional 252. Since previous studies identified primarily the most abundant proteins contained within the secreteome, a rigorous analysis to distinguish between secreted/extra-cellular proteins and other nonsecreted/intracellular proteins, which may be present due to cell death, was not necessary. In the current study, cell death was unchanged (~15%) and cytokine-independent across all treatment conditions as quantified by flow cytometry (Fig. 1 of Supporting Information) and trypan blue exclusion (data not shown). Yet, given the increased sensitivity of the current approach it was critical that the potential contribution of differences in depth of analysis be considered.
We addressed these potential differences in depth of analysis by evaluating the protein identifications using a multi-step computational workflow. For the human proteome, cellular localization for only about 30% of all proteins has been determined experimentally [29], making in silico localization prediction algorithms valuable computational tools for the analysis of secreted proteomes [30]. Since the majority of soluble proteins destined for secretion into the extracellular space contain an N-terminal signal peptide, many computational algorithms utilize this feature for sub-cellular localization prediction. The use of trained neural networks and support vector machines has improved the overall performance of these algorithms. In particular, we utilized Protein Prowler [31] for its excellent specificity (0.99) and sensitivity (0.91; Nonmembrane) [30]. Protein Prowler analysis predicted 149 proteins to contain an N-terminal signal peptide (Table 2 of Supporting Information). Yet recent studies have clearly documented that not all extracellular/secreted proteins adhere to the N-terminal rule [32]. To maximize inclusion of secreted proteins that may lack an N-terminal signal, we utilized two complementary approaches. First, GO analysis was performed using Cytoscape network visualization software implementing the BiNGO plug-in. An additional 14 proteins were classified that lacked an identifiable signal peptide, but had been annotated to the extra-cellular region (GO: 5 576) or the cell surface (GO: 9 986). Second, a sequence-based prediction algorithm for non-classical secretion, SecretomeP [33], was employed in conjunction with existing experimental evidence. An additional six proteins that likely proceed via nonclassical secretion were included as a result of this analysis, including vimentin, an intermediate filament protein secreted by activated macrophages [34], as well as annexin A2 [35] and cyclophilin A [36].
Given that the majority of secreted proteins become enriched in conditioned media between 1 day and 7 days compared to nonsecreted proteins, the average fold change in relative protein abundance for proteins classified as secreted should be significantly greater than the changes in relative protein abundance of nonsecreted proteins, which are largely identified due to uniform cell death (Fig. 1 of Supporting Information). To test this hypothesis, semi-quantitative MS based on label-free spectral counting was employed. This method has been previously used as an effective means to estimate relative protein abundance [37–42]. While semi-quantitative MS based on spectral counting can be used to compare the relative abundance between different proteins, for example by normalizing spectral counts by either the protein molecular weight or by the number of observable tryptic peptides, our goal was to compare relative changes of the same protein across experimental conditions. Therefore, we simply compared the number of redundant peptides, i.e., spectral counts, for each protein between experimental conditions (Tables 3 and 4 of Supporting Information). Supporting this hypothesis, the average, absolute fold change of spectral counts from 1 day to 7 days was significantly different (P<0.0001) for the proteins classified in the secretome (3.9 ± 0.4, mean ± SEM, N = 79) (Table 3 of Supporting Information) than for the proteins that were assigned as “nonsecretory” (2.1 ± 0.1, mean ± SEM, N = 84) (Table 4 of Supporting Information).
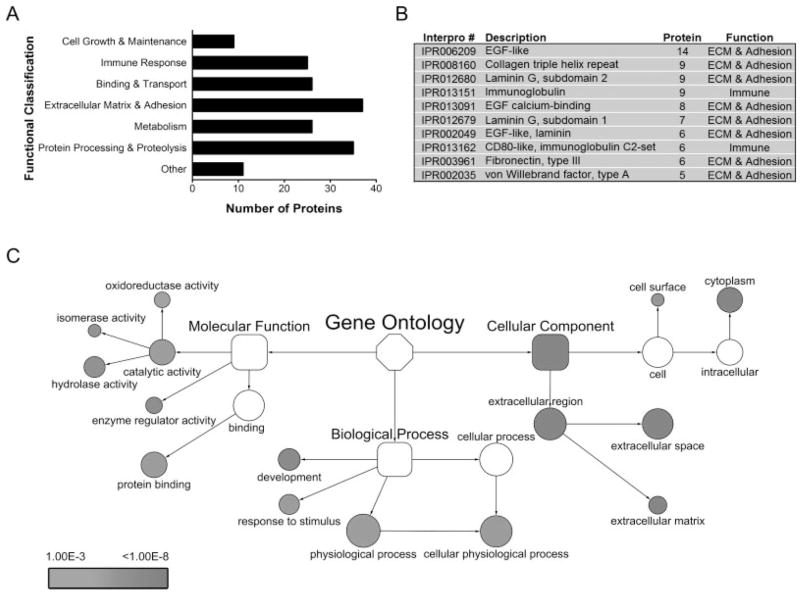
While post-hoc analyses cannot achieve complete sensitivity for the classification of secretory proteins, by utilizing multiple complementary analyses, namely in silico cellular localization prediction algorithms, functional GO classification, and published experimental evidence, we generated a list of 169 high confidence secretory proteins, which could be assigned to seven broad functional categories (Fig. 2A). The list included expected extracellular matrix proteins, such as laminins and collagens, proteins involved in processing and proteolysis, such as matrix metalloproteinase-3 (MMP-3) and cathepsins, as well as proteins that play critical roles in the immune response, such as the complement components and chemokine ligands. A complete list of these proteins and their corresponding numbers of unique peptides are reported in Table 1. InterPro domain analysis of these proteins was consistent with the InterPro domains of 2 033 proteins that were computationally predicted to be soluble, secreted proteins from the mouse genome [43]. Significantly, the EGF-like domain (IPR000561) was the most common domain in both the theoretical [43] and experimental mouse secretomes (Fig. 2B). To ascertain specific molecular and biological processes that were represented among the proteins identified in the astrocyte secretome (Table 1), we used GO classification to assign proteins into molecular, biological, and cellular subcategories, followed by functional network analysis using hypergeometric statistics paired with multiple testing correction (p<0.001) (see Materials and Methods). As shown in Fig. 2C, proteins with molecular functions assigned to protein binding (P = 2.6E-5) as well as enzyme regulator (P = 1.5E-7), hydrolase (P = 4.9E-7), isomerase (P = 6.4E-7), and oxidoreductase (P = 1.8E-4) activities were over-represented. The biological process ontology contained proteins significantly over-represented in development (P = 4.1E-8) and response to stimulus (P = 2.6E-5). The proteins that remained unclassified (Table 1 of Supporting Information) were significantly over-represented in catabolism (P = 1.5E-26) and macromolecule metabolism (P = 3.3E-5) (data not shown), further supporting the computational and functional analysis workflow was largely successful in classifying extracellular and secretory proteins.
Figure 2.
Functional GO analysis of the astrocyte protein secretome. (A) Astrocyte protein secretome containing 169 proteins classified into broad functional categories. (B) InterPro domains (Top 10) represented by the astrocyte protein secretome. (C) Over-represented GO terms of the astrocyte protein secretome. Network visualization and statistical analysis was performed using BiNGO 2.0 implemented in the Cytoscape platform. Over-representation was determined for each GO term individually by comparing the proportion of genes assigned to each term from the astrocyte secretome to the proportion of genes assigned to that same term from the annotated mouse genome. Statistically significant over-representation was calculated by hypergeometric analysis and Benjamini & Hochberg false discovery rate (FDR) correction (p<0.001). Key represents range of p-values for significantly over-represented GO terms. To maintain hierarchical accuracy, parental GO terms that were not significantly over-represented are illustrated (white shapes). Relative sizes of shapes correspond to the number of members within that term.
3.4 Basal and cytokine-induced alterations in the astrocyte secretome
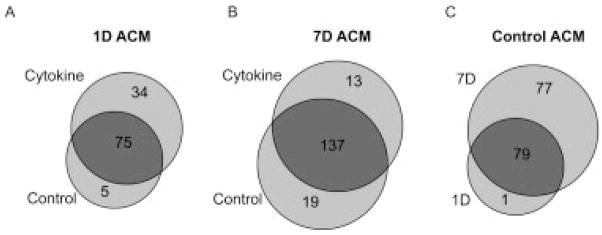
The effects of proinflammatory cytokines on the astrocyte secretome (Table 1) were compared after 1 day and 7 days of conditioning. Under control conditions, 80 proteins were identified from 1 day ACM, which subsequently increased to 109 upon cytokine stimulation (Fig. 3A). In contrast, the total and unique numbers of proteins identified in 7 days ACM between control and cytokine conditions were more similar (Fig. 3B). The relative decrease in unique proteins at 7 days versus 1 day post cytokine treatment can be partially attributed to the substantial basal protein secretion, as evidenced by the 77 proteins that were unique to 7 days control versus 1 day control (Fig. 3C) and by the related increase in total secreted protein in 7 days ACM as visualized by SDS–PAGE (Fig. 2A of Supporting Information). Yet this did not preclude identification of proteins which were unique to 1 day or 7 days conditions. Overall, 15 proteins were exclusively detected in control ACM (“d” in Table 1), while 12 proteins were exclusively detected in ACM only after cytokine treatment (“c” in Table 1). These cytokine-specific proteins included MMP-3 and four members of the chemokine ligand family, consistent with their central roles in immune response. Interestingly, three of the chemokine ligands, chemokine (C–C motif) ligand 7, chemokine (C–C motif) ligand 8, and chemokine (C–X3–C motif) ligand 1, were detected only after 7 days cytokine treatment, while chemokine, (C–X–C motif) ligand 1 was also identified under the 1 day exposure condition.
Figure 3.
Basal and cytokine-induced protein identifications in the astrocyte protein secretome. (A) Comparison of 1 day ACM between control and cytokine-treated astrocytes. (B) Comparison of 7 days ACM between control and cytokine-treated astrocytes. (C) Evaluation of control ACM between 1 day and 7 days cultured astrocytes.
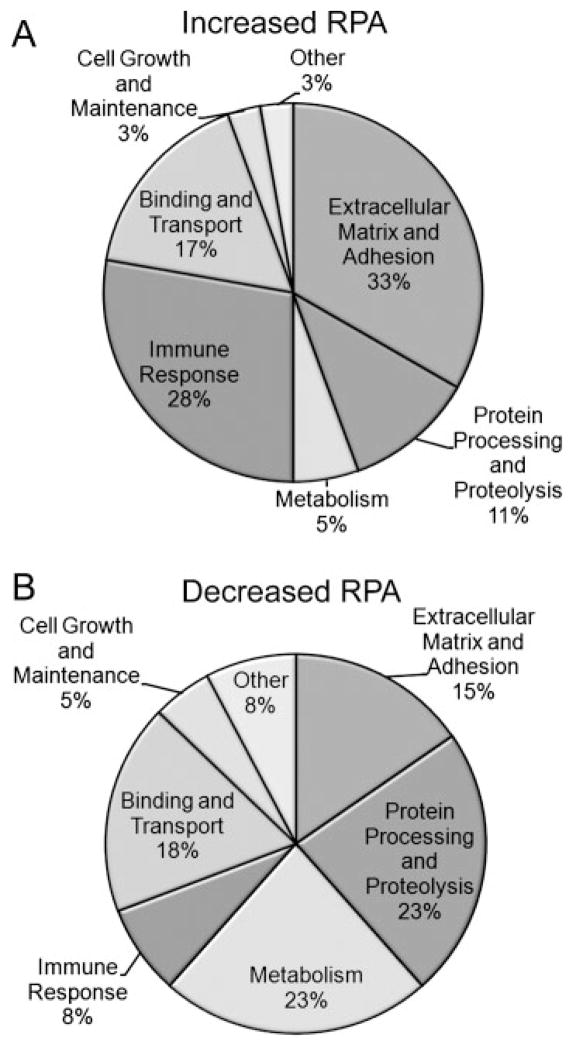
To reveal potential functional alterations in the population of secretory proteins resulting from 7 days cytokine, changes in relative protein abundance were examined with respect to functional category. In this analysis, a protein was considered increased relative to control if it (i) was exclusively detected in 7 days cytokine samples or (ii) had a ≥2.5-fold increase in relative protein abundance (RPA) as assessed by the number of redundant peptides (spectral counts) (Table 3 of Supporting Information). A protein was considered significantly decreased relative to control if it (i) was exclusively detected in 7 days control samples or (ii) had a ≥2.5-fold decrease in RPA (Table 3 of Supporting Information). RPA changes were calculated for the shared protein IDs between 7 days control and 7 days cytokine conditions (Fig. 3B, circle overlap). Using these criteria, a total of 36 proteins (13 proteins unique to 7 days cytokine ACM plus 23 proteins with increased RPA) identified after 7 days cytokine treatment were considered significantly increased relative to 7 days control. Of these, 28 and 33% were associated with immune response and extracellular matrix and adhesion, respectively (Fig. 4A). In contrast, 40 proteins (19 proteins unique to 7 days control ACM plus 21 proteins with decreased RPA) were considered significantly decreased after 7 days cytokine stimulation. Interestingly, compared to the group of protein with increased RPA, proteins with decreased RPA comprised a smaller portion of immune response (8%) and extracellular matrix and adhesion (15%), whereas those associated with metabolism (23%) were now the most prominent (Fig. 4B). Notably, there were no significant decreases in RPA under control conditions from 1 day to 7 days suggesting that the observed decreases in RPA of metabolic enzymes after 7 days cytokine treatment was due to the cytokine exposure and not to a decreased secretion over time. A similar analysis was not performed at the 1 day time point, as there were too few proteins with significant decreases in RPA to perform functional comparisons.
Figure 4.
Functional comparison of proteins with RPA changes after 7 days cytokine treatment. (A) Functional classification of proteins with increased RPA. Thirty-six proteins were classified, composed of 23 proteins with ≥2.5-fold increase in redundant peptides and 13 proteins that were unique to 7 days cytokine ACM. (B) Functional classification of proteins with decreased RPA. A total of 40 proteins were classified, composed of 21 proteins with ≥2.5-fold decrease in redundant peptides and 19 proteins that were unique to 7 days control ACM.
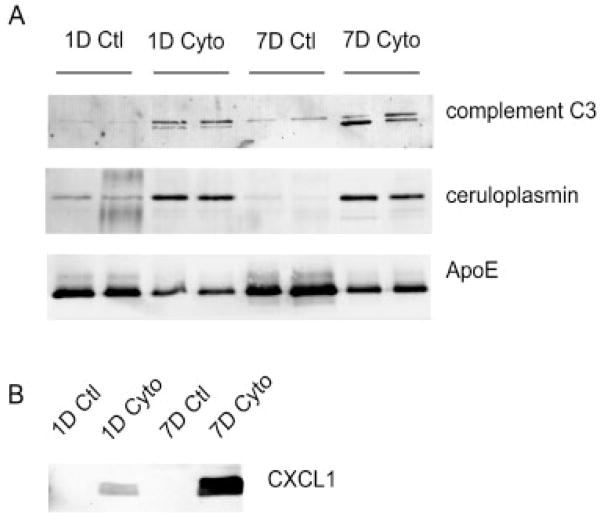
Western blot analysis was performed for several of the proteins identified in the astrocyte secretome to (i) confirm their identification by MS and (ii) corroborate the spectral counting analysis. Consistent with the increase in spectral counts, complement C3 and ceruloplasmin show an increase upon cytokine treatment, while the cytokine-induced decrease in ApoE protein levels support the observed decrease in spectral counts (Fig. 5A). Importantly, chemokine ligand 1, which was exclusively identified by MS under cytokine-treated conditions (Table 1), was also only detected by Western analysis under these conditions (Fig. 5B).
Figure 5.
Western blot validation of Gel/LC-MS/MS analysis. (A) Western blots for proteins predicted to have increased (complement C3, ceruloplasmin) or decreased (ApoE) RPA based on spectral count analysis. For each treatment condition, equal volumes of biological duplicates were loaded, corresponding to the identical conditions under which the MS analysis was performed. (B) Western blot for chemokine ligand 1 (CXCL1) showed detection only after cytokine stimulation. Equal protein (25 μg) was loaded per lane.
4 Discussion
The astrocyte secretome represents a relatively unexplored proteome but one with increasing interest and importance as astrocytes play vital roles in CNS development and synaptic communication. While activated astrocytes are a hallmark of many CNS pathologies, and as such their responses to inflammatory mediators have been studied extensively [44], a broad characterization of astrocyte-secreted biomolecules had not been performed. Here, we report a systematic secretome analysis from murine astrocytes under control and cytokine-treated conditions, which mimicked both acute (1 day) and more sustained (7 days) exposure to inflammatory mediators. Using validated proteomic approaches coupled with stringent selection criteria and a multistep bioinformatic workflow, 169 extracellular/secreted proteins were identified from ACM. This study confirmed a majority of known astrocyte-secreted proteins [9–12], while identifying more than 100 proteins not previously ascribed to astrocyte protein secretion. Proteins identified in the secretome included components of the extracellular matrix and proteins involved in extracellular protein processing and metabolism, which are consistent with known functions of astrocytes in the maintenance and restructuring of the extracellular scaffolding. The secretome also included members of the insulin-like growth factor binding proteins and cystatin C, known to provide growth support for stem cells, neurons and astrocytes. In addition, we identified many lesser-known or studied components of the astrocyte ECM such as nidogen-2, cathepsins, proteinase inhibitors, and glucosamine transferases, which may not only regulate the composition of the ECM, but also serve as signaling molecules in a paracrine or autocrine fashion. Interestingly, one of the largest functional categories containing protein with decreased RPA was metabolism (Fig. 4B). This included several enzymes, such as hexosaminidase B and β-glucuronidase, involved in protein glycosylation as well as the processing of glycosaminoglycans (GAGs). Deficiencies in these classes of proteins are associated with various lysosomal storage diseases, such as Sandhoff’s disease and the mucopolysaccharidosis disorders, which can exhibit a diversity of CNS deficits [45, 46].
The study also found secretion of four ligands from the C–C, C–X–C, and C–X3–C chemokine ligand families upon cytokine stimulation. These low molecular weight proteins have diverse biological function in the CNS, including the regulation of inflammation [47, 48] and the migration of oligondendrocyte precursors [49] and neural stem cells [50]. The C–X–C ligand 1, which was the only chemokine identified 1 day after inflammatory stimulation, is expressed in reactive astrocytes in mice models of multiple sclerosis [51] and although not toxic to oligodendrocytes may prevent their migration into demyelinated regions. The secretion of these chemokines may also have autocrine functions resulting in the elaboration of other inflammatory mediators such as products of the cyclooxygenase pathway and nitric oxide synthases. Quantitative lipidomics determined that PGE2, and PGF2α but not other lipid mediators of arachidonic acid metabolism were selectively secreted by astrocytes, and that only PGE2 is significantly elevated upon cytokine treatment. Interestingly, elevated levels of PGE2 have been documented in the cerebrospinal fluid (CSF) of patients with neurode-generative diseases [52].
While the majority of classically secreted proteins are produced as unprocessed precursors that contain signal sequences, directing them through the traditional ER/Golgi secretion pathway, a limited number of proteins have been identified that do not contain signal sequences, but yet are still actively secreted [32]. Of the 169 proteins reported in the astrocyte secretome, 12% did not contain an N-terminal signal sequence. Many of these proteins have been documented as proceeding through nonclassical secretory pathways including macrophage inhibitory factor (MIF), galectin-3, phosphatidylethanolamine-binding protein 1 (PEBP-1), vimentin, meteorin, and acyl CoA-binding protein (ACBP) [32, 34, 53–55]. Interestingly, the secretion of ACBP, also known as diazepam binding inhibitor (DBI), was originally described as a glial derived factor [54], and recent findings suggest that its secretion may require the Golgi-associated protein GRASP secretory pathway [56]. Another protein of potential interest, meteorin, has been previously identified as a developmentally secreted protein that facilitates astrocyte formation and glial cell differentiation and can support neuron axonal extension [57].
Global proteomic analyses of conditioned cellular media resulted in the identification of secreted and extra-cellular proteins as well as nonsecreted proteins, which in part can be ascribed to cellular death. Given the depth of analysis afforded by current proteomic approaches, computational means are necessary for rapid, unbiased evaluation of protein secretomes. The current approach provides a computational workflow that can be applied in a systematic fashion to analyze protein identifications from any biological secretome. Importantly, the identification of secreted proteins with distinct export mechanisms highlights the utility of this model system for elucidating the molecular mechanisms that govern the regulation and functionality of protein secretion. Overall, this systematic proteomic analysis provides a comprehensive profile of the astrocyte secretome that can be used as a reference for evaluating the impact of individual or multiple components on CNS physiology.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Ian Blair for his assistance and expertise in lipidomic profiling and Kaye Speicher for her helpful discussions on the proteomic analysis. The work was supported by grants from the National Institutes of Health NIA AG13966 and ES013508 NIEHS Center of Excellence in Environmental Toxicology. HI is the Gisela and Dennis Alter Research Professor of Pediatric Neonatology at the Children’s Hospital of Philadelphia.
Abbreviations
- ACM
astrocyte-conditioned media
- ALS
amyotrophic lateral sclerosis
- APCI/MRM/MS
atmospheric pressure chemical ionization/multiple reaction monitoring/MS, CNS
- GO
gene ontology
- IFN
interferon
- MMP-3
matrix metalloproteinase-3
- NGF
nerve growth factor
Footnotes
The authors have declared no conflict of interest.
References
- 1.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 2.Mauch DH, Nagler K, Schumacher S, Goritz C, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 3.Pascual O, Casper KB, Kubera C, Zhang J, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 4.Volterra A, Meldolesi J. Astrocytes from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 6.Zonta M, Angulo MC, Gobbo S, Rosengarten B, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 7.Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escartin C, Valette J, Lebon V, Bonvento G. Neuron-astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem. 2006;99:393–401. doi: 10.1111/j.1471-4159.2006.04083.x. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Koistinaho M, Lin S, Wu X, Esterman M, et al. Apolipo-protein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 11.Delcourt N, Jouin P, Poncet J, Demey E, et al. Difference in mass analysis using labeled lysines (DIMAL-K): a new, efficient proteomic quantification method applied to the analysis of astrocytic secretomes. Mol Cell Proteomics. 2005;4:1085–1094. doi: 10.1074/mcp.M500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Lafon-Cazal M, Adjali O, Galeotti N, Poncet J, et al. Proteomic analysis of astrocytic secretion in the mouse. Comparison with the cerebrospinal fluid proteome. J Biol Chem. 2003;278:24438–24448. doi: 10.1074/jbc.M211980200. [DOI] [PubMed] [Google Scholar]
- 13.Egnaczyk GF, Pomonis JD, Schmidt JA, Rogers SD, et al. Proteomic analysis of the reactive phenotype of astrocytes following endothelin-1 exposure. Proteomics. 2003;3:689–698. doi: 10.1002/pmic.200300407. [DOI] [PubMed] [Google Scholar]
- 14.Yang JW, Rodrigo R, Felipo V, Lubec G. Proteome analysis of primary neurons and astrocytes from rat cerebellum. J Proteome Res. 2005;4:768–788. doi: 10.1021/pr049774v. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 16.Antony JM, van Marle G, Opii W, Butterfield DA, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7:1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 17.Cassina P, Pehar M, Vargas MR, Castellanos R, et al. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93:38–46. doi: 10.1111/j.1471-4159.2004.02984.x. [DOI] [PubMed] [Google Scholar]
- 18.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Noncell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai M, Re DB, Nagata T, Chalazonitis A, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vargas MR, Pehar M, Cassina P, Martinez-Palma L, et al. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–25579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- 21.Tang HY, Ali-Khan N, Echan LA, Levenkova N, et al. A novel four-dimensional strategy combining protein and peptide separation methods enables detection of low-abundance proteins in human plasma and serum proteomes. Proteomics. 2005;5:3329–3342. doi: 10.1002/pmic.200401275. [DOI] [PubMed] [Google Scholar]
- 22.Speicher K, Kolbas O, Harper S, Speicher D. Systematic analysis of peptide recoveries from in-gel digestions for protein identifications in proteome studies. J Biomol Tech. 2000;11:74–86. [PMC free article] [PubMed] [Google Scholar]
- 23.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 24.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by MS/MS. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 25.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 26.Chukkapalli G, Guda C, Subramaniam S. SledgeHM-MER: a web server for batch searching the Pfam database. Nucleic Acids Res. 2004;32:W542–W544. doi: 10.1093/nar/gkh395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villoslada P, Genain CP. Role of nerve growth factor and other trophic factors in brain inflammation. Prog Brain Res. 2004;146:403–414. doi: 10.1016/S0079-6123(03)46025-1. [DOI] [PubMed] [Google Scholar]
- 28.Lukes A, Mun-Bryce S, Lukes M, Rosenberg GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- 29.Nair R, Rost B. Mimicking cellular sorting improves prediction of subcellular localization. J Mol Biol. 2005;348:85–100. doi: 10.1016/j.jmb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Klee EW, Sosa CP. Computational classification of classically secreted proteins. Drug Discov Today. 2007;12:234–240. doi: 10.1016/j.drudis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins J, Boden M. Detecting and sorting targeting peptides with neural networks and support vector machines. J Bioinform Comput Biol. 2006;4:1–18. doi: 10.1142/s0219720006001771. [DOI] [PubMed] [Google Scholar]
- 32.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 33.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of nonclassical and leaderless protein secretion. Protein Eng Des Sel. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 34.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 35.Zhao WQ, Chen GH, Chen H, Pascale A, et al. Secretion of Annexin II via activation of insulin receptor and insulin-like growth factor receptor. J Biol Chem. 2003;278:4205–4215. doi: 10.1074/jbc.M210545200. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Sadygov RG, Yates JR., III A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 38.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, et al. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;12:1231–1245. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt MW, Houseman A, Ivanov AR, Wolf DA. Comparative proteomic and transcriptomic profiling of the fission yeast Schizosaccharomyces pombe. Mol Syst Biol. 2007;3:79. doi: 10.1038/msb4100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Tan G, Levenkova N, Li T, et al. The proteome of the mouse photoreceptor sensory cilium Complex. Mol Cell Proteomics. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishihama Y, Oda Y, Tabata T, Sato T, et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Grimmond SM, Miranda KC, Yuan Z, Davis MJ, et al. The mouse secretome: functional classification of the proteins secreted into the extracellular environment. Genome Res. 2003;13:1350–1359. doi: 10.1101/gr.983703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 45.Donsante A, Levy B, Vogler C, Sands MS. Clinical response to persistent, low-level beta-glucuronidase expression in the murine model of mucopolysaccharidosis type VII. J Inherit Metab Dis. 2007;30:227–238. doi: 10.1007/s10545-007-0483-4. [DOI] [PubMed] [Google Scholar]
- 46.Denny CA, Alroy J, Pawlyk BS, Sandberg MA, et al. Neurochemical, morphological, and neurophysiological abnormalities in retinas of Sandhoff and GM1 gangliosidosis mice. J Neurochem. 2007;101:1294–1302. doi: 10.1111/j.1471-4159.2007.04525.x. [DOI] [PubMed] [Google Scholar]
- 47.Bezzi P, Domercq M, Brambilla L, Galli R, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 48.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai HH, Frost E, To V, Robinson S, et al. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- 50.Imitola J, Raddassi K, Park KI, Mueller FJ, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental auto-immune encephalomyelitis. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- 52.Combrinck M, Williams J, De Berardinis MA, Warden D. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:85–88. doi: 10.1136/jnnp.2005.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flieger O, Engling A, Bucala R, Lue H, et al. Regulated secretion of macrophage migration inhibitory factor is mediated by a nonclassical pathway involving an ABC transporter. FEBS Lett. 2003;551:78–86. doi: 10.1016/s0014-5793(03)00900-1. [DOI] [PubMed] [Google Scholar]
- 54.Guidotti A, Forchetti CM, Corda MG, Konkel D, et al. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci USA. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hengst U, Albrecht H, Hess D, Monard D. The phosphatidylethanolamine-binding protein is the prototype of a novel family of serine protease inhibitors. J Biol Chem. 2001;276:535–540. doi: 10.1074/jbc.M002524200. [DOI] [PubMed] [Google Scholar]
- 56.Kinseth MA, Anjard C, Fuller D, Guizzunti G, et al. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 57.Nishino J, Yamashita K, Hashiguchi H, Fujii H, et al. Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 2004;23:1998–2008. doi: 10.1038/sj.emboj.7600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.