Introduction
Radiofrequency ablation is becoming an accepted treatment option for many tumors in the liver, lung, kidney and bone (1-4). As RF ablation technologies improve, larger tumors in more difficult anatomic locations are being treated. In many centers large tumors are treated by sequentially overlapping single-electrode ablations where, after each ablation, the electrode is repositioned and activated in untreated tumor adjacent to the prior zone of ablation (5). This process is repeated multiple times until the entire tumor volume plus an ablative margin has been covered. However, the process of overlapping ablations is plagued by difficulties locating residual viable tumor, repositioning and guiding the electrode to an untreated site, and long procedure times even on relatively small tumors.
Alternatively, multiple applicators applied simultaneously have been shown to increase the ablation zone volume when a single device is incapable of ablating an entire tumor (6-8). Using multiple applicators in concert has several potential advantages. First, thermal synergy between the growing ablation zones increases the temperature boundary for each ablation zone which, in turn, may allow more heat to be contained to the target area, resulting in more confluent zones of ablation (9-11). Second, local ischemia caused by adjacent ablations may reduce perfusion-mediated cooling in each ablation zone. Third, placement of multiple applicators prior to any tissue heating may be simpler than repositioning after each ablation since the tumor is not obscured by previous ablations (7). The location of the first placement can be relatively imprecise and used as a guide, with subsequent applicators placed relative to this guide to create an ideal array in and around the tumor. Finally, simultaneous ablations are inherently faster than sequential ablations when sufficient power is available.
Recently, an RF ablation system has become available that allows multiple electrodes to be applied simultaneously using a switching algorithm. The algorithm uses the inherent “off” time associated with pulsed single-electrode application to deliver power through additional electrodes (12). This switching algorithm has been shown to create large, confluent zones of ablation in both preclinical and clinical studies (7, 9, 13). However, little work has been performed to directly compare simultaneous application of multiple electrodes to the sequential technique, and even less to define the roles of thermally synergistic interactions between multiple-electrodes or reduced perfusion caused by nearby ablations. The purpose of this study was to compare radiofrequency ablations created using a sequential technique to those created simultaneously using a switching algorithm in ex vivo and in vivo liver models.
Materials and Methods
All animal studies were performed with approval from our institution's Animal Care and Use committee and in accordance with the Guide for Care and Use of Laboratory Animals issued by the National Research Council (14).
Ex vivo experimental setup
Radiofrequency ablations were performed in ex vivo bovine liver at 20-22 °C in two groups. In Group 1a (n = 14 ablations), zones of ablation were created by sequentially overlapping single-electrode ablations (Cool-tip™; Valleylab/Covidien, Boulder, CO). Each ablation was performed for 12 min with a 5 min wait time before inserting the subsequent electrode simulate electrode repositioning. A maximum power of 200 W was applied using the generator's internal impedance feedback algorithm. In Group 2a (n = 14 ablations), zones of ablation were created by powering three electrodes for 12 min using a switching algorithm with a maximum power of 200 W (Cool-tip™ Switching Controller™; Valleylab/Covidien). Cooled needle electrodes with 3 cm active tips were used for all parts of this study. Electrodes were placed 2 cm apart in a triangular configuration maintained by an acrylic template in both groups (Figure 1). Electrodes were cooled with chilled water at 5 °C at a flow rate of approximately 100 ml/min.
Figure 1.
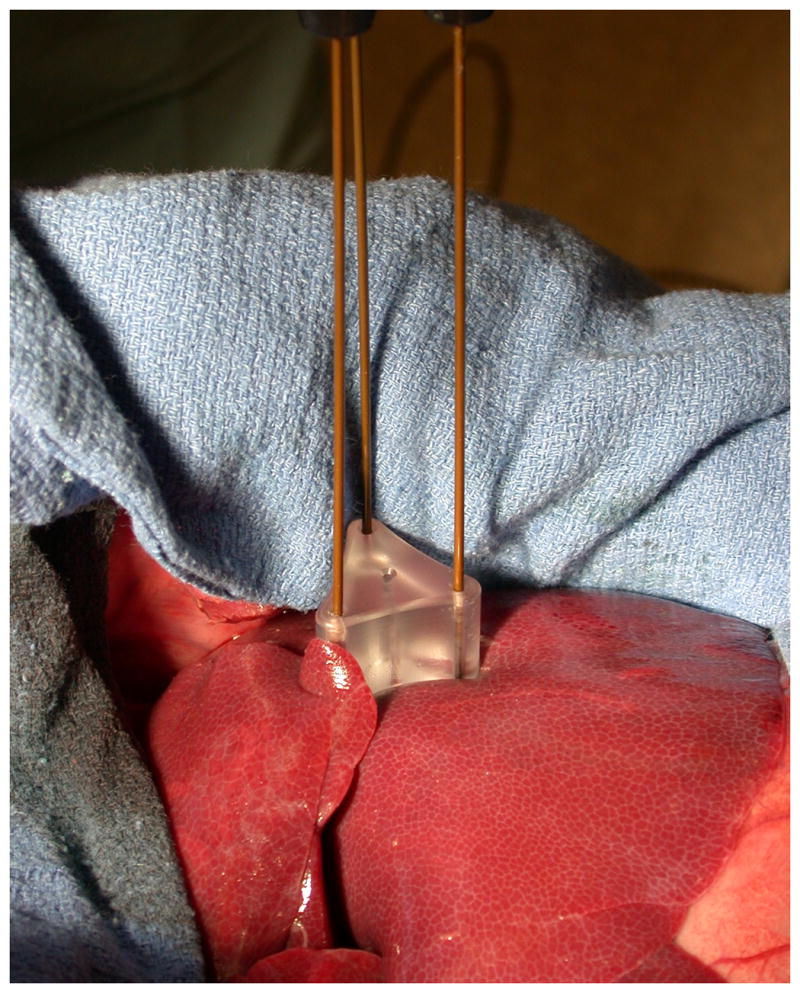
Experimental setup for both groups. A spacer was used to ensure parallel insertion and maintain the 2.0 cm spacing between electrodes. All in vivo procedures were performed at open surgery.
In vivo experimental setup
A total of six female domestic swine (50-60 kg) were utilized for this study. Pre-anesthetic sedation was achieved with 7 mg/kg intramuscular tiletamine hydrochloride/zolazepam hydrochloride (Telazol; Fort Dodge, IA) and 2.2 mg/kg xylazine hydrochloride (Xyla-Ject; Phoenix Pharmaceutical, St. Joseph, MO). Endotracheal intubation was performed in the standard fashion, facilitated by 0.05 mg/kg atropine (Phoenix Pharmaceutical, St. Joseph, MO). Anesthesia was then induced and maintained with inhaled isofluorane to effect (Halocarbon Laboratories, River Edge, NJ). The liver was surgically exposed through a midline incision to allow electrode positioning by visual inspection.
Radiofrequency ablations were performed in two groups (Groups 2a and 2b, n = 6 ablations each, one per group per animal), using the same setup and heating parameters outlined for the ex vivo study (Figure 1). Ablations were performed in the medial lobes of the liver. To reduce the number of animals required and limit the influence of inter-animal variability in tissue composition, blood flow, anatomy, etc., one ablation from each group was performed in each animal. Because of the relative time difference between groups, ablation order was alternated between animals so that an equal number of experiments were performed starting with either sequential or switched ablations. The order of sequential application was selected randomly to minimize bias caused by local ischemia induced from adjacent ablations and anisotropic blood flow. After completing the ablations, animals were sacrificed using an intravenous injection of 0.2 ml/kg Beuthanasia-D (390 mg/ml pentobarbital sodium and 50 mg/ml phenytoin sodium; Schering-Plough, Kenilworth, NJ).
Measured variables
In the ex vivo study, temperatures were measured in 1 s intervals at distances of 0 cm, 1 cm, 2 cm and 3 cm along a line emanating from the isocenter of the electrode array and bisecting two of the electrodes using fiber optic temperature sensors (Lumasense Technologies, Santa Cruz, CA). Due to limited spatial resolution of this technique, the temperature distribution was assumed to have threefold rotational symmetry (ie, temperature distributions were identical between each pair of electrodes).
Ablation zones from both ex vivo and in vivo studies were sliced transverse to the electrode insertion track in approximately 5 mm increments. Samples were placed onto a flatbed scanner and digital images saved electronically. Measurements were performed with consensus between two observers on digital images using the software ImageJ v1.39e (National Institutes of Health, Bethesda, MD). Cross-sectional measurements included minimum diameter, maximum diameter, area, isoperimetric ratio and the diameter of the maximum circle inscribed by the ablation zone (inscribed diameter). Distances between electrode tracks were recorded as a surrogate for tissue shrinking measurements. Each component of sequential ablations was also measured individually for mean diameter, area and isoperimetric ratio when the ablation was not fully confluent (n = 3). Measurements were made by extrapolating the ablation shape around each electrode based on visual inspection. Procedure time was defined as the time between first power application and final shutdown, which included repositioning intervals in sequential groups.
Statistical Analysis
Descriptive statistics were calculated for each measurement, including temperatures for each time point. Ex vivo study groups were compared using a one-tailed Mann-Whitney test, based on the hypothesis that the switched group must have larger dimensions and circularity than the sequential group (15). Post-ablation inter-electrode distances were also compared between groups using a two-tail Welch corrected test. Ex vivo study results were used to predict the minimum number of samples required for the in vivo study under the assumption that ablations created in vivo are 25 percent smaller in diameter than those created ex vivo using the same input parameters. With data paired by animal, we determined that a minimum of six pairs would be required for 80 percent statistical power. In vivo study groups were compared using the one-tailed Wilcoxon matched pairs test with data paired by animal. ANOVA and post-hoc t-tests was used to identify differences between the individual components of sequential ablations. P-values less than 0.05 were considered significant.
Results
Animals tolerated the procedures well and no complications were encountered during the course of this study.
Ex vivo study
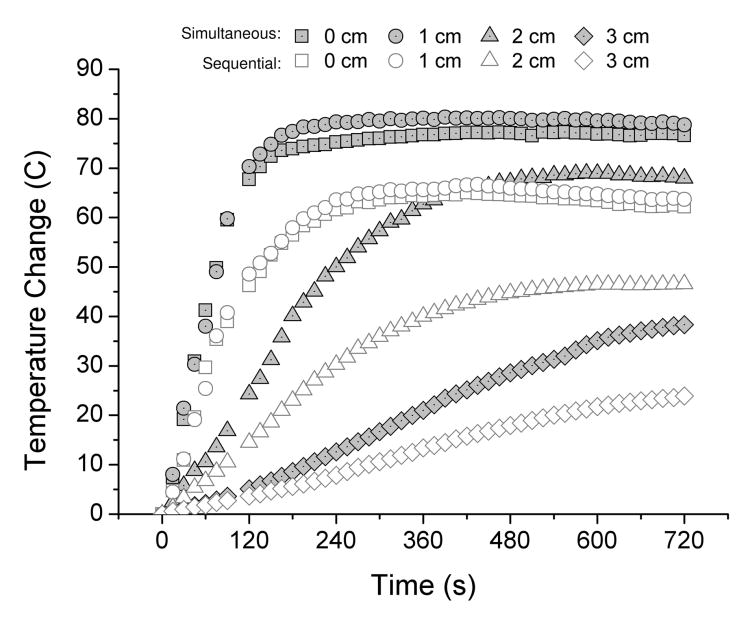
Ablations created in the switched group were significantly larger and more circular than those created in the sequential group (Table 1, Figure 2). Mean (± standard deviation) ablation diameters in Group 1a and Group 2a were 4.71 ± 1.0 cm and 5.70 ± 0.5 cm, respectively (P < .01). Cross sectional areas were 18.8 ± 6.6 cm2 and 25.4 ± 5.3 cm2, respectively (P = .001), representing a mean growth in area of 35.1 percent when ablations are created simultaneously by switching, without the influence of blood flow. Measurement standard deviations in Group 2a were consistently smaller than in Group 1a, indicating more consistency when creating ablations simultaneously by switching. Mean temperatures measured during switched ablations were correspondingly higher and elevated faster than during sequential ablations at each spatial location (Figure 3). Total procedure time was 46 min for sequential activation and 12 min in for switched activation.
Table 1. Summary of ex vivo ablations given as mean ± standard deviation. Pre-ablation electrode spacings are assumed to be 2.0 cm.
| Group | Sample size |
Treatment time (min) |
Mean Diameter (cm) |
Minimum Diameter (cm) |
Maximum Diameter (cm) |
Area (cm2) |
Circularity | Inscribed Diameter (cm) |
Post- ablation Electrode Spacing (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Single† | 2.5 | 14.7 | |||||||
| Sequential | 14 | 46 | 4.71 ± 1.0 | 4.29 ± 1.0 | 5.13 ± 1.1 | 18.8 ± 6.6 | 0.88 ± 0.09 | 4.40 ± 0.9 | 1.75 ± 0.1 |
| Simultaneous | 14 | 12 | 5.70 ± 0.5 | 5.33 ± 0.4 | 6.07 ± 0.6 | 25.4 ± 5.3 | 0.97 ± 0.01 | 5.41 ± 0.5 | 1.66 ± 0.1 |
| P-value | .0067 | .0020 | .0123 | .0012 | .0001 | .0034 | .0055 |
Historical data based on previous studies in the same animal model and experimental conditions (17). Areas provided are three-times the area of a single ablation for easier comparison to data from the present study.
Figure 2.
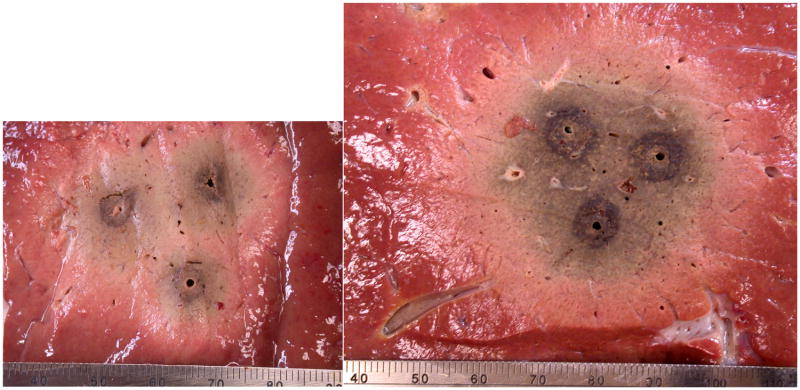
Representative ablations created in ex vivo bovine liver tissue using sequential (left) and simultaneous (right) application of three electrodes. Ablations created simultaneously were consistently larger and more circular in cross section, had better temperature profiles and could be created much faster than those created sequentially.
Figure 3.
Mean temperature elevations recorded at each time point for sequential and simultaneous application of three electrodes ex vivo. Temperatures were consistently higher and rose faster at each measurement point when using simultaneous application.
At gross inspection, the central portions of switched ablations were denser and more desiccated than in Group 1a (Figure 2). Swiched ablations were characterized by sharp contrast between the zone of ablation and normal tissue whereas the peripheries of sequential ablations were often diffuse in appearance. The mean (± standard deviation) distance between electrode tracks post-ablation were significantly smaller in Group 2a (1.66 ± 0.12) than in Group 1a (1.75 ± 0.11, P < .01), indicating that tissues collapsed and shrank more during switched ablations (Table 1). These observations were supported by temperature measurements (Figure 3).
In vivo study
As in the ex vivo study, ablations created in vivo were significantly larger and more circular when using switched application (Table 2,Figure 4). Mean (± standard deviation) diameters in Group 1b and Group 2b were 3.55 ± 0.6 cm and 4.56 ± 0.9 cm, respectively (P < .05). Cross sectional areas were 13.2 ± 4.2 cm2 and 17.1 ± 5.1 cm2, respectively (P < .05), representing a mean growth in area of 29.5 percent when ablations are created simultaneously by switching in live animals. Mean cross-sectional areas decreased by 30 and 33 percent in the Group 1b and Group 2b, respectively, when comparing the ex vivo to the in vivo model.
Table 2. Summary of in vivo ablations given as mean ± standard deviation.
| Group | Sample size | Treatment time (min) |
Mean Diameter (cm) |
Minimum Diameter (cm) |
Maximum Diameter (cm) |
Area (cm2) |
Circularity | Inscribed Diameter (cm) |
|---|---|---|---|---|---|---|---|---|
| Single† | 2.0 | 9.4 | ||||||
| Sequential | 6 | 46 | 3.55 ± 0.6 | 2.48 ± 1.0 | 4.63 ± 0.4 | 13.2 ± 4.2 | 0.72 ± 0.15 | 2.99 ± 0.9 |
| Simultaneous | 6 | 12 | 4.59 ± 0.9 | 3.89 ± 1.1 | 5.28 ± 0.6 | 17.1 ± 5.1 | 0.83 ± 0.09 | 3.93 ± 1.0 |
| P-value | .0156 | 0.0156 | 0.0156 | 0.0313 | 0.0313 | 0.0469 |
Historical data based on previous studies in the same animal model and experimental conditions (17). Areas provided are three-times the area of a single ablation for easier comparison to data from the present study.
Figure 4.
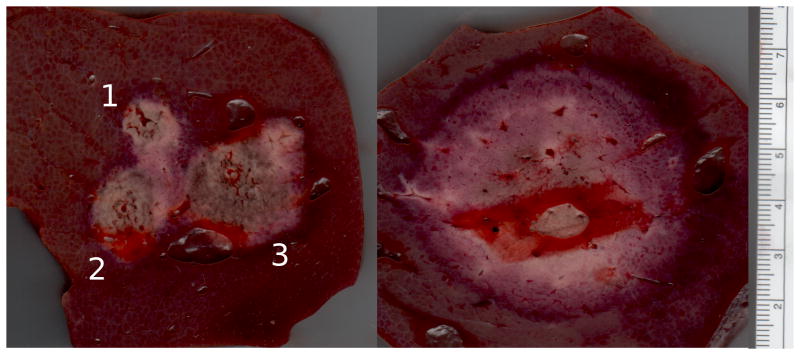
Representative ablations created in vivo using sequential (left) and simultaneous (right) application of three electrodes. As in the ex vivo study, ablations created simultaneously were again larger and more circular in cross section than those created sequentially. Note that each subsequent ablation in the sequential image is larger than previous ablations. This effect is most likely a result of local ischemia created by previous ablations.
Significantly greater cross-sectional areas were observed in subsequent components of sequential ablations; that is, the second ablation was larger than the first, and the third ablation was larger than the second (ANOVA P = .001; Table 3). Anecdotally, this seemed more evident when the subsequent ablation was placed peripheral to the previous ablation, though not enough data was available to test this hypothesis statistically (Figures 4-5). No significant differences were noted in isoperimetric ratio.
Table 3. Breakdown of sequential ablation components given as mean ± standard deviation.
| Ablation Sequence Number | Sample size | Mean Diameter (cm) |
Area (cm2) |
Circularity |
|---|---|---|---|---|
| 1 | 3 | 1.70 ± 0.3 | 2.35 ± 1.0 | 0.94 ± 0.01 |
| 2 | 3 | 1.92 ± 0.2 | 2.98 ± 0.8 | 0.92 ± 0.04 |
| 3 | 3 | 2.43 ± 0.2 | 4.68 ± 0.5 | 0.88 ± 0.02 |
| P-value | .0049 | .0010 | .1506 |
Figure 5.
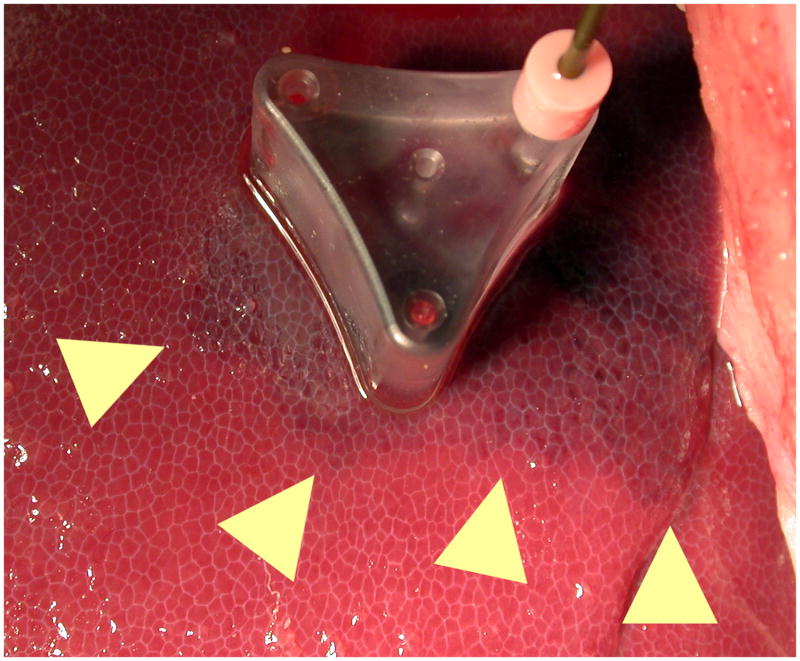
Local ischemia induced after the first ablation of a sequential ablation (edges marked with arrows). The reduced perfusion caused by this ablation allowed subsequent ablations to grow larger in size, due to the lack of perfusion-mediated cooling. Ischemia is more pronounced in areas peripheral to the ablation.
Discussion
The principal finding of this study was that simultaneous application of multiple electrodes using a switching algorithm creates ablations that are larger, more confluent, more consistent and faster than sequentially overlapping single-electrode ablations in the same configuration. We also noted an increased size in subsequent ablations when using the sequential technique. We attribute the increasing size primarily to reduced perfusion-mediated tissue cooling caused by the previous ablations. While thermal synergy between ablation zones could be a contributing factor, the five-minute delay between ablations most likely reduced the temperature of the previous ablation substantially, thereby reducing the effects of thermal synergy.
Comparison to historical single-electrode data was also striking. In the ex vivo model, sequential ablations were 28 percent larger in area than historical controls (18.8/14.7), while switched ablations were 73 percent larger (25.4/14.7). Similarly, in the in vivo model, sequential ablations were 40 percent larger in area than historical controls (13.2/9.4), while switched ablations were 82 percent larger (17.1/9.4). Since perfusion is absent from the ex vivo model, our data indicates that simultaneous application of multiple electrodes by switching creates more thermal synergy between growing ablations than sequential application, but that sequential ablations are also larger than would be expected based on single-electrode data alone. The effects of using multiple electrodes are more pronounced in vivo, with additional benefits gained by ablation-induced ischemia for both switched and sequential multiple-electrode ablation. Maximal benefit can be expected when using switched application of multiple electrodes.
A prior analysis assuming a static ablation zone size showed that a large number of ablations may be required when using the sequential technique, even on relatively small tumors (5). However, that study was limited by the use of geometric modeling based on theoretical, idealized ablation zones. Our ex vivo and in vivo study accounts for both thermal synergy inherent in multiple-applicator ablations, and ischemia created by ablation zones which decreases perfusion mediated cooling on subsequent ablations. In our study, the second ablation was 28 percent larger in cross-sectional area than the first ablation; the third ablation was 56 percent larger than the second and approximately twice the size of the first ablation. Under these circumstances, the number of required ablations estimated by geometrical modeling must be reconsidered. As a simplified example, consider the cross-section of a 3 cm tumor. The static-size assumption would require seven 1.8 cm ablations to cover the tumor area with a small ablative margin, but using the growth trends observed in this study, only four ablations would be required to cover the same area (Figure 6). A new analysis of the overlapping technique is warranted if it is to be used for treatment planning. Note that, as shown in this study and others, a single switched application of three electrodes would easily encompass this tumor and margin (7, 9).
Figure 6.
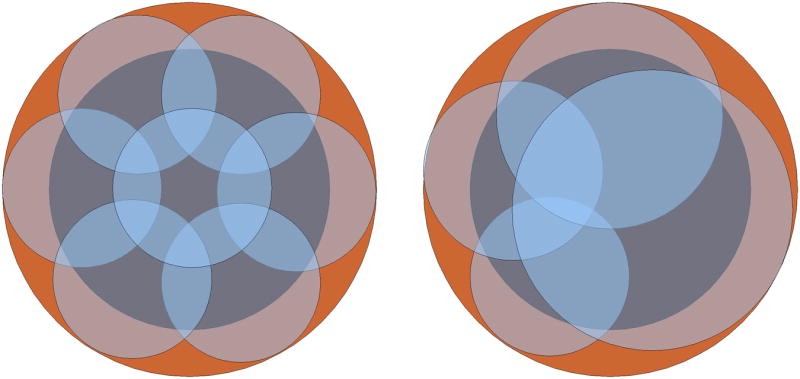
Simplified example comparing a static-size model (left) to a more realistic model (right) that accounts for changes in ablation size to predict coverage of a 3 cm tumor using the overlapping ablation technique. The initial ablation size was equivalent in both models (1.8 cm diameter) but in the dynamic-size model the ablation diameter grew by 13, 25 and 25 percent, based on the results of the present study. The static-size model requires seven ablations to cover the tumor with a small margin whereas the model based on in vivo results requires only four.
The strategy of sequentially overlapping ablations is time-consuming, and presents technical and safety concerns. Residual tumor left after a particular ablation can be extremely difficult to locate by imaging, especially if the residual volume is small. Bubbles created during RF heating tend to obscure ultrasound images and, after bubbles dissipate, leftover tumor is not well-visualized. Locating and guiding an electrode into residual tumor post-ablation can be challenging. In addition, covering even a 3 cm tumor with an appropriate ablative margin may require several individual ablations (5). This underscores the daunting prospect of adequately treating larger tumors with the overlapping technique. Finally, needle repositioning during percutaneous ablation may be linked to tumor seeding (16). For these reasons, using multiple-electrodes with switching seems to be a safer, more effective alternative when available.
To our knowledge, only one study has compared the techniques of sequentially overlapping ablations to simultaneous application of multiple electrodes by switching (15). In that study, it was also concluded that switched ablations were larger, more confluent, and could be performed faster than sequential ablations. However, that study by Lee et al. was limited by technique. Sequential ablations were performed using no time interval between heating periods. In clinical situations, however, 5-10 min or more are often required to reposition electrodes between ablations. During this period, blood flow can cool the prior ablation, decreasing some of its influence on subsequent ablations. In Lee et al., ablation times in the switched groups were also longer than each of the sequential ablations. However, it has been shown using a similar system that three ablation zones created simultaneously in separate parts of the liver are no different in size or shape than a single-electrode ablation (17). This is largely because electrode “off-time” inherent to the RF power pulsing algorithm can be used to activate additional electrodes, thereby increasing the duty cycle of the generator. The use of a longer ablation time in the switched groups of Lee et al. (18-24 min per electrode) leaves open the possibility that each component of the confluent ablation was actually larger than would be expected from a normal single-electrode ablation (12 min per electrode). Lee et al. also noted that total ablation times were shorter in the switched groups, which is certainly clinically significant, but direct comparison of sizes and shapes using their approach may be biased by the time disparity between groups. In our study, ablation time per electrode was constant between groups to limit this bias.
Limitations to our study include the use of a normal liver model, the inherent time difference between groups, lack of data regarding pre- and post-ablation measurements and use of a single experimental configuration. Large animal tumor models are not widely available, are expensive, and may not be fully representative of human tumors. The normal porcine liver model is a suitable preclinical model for tumor ablation device characterization as noted in several previous studies. As noted above, the techniques of sequentially overlapping or simultaneously applying multiple applicators creates an inequality in procedure time that is clinically beneficial but creates potential input bias. One way to avoid this limitation would be to standardize the total output energy in each group. With the systems used in this study, ablation time is the most relevant clinical endpoint, so we chose to standardize for application time. Future studies should consider standardizing for energy output. Finally, tissues shrink during ablation, an effect that correlates with both temperature and exposure time (18). In the present study, we noted that ablations created simultaneously by switching contained higher internal temperatures, resulting in denser and more desiccated ablation zones than those created sequentially. We also observed shorter post-ablation inter-electrode spacings, suggesting that shrinking was more substantial when using switched application. If true, pre-treatment tissue volumes that were ablated would be even larger than indicated here. However, the lack of reliable markers to compare pre- and post-ablation dimensions in the present study limit the conclusiveness of these observations. Finally, only one experimental configuration was tested. Additional optimization may be possible by altering electrode length, number, spacing or generator power.
In conclusion, simultaneous application of multiple electrodes using a switching algorithm creates ablation zones that are faster, larger and more confluent than sequential ablations. While thermal synergy plays a considerable role in defining a multiple-electrode ablation, local ischemia caused by adjacent ablations should not be overlooked. If the sequential technique must be used to maximize ablation zone size, subsequent ablations should be placed in the ischemic zones created by previous ablations whenever possible.
Acknowledgments
This work funded in part by the National Institutes of Health, R01-CA108869-01
Footnotes
Portions of this material presented at the SIR 2008 Annual Meeting in Washington, DC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cantwell CP, O'Byrne J, Eustace S. Radiofrequency ablation of osteoid osteoma with cooled probes and impedance-control energy delivery. AJR Am J Roentgenol. 2006;186:S244–248. doi: 10.2214/AJR.04.0938. [DOI] [PubMed] [Google Scholar]
- 2.Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 4.Rose SC, Thistlethwaite PA, Sewell PE, et al. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927–951. doi: 10.1097/01.RVI.0000222707.44902.66. [DOI] [PubMed] [Google Scholar]
- 5.Dodd GD, 3, Frank MS, Aribandi M, et al. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 6.Rewcastle JC, Sandison GA, Muldrew K, et al. A model for the time dependent three-dimensional thermal distribution within iceballs surrounding multiple cryoprobes. Med Phys. 2001;28:1125–1137. doi: 10.1118/1.1374246. [DOI] [PubMed] [Google Scholar]
- 7.Laeseke PF, Frey TM, Brace CL, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol. 2007;188:1485–1494. doi: 10.2214/AJR.06.1004. [DOI] [PubMed] [Google Scholar]
- 8.Vogl TJ, Straub R, Zangos S, et al. Mr-guided laser-induced thermotherapy (litt) of liver tumours: experimental and clinical data. Int J Hyperthermia. 2004;20:713–724. doi: 10.1080/02656730400007212. [DOI] [PubMed] [Google Scholar]
- 9.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241:116–124. doi: 10.1148/radiol.2411051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright AS, Lee FTJ, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 12.Lee FTJ, Haemmerich D, Wright AS, et al. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–1442. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- 13.Laeseke PF, Sampson LA, Frey TM, et al. Multiple-electrode radiofrequency ablation: comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol. 2007;18:1005–1010. doi: 10.1016/j.jvir.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 15.Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 16.Stigliano R, Marelli L, Yu D, et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. what is the risk and the outcome? seeding risk for percutaneous approach of hcc. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation: simultaneous production of separate zones of coagulation in an in vivo porcine liver model. J Vasc Interv Radiol. 2005;16:1727–1735. doi: 10.1097/01.rvi.000018362.17771.b0. [DOI] [PubMed] [Google Scholar]
- 18.Diaz TA, Sampson LA, Hinshaw JL, et al. Ablation-induced tissue shrinking: Ablation zone measurements do not accurately reflect pre-ablation volumes in liver and lung tissues. Presented at the World Congress on Interventional Oncology; June 22-25; CA: Lox Angeles; 2008. [Google Scholar]