Introduction
Products of the Neuregulin-1(Nrg-1) gene, along with the ErbB family of receptor tyrosine kinases through which Nrg-1 ligands signal, play a critical role during cardiovascular development. Through studies of genetically manipulated mice, as well as studies in cells isolated from adult hearts, it appears that Nrg-1/ErbB signaling is an essential paracrine mediator of cell-cell interactions that not only regulates tissue organization during development, but also helps to maintain cardiac function throughout an organism’s life. Studies in cells isolated from the heart demonstrate that Nrg-1 can activate a number of signaling pathways, which mediate cellular adaptations to stress in the myocardium. These observations provide insight as to why ErbB2-targeted cancer treatments have deleterious effects on cardiac function in some cancer patients. Moreover emerging data suggest that Nrg-1 ligands might be useful clinically to restore cardiac function after cardiac injury. In this review we will attempt to synthesize the literature behind this rapidly growing and exciting area of research.
Nrg-1/ErbB signaling during cardiac development and in the adult heart: lessons learned from genetically engineered mice
Myocardial Nrg-1/ErbB literature has expanded over the past 13 years to span from bench to bedside, and visa versa. In this review we will start with a discussion of results from studies in genetically engineered mice that demonstrate the critical role of Nrg-1/ErbB signaling in the developing and adult heart. The data are striking. However, in that cardiac development is incompletely understood, the risk of our approach is that we leave the reader like Dante, lost “halfway through the journey”. To pass through this “ darkened forest” and emerge “out to see once more the stars2”, the reader will need to integrate this section with the discussions that follow on signaling and clinical implication.
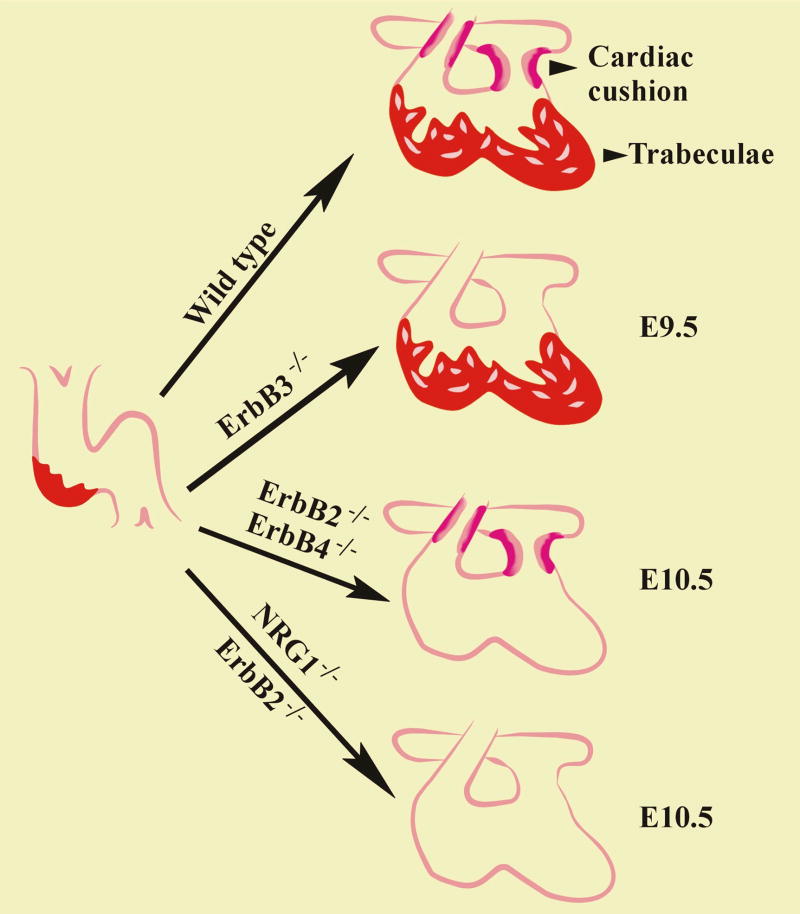
The first demonstration that the Nrg-1/ErbB signaling system plays a role in the heart came from the observations that mice with disrupted expression of Nrg-1, ErbB2, or ErbB4 die in uterus with failure of cardiac development [1–3] (Figure 1). Mice lacking functional ErbB2[2] or ErbB4[1] demonstrate a very similar cardiac phenotype, with fetal death at the age of embryonic day 10.5 due to lack of trabeculation, a process that involves myocyte proliferation resulting in thickening of the muscular ventricular wall. Immunohistochemical analysis of control littermates showed that ErbB2 and ErbB4 are expressed in the myocytes of the ventricular trabeculae [1]. Mice lacking ErbB2 or ErbB4 arrest development with a thin, single-cell layer of myocytes forming the ventricle, which appears insufficient to maintain blood circulation. Some mice lacking ErbB2 also had disruption of the endocardial cushion, a structure required for formation of the partition between the right and left chambers [2].
Fig 1. Role of Nrg-1/ErbB signaling during cardiac development.
Wild type mouse heart develops cardiac cushion and trabeculae between day 9.5 and day 10.5 of fetal development. Lack of Nrg-1 effectively blocks the formations of such structures. Lack of ErbB2 leads to impaired formation of trabeculae and in some hearts also of cardiac cushion. ErbB4 null mice specifically display lack of trabeculation whereas ErbB3 null mice develop normal trabeculae but they do not form a normal cardiac cushion.
Expression of a kinase-dead ErbB2 receptor in mouse embryos leads to a similar phenotype with death in utero, between day 10.5 and 11.5 [4]. The mutant ErbB2 was expressed under control of its own promoter, but its signaling activity was ablated as demonstrated by its inability to transform cancer cells. At the developmental stage of day 10.5 whole embryos appeared normal and homozygotes were recovered at Mendelian rates, even though hearts appeared enlarged and had irregular beats. Between day 11.5 and day 13.5 no heartbeat was detected in the homozygotes, with premature in utero death. Mice display a similar phenotype as observed in the ErbB2 null mice, suggesting that the kinase activity of ErbB2 is needed for the correct development of the trabeculae [4].
ErbB3 null mice reveal another critical step in cardiac development where Nrg-1/ErbB signaling acts (Fig1) [5]. Mice with a null mutation for ErbB3 die in uterus at day 13.5. The heart displays a less severe malformation with higher variability compared to ErbB2 and ErbB4 null mice. Examination of the heart in ErbB3 −/− mice at day 9.5 shows defects in the endocardial cushion, tissue that forms the valves of the heart. As the ErbB3 −/− fetus advances the valves are hypoplastic, do not function properly, and contribute presumably to the early fetal death at day 13.5. Trabeculae were essentially normal, suggesting that ErbB3 does not play a role at this stage (Fig 1).
Nrg-1 null mice have both trabeculae and endocardial cushion defects (Fig1), consistent with the conclusion that Nrg-1 gene products are the requisite ligands during both developmental events [3]. Inactivation of all Nrg-1 isoforms, via deletion of the EGF domain, leads to a phenotype with lack of trabeculation as observed in the ErbB2 and ErbB4 null mice, in addition to defective endocardial cushion as observed in ErbB3 −/− mice. Nrg-1 null mice survived up to day 10.5, and die between days 10.5 and 11.5. At day 10.5 mice are normal in size and overall structure, but they have irregular heartbeat and enlarged chambers. Histological analysis showed lack of trabeculation, and the endocardial cushion was not closed, leading to a non-functional heart. This phenotype, as for ErbB2 and ErbB4 null mice, appears to be the primary cause of fetal death. A detailed analysis of Nrg-1 expression revealed Nrg-1 is present in the endocardial cushion, ventricle, and atrium [3].
The similar phenotype displayed between Nrg-1, ErbB2, and ErbB4 null mice implicate these proteins as a signaling cassette required for ventricular trabeculation. Thus the presence of both ErbB1 and ErbB3 is not sufficient to replace ErbB4 at this stage of cardiac development. By similar logic, while there are other known ErbB4 ligands, there are not sufficient to replace Nrg-1 during cardiac development. Finally, while ErbB4 is capable of homodimerizing and activating signaling, this is insufficient to move the heart throughout the trabeculation phase of myocardial development. The exact cellular events mediated by this cassette during trabeculation (myocyte proliferation, organization?) remain unclear. Likewise, a Nrg-1/ErbB2/ErbB3 signaling cassette appears necessary for the correct development of the cardiac cushion, suggesting that this is a separate event from trabeculation and is differently modulated during heart development (Fig1). Again, it remains unclear what cellular events during cushion formation are mediated by this signaling system.
Nrg-1 is encoded by a large gene (1400 Kb) located in chromosome 8p12, with several promoters and alternative splicing that produces several isoforms [6, 7]. In the adult heart at least 3 different Nrg-1α isoforms and 8 Nrg-1β isoforms are expressed as mRNA in the cardiac microendothelial cells, but not in the myocytes. In the adult heart these isoforms are expressed specifically in the cardiac microendothelial cells but not in the myocytes. Although the β isoforms are 10 to 100 times more bioactive, with a higher affinity for ErbB3 and ErbB4, the predominant isoform expressed was the type I Nrg-1 EGFα, with no preferential cytoplasmatic tail detected [8]. The role of specific Nrg-1 isoforms in cardiac development can be inferred from multiple studies where specific exons have been inactivated. Null mice for the α isoform are viable at birth and survive to adulthood, showing that Nrg-1α is not needed for cardiac development. Interestingly the only abnormalities observed in these mice is a marked reduction of the alveolar proliferation during pregnancy [9]. Mice lacking type I and type II (called Ig-like neuregulins) die at day 10.5 with heart defects similar to the ones observed in ErbB2, ErbB4, and pan-Nrg-1 null mice [10–12]. Deletion of the cytoplasmatic and transmembrane domains results in early death of embryos at day 10.5 as well, due to lack of cardiac ventricular trabeculation [13]. No cardiac defects were associated with the lack of the type III Nrg-1; however mice die soon after birth, do not breath, and lack functional neuromuscular synapses. Overall these data demonstrated that Type I and II-β isoforms with transmembrane and intracellular domain of Nrg-1 are needed for the correct development of the heart [9, 14].
The 3 other Nrg genes that have been identified have no known function in the heart. Nrg-2 and Nrg-3 are mainly expressed in the central nervous system, in particular in the cerebellum and hippocampus. Nrg-2 is also expressed in motoneurons, terminal Schwann cells, and is present at synaptic sites [15]. Nrg-3 is expressed during mammary gland development and promotes morphogenesis. Unlike the other Nrgs, Nrg-4 is mildly expressed in the skeletal muscle and is highly expressed in the pancreas with three different isoforms [16, 17]. As demonstrated by Nrg-1 null mouse, none of these is able to replace the absolute requirement for Nrg-1 during cardiac development.
Most Nrg-1 isoforms are expressed as membrane bound proteins and cleaved by proteases, mainly members of the ADAM (A Disintegrin And Metalloproteinases) family. Montero et al showed that Nrg-1 processing leads to the release of the extracellular domain and the formation of a membrane bound truncated fragment [18]. It was also observed that in TACE(Tumor necrosis factor-α Converting Enzyme)/ADAM 17 null mice processing of Nrg-1 was defective [18, 19]. Mice died soon after birth with several defects in endothelial cells. Analysis of the heart revealed an increase in myocardial trabeculation and reduced cell compaction [19]. This phenotype is quite distinct from Nrg-1 null mice, where there is an earlier defect in trabeculation. Expression pattern of meltrin β/ADAM 19 during mouse development is similar to that of Nrg-1. In vitro overexpression of wild type ADAM 19 enhances Nrg-1 processing, whereas expression of a protease-deficient mutant inhibits its release in the medium [20]. Mouse deficient for ADAM 19 dies few days after birth and display several heart and cardiovascular defects. For instance, the cardiac cushion is thinner compared to control littermates, with septal defects; the coronary arteries show abnormalities, and malrotation of the cardiac outflow tract was observed also [21, 22]. The fact that embryos pass successfully through trabeculation phase of cardiac development suggests that ADAM 19 are not required for Nrg-1 processing at this stage. The cardiac cushion defect in ADAM 19 null mice, while not completely similar to the Nrg-1 null mice, supports the notion that Nrg-1 processing by this protease is involved in this developmental step.
To understand the role of ErbB2 in the post-natal heart, Crone et al [23] used the Cre-lox system to specifically delete myocyte ErbB2 after trabaculation, by mating mice carrying a floxed ErbB2 allele with mice expressing Cre-recombinase under the control of the α-Myosin Heavy Chain (MHC) promotor. The α-MHC promotor is active at a perinatal stage, when the heart is fully developed and traebeculation is complete. At birth these mice were viable and genotypes were harvested at Mendelian rates. By 8 weeks of life, however, ErbB2 conditional knock-out mice spontaneously developed dilated cardiomyopathy, with enlarged cardiac chambers, thinning of the walls, reduction of fractional shortening, and an increase in the heart:body-weight ration. At this stage, ANP (Atrial Natriuretic Peptide) and skeletal α-actin genes where expressed in the heart consistent with development of heart failure. Electron microscopic analysis showed an increase in the volume fraction of mitochondria and vacuoles, and concomitant decrease in fraction occupied by myofilaments. ErbB2 deficient mice were unable to survive pressure overload induced by aortic binding. Similarly, isolated myocytes were more sensitive to the cardiotoxic chemotherapeutic doxorubicin, suggesting a critical role for ErbB2 in the ability of myocytes to withstand stress conditions [23, 24].
ErbB4 is also required for the maintenance of the adult heart [25]. Using a similar method as for the conditional deletion for ErbB2, Garcia-Rivello et al were able to specifically delete ErbB4 in the post-natal mouse heart. Immuno-histochemical analysis of these hearts showed an abnormal distribution of the ErbB2 receptor and an increase of vinculin at the intercalated disks. These mice developed dilated cardiomyopathy by the third month of life with impaired contractility and delayed conduction [25]. Although the exact cellular events that are mediated by ErbB2 and ErbB4 is not clear from these studies, we can conclude that they are required in the post-natal heart to maintain ventricular structure and function.
A role of EGFR(Epidermal Growth Factor Receptor)/ErbB1 in heart development has also been demonstrated. There is clear evidence that EGFR plays a role during semilunar (but not atrioventricular) valve development [26–28]. Chen et al [27] expressed an EGFR point mutation in the kinase domain, which lead to a reduction in its kinetic activity to 10–20% of wild-type. These mice show a significant thickening of the semilunar valves, with no other cardiac defects observed. Sibilia et al [28] observed a similar phenotype when they overexpressed the hEGFRKI gene (a human kinase inactive isoform) or deleted the EGFR gene. Confusing the picture, the hEGFRKI mice developed cardiac hypertrophy by the age of 3 months, a phenotype not observed in the EGFR−/− mice. Chronic pharmacological inhibition of ErbB1 also leads to changes in left ventricular wall thickness and function [29]. Apcmin mice treated with ErbB1 tyrosine kinase inhibitor AG-1478 or EKB-569 showed a decrease in the fractional shortening, as assessed by echocardiography, and an increase in the left ventricle end diastolic diameter, confirmed by histological analysis. TUNEL assay for cardiac sections demonstrated an increase in the number of apoptotic cells associated with a decrease in the expression of Bcl2. Thickening in the aortic valves are also present, similar to the mice expressing EGFR mutants [29]. Inhibition of EGFR in the early stages of development of zebrafish led to enlarged pericardial sacs, reduction in the blood circulation, due to the lack of flow from the heart to the aorta. [30]. One consideration that has not been fully examined is that the effect of these inhibitors on cardiac function is due to cross-reacting with other tyrosine kinases, including ErbB4, which has been observed in vitro [31].
Neuregulin-1/ErbB signaling in the heart: lessons from studies of myocyte biology
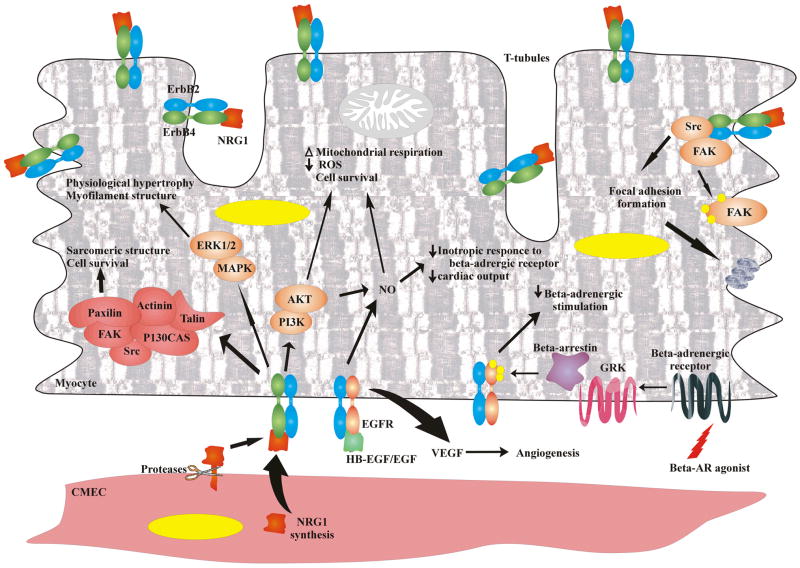
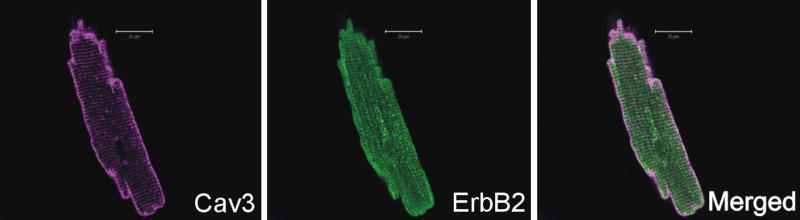
Following Dante’s journey, we will move to the arena of experimental cell research, a ‘purgatory’ of sorts, where many ideas develop that might help to explain the developmental studies discussed above, and provide ideas for how to translate from basic to clinical research. Cells from the intact heart can be separated and kept in culture for days to weeks [32–37]. Because this experimental system leads to relatively cell populations, the possible signaling mechanisms and cellular responses to Nrg-1 responsible for mouse phenotype and its receptors can be investigated (Fig 2). These efforts have shown that in the adult heart microvascular endothelial cells express multiple Nrg-1 isoforms [38–40], along with ErbB1, ErbB2 and ErbB3 receptors (Cote, Fukuzawa, and Sawyer, unpublished observations). Although ErbB3 is expressed in prenatal myocytes, adult ventricular myocytes express only ErbB1, ErbB2, and ErbB4 [38]. Immunostaining of adult myocytes reveals ErbB2 expression follows a pattern suggesting receptors are present throughout the sarcolemmal membrane, including the t-tubule system (Fig 3). ErbB4 expression overlaps in location with ErbB2, thought is somewhat distinct with high levels present at the intercalated disk.
Fig 2. Nrg-1/ErbB signaling in myocytes.
Nrg-1/ErbB activated a large number of signaling cascades, such as Erk 1/2, Akt, and FAK. Moreover this complex interacts with other systems as Focal Adhesion Complex and β-adrenergic stimulation.
Fig 3. ErbB2 and Caveolin 3 colocalization.
Freshly dissociated myocytes form rat heart were fixed and stained for ErbB2 (green) and Caveolin 3 (violet), a marker for T-tubules. ErbB2 present a diffused and striated distribution suggesting the ErbB2 presence is not limited to the T-tubules.
Based upon work on isolated cell systems, a number of processes appear to be regulated by Nrg-1/ErbB signaling, including cell growth [38], myofilament structure and organization [41, 42], survival [38], myocyte-matrix coupling [43], glucose uptake [8], and angiogenesis [44]. In prenatal myocytes, DNA synthesis is increased by Nrg-1 stimulation, athough this is not seen in post-natal cells [38]. Thus inadequate myocyte proliferation may account for the defect in trabeculation as well as cardiac cushion formation in mice lacking components of this pathway discussed above. In postnaltal myocytes, the individual data supporting multiple functions for Nrg-1 are robust. However it seems improbable that Nrg-1/ErbB signaling orchestrates all of these processes simultaneously. More than likely there are constraints in space and time, as well as competitive signaling systems that modulate the relative amplitude of each Nrg-1 dependent response in the intact heart.
In neonatal [45] and adult myocytes [42], Nrg-1 stimulates activation of MEK/Erk 1/2 with subsequent induction of protein synthesis [46] and hypertrophic gene expression [45]. In neonatal myocytes this is associated with myofilament organization, which is also MEK dependent [45]. Treatment of myocytes with an anti-ErbB2 that induces receptor phosphorylation (so-called activating antibody), along with activation of Erk 1/2 [43, 47], demonstrating that ErbB2 phosphorylation is sufficient for activation of this pathway. In cultured adult myocytes, specific inhibition of Erk 1/2 with either PD98059 or U129 causes myofilament disarray. A similar phenotype was observed when myocytes where treated with a non-activating ErbB2 specific antibody (clone 7.16.4, Pentassuglia and Suter, unpublished observations). In adult myocytes ErbB2 inhibition reduces basal phosphorylation of Erk 1/2. Concomitant inhibition of ErbB2 and treatment with chemotherapeutic agents, like doxorubicin and taxol, significantly increases myofilament disarray [41, 42]. These multiple lines of evidence support the conclusion that ErbB2 couples to Erk 1/2 in cardiac myocytes, and this pathway regulates sarcomere synthesis/organization/stability.
The details of how ErbB2 phosphorylation couples to Erk 1/2 in myocytes remains to be fully worked out. Members of the Grb family, namely Grb2/Shc and Grb7, are able to bind to the ErbB2 receptor, and are known to couple to Ras/Mek/Erk 1/2. Shc, another ErbB2 adaptor protein, has been implicated in cardiac and vascular hypertrophy [48–53]. It is interesting that the Nrg-1 concentration dependence of myocyte hypertrophy is distinct from that for other signals and responses. Erk 1/2 phosphorylation and protein synthesis display a biphasic dose response to Nrg-1, with a declining response at higher Nrg-1 concentrations [54, 55]. One explanation for this phenomenon is ErbB-dependent activation of growth suppressing signals at higher Nrg-1 concentrations. An attractive mechanism for this phenomenon involves concentration dependent activation of ErbB4/ErbB4 homodimers, which a priori require two Nrg-1 ligands per receptor dimer (vs. only one Nrg-1 ligand should be sufficient for activation of ErbB2/ErbB4 heterodimers).
Another downstream signaling pathway induced by Nrg-1 in myocytes is the PI3-kinase/Akt pathway, which appears to be involved in the protection of cardiac myocytes against cell death, as well as regulation of metabolism and growth. Nrg-1 induces PI3-kinase dependent activation of Akt in cardiac myocytes, a pathway that has been heavily implicated in cell survival [31, 41, 42, 56–59]. Nrg-1 protects cardiac myocytes in primary culture from cell death induced by serum starvation [38], β-adrenergic receptors activation [60, 61], and the cardiotoxic chemotherapeutic doxorubicin [31, 41, 57, 58]. Overexpression of a dominant negative Akt isoform prevents Nrg-1 protection [31, 41, 57, 58]. The exact mechanism by which Nrg-1 dependent Akt signaling protects myocytes is incompletely understood. Akt-dependent change in bcl-2 family expression has been implicated [62–64]. In addition, Akt enhances glucose uptake [8] and Akt-dependent activation of eNOS (endothelial Nitric Oxide Synthase) [65] triggering changes in mitochondrial respiration, which may also play a role in regulating cell survival in the setting of metabolic stress.
Sequence analysis supports the notion that ErbB4 but not ErbB2 is required for coupling to the PI3-kinase pathway. Unlike ErbB2, ErbB4 (Cyt-1 variant) has a consensus sequence for PI3-kinase homologous with that on ErbB3. Thus ErbB2 may not be required for Nrg-1 induced activation of PI3-kinase and Akt, so long as Nrg-1 is available at sufficient concentration to activate ErbB4/ErbB4 homodimers, promoting cytoprotection. Perhaps this explains why experimental studies examining whether inhibiting ErbB2 alters cell survival have delivered mixed results [31, 39, 66].
A third pathway activated by Nrg-1 in myocytes involves FAK (Focal Adhesion Kinase) (Fig 2). FAK is activated by integrin receptors and is critical for the formation of focal adhesion complexes [43, 67, 68]. FAK is a known substrate for the adaptor protein Src [69, 70], and is activated in ErbB2 overexpressing breast cancer cells [71]. In cardiac myocytes, FAK is involved in the maintenance of sarcomeres [72, 73] as well as cell survival [74, 75]. Cardiac specific inactivation of FAK in mice leads to increased chamber dimensions associated with re-expression of fetal genes and hypertrophic markers ANP, BNP, skeletal α-actin and β-MHC, similar to the ErbB2 and ErbB4 cardiac restricted deletion [76, 77]. Histological analysis of such hearts revealed disorganized myofibrils and swollen mitochondria. Intercalated disks were also affected by FAK loss; with replacement of the normally serpentine structure by sharp angles [77]. It is therefore interesting that recombinant Nrg-1 induces Src-dependent phosphorylation of FAK at tyrosine residues 861 in myocytes with formation of focal adhesion complexes as demonstrated by the co-immunoprecipitation of p130CAS and Src with FAK [43]. Nrg-1 also promotes accumulation of p-FAK at the former intercalated disks, and formation of lamellipodia from these sites. Pretreatment with either an ErbB2 specific antibody effectively blocked Nrg-1 induced FAK phosphorylation and focal adhesion complex formation [43].
Studies in undifferentiated cell lines from heart cells implicate several other signaling pathways and biological functions of Nrg-1/ErbB signaling. For example, inhibition of ErbB1 and ErbB2 with a tyrosine kinase inhibitor in embryonic human primary cardiacmyocytes leads to AMPK activation, activation of the fatty acid oxidation, increase in ATP production, and subsequent protection against TNFα-induced cell death [78]. Weather AMPK is regulated by Nrg-1 in differentiated adult myocytes is not clear. The Gab (Grb2-associated Binder) family of adaptor proteins seems to relate to Nrg-1/ErbB signaling as well [79]. Three members of this family have been indentified so far (Gab1, Gab2, and Gab3). Gab1 is required for fetal development, whereas both Gab2 and Gab3 null mice develop normally. Gab1 and Gab2 but not Gab3 mRNA has been detected in the adult heart and associate with SHP2 and p85 upon ErbB activation with Nrg-1β, HB-EGF (heparin binding EGF), and EGF. To evaluate the role of Gab1 and Gab2 in the adult heart a cardiac restricted double knock-out was generated using the cre-lox technology. These mice developed dilated cardiomyopathy similar to the cardiac restricted ErbB2 and ErbB4 knockouts, along with endocardial fibroelastosis. Nrg-1β injections failed to activate Erk 1/2 and Akt in Gab 1/2 double knock out mice even though both ErbB2 and ErbB4 were phosphorylated. These data suggest that Gab1 and Gab2 are needed to successfully couple Nrg-1β to downstream signaling [79].
The micro-endothelial cells (CEMC) of the heart express multiple isoforms of Nrg-1, the majority of which are membrane bound ligand [8]. Oxidative stress [39] and endothelin-1 [38] significantly increase the activity and expression of Nrg-1 in CEMC. Conditioned medium collected from cultured CEMC activates the ErbB2 receptors and downstream signaling pathways, as assessed by immunoblot [8, 38, 39]. This endothelial derived Nrg-1 induces expression of hypertrophic markers, like BNP (Brain Natriuretic Peptide), in cultured myocytes, confirmed by an increase in cell surface area. Released Nrg-1 is also protective against cytotoxic stimuli, such as oxidative stress [39] and doxorubicin [40]. Nrg-1β treatment of myocytes in vitro alters the expression of a number of genes, with unknown and known consequences [38, 45]. Several genes related to regulation of cellular oxidative stress, such as catalase and SOD, are significantly upregulated after treatment with Nrg-1β, supporting the idea that Nrg-1β plays an important role in regulation of myocardial oxidative stress. Other genes found upregulated were ubiquitin, cyclin D1, elogation factor 2, and genes involved in energy metabolism [46]. The full implications of these findings have yet to be explained.
There is also growing evidence that myocardial Nrg-1/ErbB signaling is dynamically regulated by receptor expression in myocytes. ErbB2 stability in cancer cells is in part regulated by the chaperone activity of Hsp90 (Heat Shock Protein 90) [80]. Inhibiting Hsp90 in myocytes with geldonamycin likewise causes degradation of ErbB2 by releasing Hsp90 from ErbB2 itself, and reduced cell responsiveness to Nrg-1 [81]. Hsp90 requires ATP for chaperone activity, and reducing ATP production in myocytes has a similar effect on Hsp90/ErbB2 association with induction of ErbB2 degradation [81]. Thus small shifts in energy status of myocytes have the potential to inhibit Nrg-1/ErbB signaling via induction of receptor degradation. This may have implications for mechanisms of chronic cardiac remodeling in the setting of metabolic stress (discussed further below).
ErbB4 activity is also dynamically regulated. Nrg-1 activation of ErbB4 is associated with translocation of receptor to a triton-insoluble membrane fraction, likely caveolae [25]. The implication for this is still not clear. In addition, ErbB4 alternative spicing at the juxtamembrane site leads to the expression of two distinct isoforms, the JM-A and JM-B [82]. While the JM-A isoform is cleavable by TACE and γ-secretase and the cytosolic fragment can localize in the nucleus, JM-B is not cleavable. In the adult heart only the latter is present [82, 83].
Growing evidences support a role for Nrg-1 in angiogenesis with clear implications for cardiovascular biology [44]. In vivo stimulation of corneal vessels with either VEGF (Vascular Endothelial Growth Factor) or Nrg-1 induces formation of new capillaries in a dose dependent manner [44]. Trastuzumab significantly reduces the vessel diameter in ErbB2-expressing tumors. VEGF expression in cancer cells was also reduced both in vivo and in vitro, supporting previous observations that suggested a possible role of ErbB2 in VEGF modulation [84–86]. Thus ErbB2 may regulate angiogenesis via inducing the expression and release of VEGF, a well-known angiogenic factor [87]. A second possible mechanism involves ErbB coupling directly to the process of angiogenesis, via one of several pathways including eNOS. In support of this, inhibition of eNOS effectively blocks HB-EGF and EGF induced angiogenesis [88].
Nrg-1 activates NO production via eNOS in myocytes [89], with interesting implications for cardiac function. NO is well-known to modulate the inotropic state of the heart, inducing a negative inotropic effect that decreases active tension of the heart [65]. Nrg-1 has been shown to reduce the myocardial inotropic response to adrenergic stimulation, mimicking the effect of the muscarinic cholinergic receptor. Thus Nrg-1 released by endothelial cells may reduce cardiac output and blood pressure, regulating the activity of neurohormonal agonists.
ErbB receptors have also been implicated in adrenergic signaling via ligand independent transactivation (Fig 2). β-adrenergic stimulation activates EGFR via the G protein-coupled receptors kinase (GRK) β-arrestin, which exerts a protective effect on the heart. Disruption of the crosstalk by inhibiting GRK or EGFR leads to a significant deterioration of cardiac function with LV dilation and decrease in fractional shortening [90]. Transactivation of ErbB receptors also occurs in response to GPCR agonists, such as lysophosphatidic acid, carbachol or thrombin [91]. Although the exact mechanism and consequences of this signalling are not completely clear, src is involved in this crosstalk. Thus ErbB receptors may modulate a number of response to diverse stimuli independent of Nrg-1.
An interaction between erbB signalling and integrins has not been fully explored, but appears to be present in the heart. ErbB2 and integrins interact in cancer cells, where coexpression is associated with aggressive growth [92], matrix molecule are able to induce transactivation of ErbB2 via integrin interactions [93]. Integrins and ErbB2 co-localize [94–96] and form aggregates with tyrosine kinase proteins [43, 97]. These demonstrate the two different types of integrin-RTK interactions that have been described [98]. “Collaborative” signalling appears to occur when both integrins and receptor tyrosine kinases are activated by their respective ligands, form a cluster via activation of FAK. In “direct” signalling, integrins phosphorylate RTK without the need of growth factors and FAK signalling [99, 100]. Emerging data support a role for integrin/ErbB2 cross talk in regulating myocyte-matrix force coupling in the heart via the collaborative model. Nrg induces specific phosphorylation of Src (Y215 and Y416) and FAK (Y867), leading to ErbB2 association with Src, FAK, p130CAS, and paxillin [43]. Binding of laminin to myocyte integrin heterodimers causes recruitment of paxillin and talin, which activate FAK via autophosphorylation at Y397. These events appear to promote the formation of an ErbB2/ErbB4/integrin complex via FAK, recruitment and phosphorylation of p130CAS, and modulation of focal adhesion complex (FAC) and mechanical coupling (Pentassuglia and Sawyer, unpublished observation). The role this plays in Nrg-1 regulation of myocardial structure and function remains to be fully explained.
Nrg-1 paracrine signalling has also been demonstrated to be a factor that promotes for development of the cardiac conduction system. Rentschler et al. demonstrated that Nrg-1 induces differentiation of myocytes into cardiac conduction system as measured by use an ectopic reporter system in mice embryos [101]. Studies in an embryonic cell culture system by Patel and Kos demonstrate that Nrg-1 induces a number of conduction specific markers such as Connexins 40 and 45 [102]. Milan et al provided further support for this phenomenon using a morphilino to knock-down neuregulin expression in zebrafish [103]. Nrg-1 expression was strongest in the endocardium of the AV ring, and embryos lacking neuregulin display a slow conduction throughout the heart and a loss of physiological atria-ventricular delay. Lack of Nrg-1 zebrafish mutant cloche that lacks endothelial cell lineages, a cardiac conduction system. [104]. Collectively these studies demonstrate a role for endocardial-derived neuregulin in regulating the differentiation of myocytes into specific lineages required for coordinated cardiac function.
Clinical role of Nrg-1/ErbB signaling in the heart
When Dante reaches the end of his journey he recognizes that the passage through Hell and Purgatory were necessary experiences for full comprehension of the vastness of Paradise. In the same way, we like to think that efforts at understanding the role of Nrg-1/ErbB during fetal development and cell biology are the basis to dissect its role in pathological conditions of clinical significance, and to understand its potential as a molecular target for cardiovascular therapeutics. Our understanding of myocardial Nrg-1/ErbB signaling has reached the stage where it is clinically important in at least two areas. First, the biology discussed above helps to understand why cancer victims receiving treatments targeting ErbB2 (Her2/Neu) experience cardiac side-effects. Second, the beneficial effects of Nrg-1 on myocardial structure and function suggest that ErbB agonists might have therapeutic potential for patients with heart disease.
The association of ErbB2 to highly invasive and metastatic breast cancer led to the development of trastuzumab, a humanized monoclonal antibody to ErbB2 [105]. Trastuzumab improved the efficacy of classical chemotherapeutic agents, increasing the disease free time and survival rate for these unfortunate patients. However, trastuzumab significantly increases the incidence of cardiac dysfunction in those treated with prior or concomitant anthracyclines [106]. Anthracyclines are one of the most effective cancer therapeutic agents, with well-known cardiotoxic effects. In the pivotal trials that led to the approval of the FDA for trastuzumab, concomitant use of trastuzumab and anthracyclines was associated with a very high rate of cardiac events in the form of reduced cardiac function and symptomatic heart failure. Treatment with trastuzumab alone was associated with some cardiac events, although these patients had prior exposure to anthracyclines, itself a risk factor for development heart muscle weakening. When trastuzumab has been used in persons without prior or concomitant anthracyclines, very few adverse cardiac events have been seen [107]. Extrapolating from the basic science discussed previously, a number of potential mechanisms can be hypothesized to explain the cardiac effects of trastuzumab. Suppression of ErbB2 coupling to one or more intracellular signaling pathways would be expected to alter myocyte survival, or regulation of transcriptional pathways necessary for maintenance of sarcomeres. In this setting of concomitant administration of anthracyclines, which accelerate myofilament degradation [108], this could be particularly problematic and hence explain the profound cardiac dysfunction observed [106].
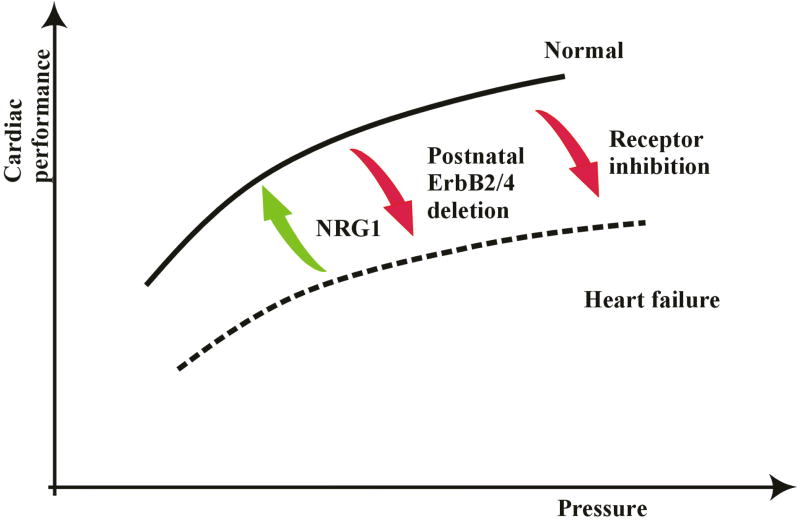
It is interesting that expression of ErbB receptors decreases in chronic heart failure [109]. A similar decrease is observed in mice subjected to chronic pressure overload [110]. These observations raise the question of whether altered ErbB signaling plays a role in progression of heart failure. As discussed previously, ErbB signaling appears to couple to muscarinic cholinergic receptor activation, which balances sympathetic tone. If reduced ErbB expression leads to reciprocal increases in sympathetic tone in the failing heart, this may promote progressive myocardial dysfunction. As a correlate, increased levels of Nrg-1 improve and decreased levels exacerbate progression of heart failure in animals [111] (Fig4). These observations have led to the possibility that recombinant Nrg-1 has therapeutic potential in patients with heart disease, an exciting possibility currently under investigation.
Fig 4. Effect of ErbB2 modulation in the heart.
Starling curves describe cardiac performance in relation to ventricular filling pressure. Inhibition of ErbB2 by trastuzumab or by restricted deletion of the receptor in the heart leads to a reduction in cardiac function. Visa versa, hearts with cardiac dysfunction can be rescued when treated with Nrg-1.
It is also interesting to consider whether endogenous Nrg-1 can be harnessed to help in recovery of myocardial function. Nrg-1 activation in the heart by whatever mechanism should offer some degree of cardioprotection or enhance recovery from injury. It is also possible that Nrg-1 from other organs, such as skeletal muscle, can act on the myocardium in an endocrine manner. Exercise is a potent activator of Nrg-1 in skeletal muscle [112], and Nrg-1 is present in the circulation of people is proportion to fitness (Moondra and Sawyer, unpublished observations). This observation has many intriguing implications, including the notion that circulating Nrg-1 plays a role in the cardioprotection associated with regular physical activity. As Neuregulin afficionados, we are often amused at the notion that the exercise recommended by health professionals, as painful as it feels, is activating our favorite myocardial protective growth factor.
Acknowledgments
This work was supported by grants HL068144 from the National Institutes of Health and an Established Investigator Award from the American Heart Association to DBS. Myocyte imaging was done at the CIRS (Cell Imaging Shared Resources) with a LSM510 confocal microscope.
Footnotes
Halfway through the journey we are living
I found myself deep in a darkened forest,
For I had lost all trace of the straight path.
Dante Alighieri
The Divine Comedy
Hell
Canto I, 1-3
Dante Alighieri
The Divine Comedy
Hell
Canto XXXIV, 138
“And I shall sing this second kingdom where
the human spirit purifies itself,
becoming fit to mount up into heaven”
Dante Alighieri
The divine Comedy
Purgatory
Canto I 4-6
The glory of Him who sets all things in motion
Cleaves through the universe, and it flames again
In different places with a different force.
Dante Alighieri
Divine Comedy
Paradise
Canto I, 1-3
References
- 1.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 2.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 3.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 4.Chan R, Hardy WR, Laing MA, Hardy SE, Muller WJ. The catalytic activity of the ErbB-2 receptor tyrosine kinase is essential for embryonic development. Mol Cell Biol. 2002;22:1073–1078. doi: 10.1128/MCB.22.4.1073-1078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Bermingham-McDonogh O, Danehy FT, Jr, Nolan C, Scherer SS, Lucas J, Gwynne D, Marchionni MA. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- 7.Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc Natl Acad Sci U S A. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Cleary S, Mandarano MA, Long W, Birchmeier C, Jones FE. The breast proto-oncogene, HRGalpha regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002;21:4900–4907. doi: 10.1038/sj.onc.1205634. [DOI] [PubMed] [Google Scholar]
- 10.Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci U S A. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 12.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Hwang H, Cao L, Buckland M, Cunningham A, Chen J, Chien KR, Graham RM, Zhou M. Domain-specific gene disruption reveals critical regulation of neuregulin signaling by its cytoplasmic tail. Proc Natl Acad Sci U S A. 1998;95:13024–13029. doi: 10.1073/pnas.95.22.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- 15.Ring HZ, Chang H, Guilbot A, Brice A, LeGuern E, Francke U. The human neuregulin-2 (NRG2) gene: cloning, mapping and evaluation as a candidate for the autosomal recessive form of Charcot-Marie-Tooth disease linked to 5q. Hum Genet. 1999;104:326–332. doi: 10.1007/s004390050961. [DOI] [PubMed] [Google Scholar]
- 16.Hayes NV, Newsam RJ, Baines AJ, Gullick WJ. Characterization of the cell membrane-associated products of the Neuregulin 4 gene. Oncogene. 2008;27:715–720. doi: 10.1038/sj.onc.1210689. [DOI] [PubMed] [Google Scholar]
- 17.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 18.Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci. 2000;16:631–648. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Chen H, Sun J, Buckley S, Zhao J, Anderson KD, Williams RG, Warburton D. TACE is required for fetal murine cardiac development and modeling. Dev Biol. 2003;261:371–380. doi: 10.1016/s0012-1606(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 20.Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A. Roles of Meltrin beta/ADAM19 in the processing of neuregulin. J Biol Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- 21.Kurohara K, Komatsu K, Kurisaki T, Masuda A, Irie N, Asano M, Sudo K, Nabeshima Y, Iwakura Y, Sehara-Fujisawa A. Essential roles of Meltrin beta (ADAM19) in heart development. Dev Biol. 2004;267:14–28. doi: 10.1016/j.ydbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Zhou HM, Weskamp G, Chesneau V, Sahin U, Vortkamp A, Horiuchi K, Chiusaroli R, Hahn R, Wilkes D, Fisher P, Baron R, Manova K, Basson CT, Hempstead B, Blobel CP. Essential role for ADAM19 in cardiovascular morphogenesis. Mol Cell Biol. 2004;24:96–104. doi: 10.1128/MCB.24.1.96-104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 24.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 26.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 28.Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130:4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- 29.Barrick CJ, Yu M, Chao HH, Threadgill DW. Chronic pharmacologic inhibition of EGFR leads to cardiac dysfunction in C57BL/6J mice. Toxicol Appl Pharmacol. 2008;228:315–325. doi: 10.1016/j.taap.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goishi K, Lee P, Davidson AJ, Nishi E, Zon LI, Klagsbrun M. Inhibition of zebrafish epidermal growth factor receptor activity results in cardiovascular defects. Mech Dev. 2003;120:811–822. doi: 10.1016/s0925-4773(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 31.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Grosso DS, Frangakis CJ, Carlson EC, Bressler R. Isolation and characterization of myocytes from the adult rat heart. Prep Biochem. 1977;7:383–401. doi: 10.1080/00327487708061656. [DOI] [PubMed] [Google Scholar]
- 33.Haworth RA, Hunter DR, Berkoff HA. The isolation of Ca2+-resistant myocytes from the adult rat. J Mol Cell Cardiol. 1980;12:715–723. doi: 10.1016/0022-2828(80)90101-7. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz P, Piper HM, Spahr R, Spieckermann PG. Adaptation phenomena of adult cardiac myocytes in culture. Basic Res Cardiol. 1985;80 Suppl 2:181–185. [PubMed] [Google Scholar]
- 35.Grafe M, Graf K, Auch-Schwelk W, Terbeek D, Hertel H, Fleck E. Cultivation and characterization of micro- and macrovascular endothelial cells from the human heart. Eur Heart J. 1993;14 Suppl I:74–81. [PubMed] [Google Scholar]
- 36.Nishida M, Carley WW, Gerritsen ME, Ellingsen O, Kelly RA, Smith TW. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol. 1993;264:H639–652. doi: 10.1152/ajpheart.1993.264.2.H639. [DOI] [PubMed] [Google Scholar]
- 37.Grafe M, Auch-Schwelk W, Graf K, Terbeek D, Hertel H, Unkelbach M, Hildebrandt A, Fleck E. Isolation and characterization of macrovascular and microvascular endothelial cells from human hearts. Am J Physiol. 1994;267:H2138–2148. doi: 10.1152/ajpheart.1994.267.6.H2138. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 39.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 40.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281:19469–19477. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 41.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 42.Pentassuglia L, Timolati F, Seifriz F, Abudukadier K, Suter TM, Zuppinger C. Inhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Exp Cell Res. 2007;313:1588–1601. doi: 10.1016/j.yexcr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 45.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 46.Giraud MN, Fluck M, Zuppinger C, Suter TM. Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1beta. J Appl Physiol. 2005;99:313–322. doi: 10.1152/japplphysiol.00609.2004. [DOI] [PubMed] [Google Scholar]
- 47.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ Res. 1999;85:672–681. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daly RJ, Sanderson GM, Janes PW, Sutherland RL. Cloning and characterization of GRB14, a novel member of the GRB7 gene family. J Biol Chem. 1996;271:12502–12510. doi: 10.1074/jbc.271.21.12502. [DOI] [PubMed] [Google Scholar]
- 50.Kauraniemi P, Barlund M, Monni O, Kallioniemi A. New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res. 2001;61:8235–8240. [PubMed] [Google Scholar]
- 51.Pero SC, Shukla GS, Cookson MM, Flemer S, Jr, Krag DN. Combination treatment with Grb7 peptide and Doxorubicin or Trastuzumab (Herceptin) results in cooperative cell growth inhibition in breast cancer cells. Br J Cancer. 2007;96:1520–1525. doi: 10.1038/sj.bjc.6603732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obreztchikova M, Elouardighi H, Ho M, Wilson BA, Gertsberg Z, Steinberg SF. Distinct signaling functions for Shc isoforms in the heart. J Biol Chem. 2006;281:20197–20204. doi: 10.1074/jbc.M601859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizumi M, Tsuchiya K, Kirima K, Kyaw M, Suzaki Y, Tamaki T. Quercetin inhibits Shc- and phosphatidylinositol 3-kinase-mediated c-Jun N-terminal kinase activation by angiotensin II in cultured rat aortic smooth muscle cells. Mol Pharmacol. 2001;60:656–665. [PubMed] [Google Scholar]
- 54.Bueno OF, De Windt LJ, Lim HW, Tymitz KM, Witt SA, Kimball TR, Molkentin JD. The dual-specificity phosphatase MKP-1 limits the cardiac hypertrophic response in vitro and in vivo. Circ Res. 2001;88:88–96. doi: 10.1161/01.res.88.1.88. [DOI] [PubMed] [Google Scholar]
- 55.Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, Yamashita T, Ishido S, Hotta H, Yokoyama M. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 56.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 57.Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol. 2000;32:2397–2402. doi: 10.1006/jmcc.2000.1283. [DOI] [PubMed] [Google Scholar]
- 58.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Kuramochi Y, Lim CC, Guo X, Colucci WS, Liao R, Sawyer DB. Myocyte contractile activity modulates norepinephrine cytotoxicity and survival effects of neuregulin-1beta. Am J Physiol Cell Physiol. 2004;286:C222–229. doi: 10.1152/ajpcell.00312.2003. [DOI] [PubMed] [Google Scholar]
- 61.Okoshi K, Nakayama M, Yan X, Okoshi MP, Schuldt AJ, Marchionni MA, Lorell BH. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation. 2004;110:713–717. doi: 10.1161/01.CIR.0000138109.32748.80. [DOI] [PubMed] [Google Scholar]
- 62.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005;288:H328–335. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 64.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724–735. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 66.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231–2238. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 67.Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 69.Takayama Y, Tanaka S, Nagai K, Okada M. Adenovirus-mediated overexpression of C-terminal Src kinase (Csk) in type I astrocytes interferes with cell spreading and attachment to fibronectin. Correlation with tyrosine phosphorylations of paxillin and FAK. J Biol Chem. 1999;274:2291–2297. doi: 10.1074/jbc.274.4.2291. [DOI] [PubMed] [Google Scholar]
- 70.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Lin HJ, Hsieh FC, Song H, Lin J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br J Cancer. 2005;93:1372–1381. doi: 10.1038/sj.bjc.6602862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 73.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288:C30–38. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 74.Kuppuswamy D. Importance of integrin signaling in myocyte growth and survival. Circ Res. 2002;90:1240–1242. doi: 10.1161/01.res.0000025080.78636.23. [DOI] [PubMed] [Google Scholar]
- 75.Pfister R, Acksteiner C, Baumgarth J, Burst V, Geissler HJ, Margulies KB, Houser S, Bloch W, Flesch M. Loss of beta1D-integrin function in human ischemic cardiomyopathy. Basic Res Cardiol. 2007;102:257–264. doi: 10.1007/s00395-006-0640-1. [DOI] [PubMed] [Google Scholar]
- 76.Peng X, Wu X, Druso JE, Wei H, Park AY, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, Guan JL. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci U S A. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spector NL, Yarden Y, Smith B, Lyass L, Trusk P, Pry K, Hill JE, Xia W, Seger R, Bacus SS. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakaoka Y, Nishida K, Narimatsu M, Kamiya A, Minami T, Sawa H, Okawa K, Fujio Y, Koyama T, Maeda M, Sone M, Yamasaki S, Arai Y, Koh GY, Kodama T, Hirota H, Otsu K, Hirano T, Mochizuki N. Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J Clin Invest. 2007;117:1771–1781. doi: 10.1172/JCI30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu W, Mimnaugh EG, Kim JS, Trepel JB, Neckers LM. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones. 2002;7:91–96. doi: 10.1379/1466-1268(2002)007<0091:hngrti>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S, Sawyer DB. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem. 2005;280:13148–13152. doi: 10.1074/jbc.M410838200. [DOI] [PubMed] [Google Scholar]
- 82.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 83.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 84.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 85.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, Le XF. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 86.Kerbel RS, Viloria-Petit A, Klement G, Rak J. ‘Accidental’ anti-angiogenic drugs. anti-oncogene directed signal transduction inhibitors and conventional chemotherapeutic agents as examples. Eur J Cancer. 2000;36:1248–1257. doi: 10.1016/s0959-8049(00)00092-7. [DOI] [PubMed] [Google Scholar]
- 87.Linderholm B, Andersson J, Lindh B, Beckman L, Erlanson M, Edin K, Tavelin B, Grankvist K, Henriksson R. Overexpression of c-erbB-2 is related to a higher expression of vascular endothelial growth factor (VEGF) and constitutes an independent prognostic factor in primary node-positive breast cancer after adjuvant systemic treatment. Eur J Cancer. 2004;40:33–42. doi: 10.1016/s0959-8049(03)00673-7. [DOI] [PubMed] [Google Scholar]
- 88.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 89.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 90.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 92.Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali PG, Sacchi A. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- 93.Tagliabue E, Ardini E, Pellegrini R, Campiglio M, Bufalino R, Jeschke M, Groner B, Colnaghi MI, Menard S. Laminin activates the p185HER2 oncoprotein and mediates growth inhibition of breast carcinoma cells. Br J Cancer. 1996;74:1427–1433. doi: 10.1038/bjc.1996.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campiglio M, Tagliabue E, Srinivas U, Pellegrini R, Martignone S, Menard S, Colnaghi MI, Lombardi L, Marchisio PC. Colocalization of the p185HER2 oncoprotein and integrin alpha 6 beta 4 in Calu-3 lung carcinoma cells. J Cell Biochem. 1994;55:409–418. doi: 10.1002/jcb.240550402. [DOI] [PubMed] [Google Scholar]
- 95.Mocanu MM, Fazekas Z, Petras M, Nagy P, Sebestyen Z, Isola J, Timar J, Park JW, Vereb G, Szollosi J. Associations of ErbB2, beta1-integrin and lipid rafts on Herceptin (Trastuzumab) resistant and sensitive tumor cell lines. Cancer Lett. 2005;227:201–212. doi: 10.1016/j.canlet.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 96.Fazekas Z, Petras M, Fabian A, Palyi-Krekk Z, Nagy P, Damjanovich S, Vereb G, Szollosi J. Two-sided fluorescence resonance energy transfer for assessing molecular interactions of up to three distinct species in confocal microscopy. Cytometry A. 2007 doi: 10.1002/cyto.a.20489. [DOI] [PubMed] [Google Scholar]
- 97.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 98.Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between alpha(6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem. 2000;275:10604–10610. doi: 10.1074/jbc.275.14.10604. [DOI] [PubMed] [Google Scholar]
- 99.Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 100.Han J, Jenq W, Kefalides NA. Integrin alpha2beta1 recognizes laminin-2 and induces C-erb B2 tyrosine phosphorylation in metastatic human melanoma cells. Connect Tissue Res. 1999;40:283–293. doi: 10.3109/03008209909000706. [DOI] [PubMed] [Google Scholar]
- 101.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel R, Kos L. Endothelin-1 and Neuregulin-1 convert embryonic cardiomyocytes into cells of the conduction system in the mouse. Dev Dyn. 2005;233:20–28. doi: 10.1002/dvdy.20284. [DOI] [PubMed] [Google Scholar]
- 103.Milan DJ, Giokas AC, Serluca FC, Peterson RT, MacRae CA. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development. 2006;133:1125–1132. doi: 10.1242/dev.02279. [DOI] [PubMed] [Google Scholar]
- 104.Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 105.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- 106.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 107.Untch M, Himsl I, Kahlert S, Lueck HJ, Eidtmann H, Du Bois A, Meerpohl HG, Thomssen C, Harbeck N, Jackisch C, Kreienberg R, Emons G, Wallwiener D, Wiese W, Schaller G, Kuhn W, Muscholl M, Pauschinger M, Langer B. Anthracycline and trastuzumab in breast cancer treatment. Oncology (Williston Park) 2004;18:59–64. [PubMed] [Google Scholar]
- 108.Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 109.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 110.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 111.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 112.Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]