Abstract
Inconsistent differences in the corpus callosum (CC) structure between dyslexic readers (DRs) and typical readers (TRs) have been reported. We examine differences in CC splenium microstructure and the association of splenium microstructure with reading related skills. Nine DRs and eighteen TRs completed a reading skills battery and diffusion tensor imaging (DTI). DRs had higher splenium fractional anisotropy (FA) and axial diffusivity (LA) as compared to TRs. Retrieval of orthographic information from the language lexicon was negatively associated with FA and LA within both reading groups. Phonological awareness was positively associated with splenium FA and LA in TDs but not DRs. This study suggests two white matter pathways that may be differentially associated with reading skills in the CC splenium.
Keywords: Dyslexia, Diffusion Tensor Imaging, Corpus Callosum, Phonological Awareness, White matter microstructure
Introduction
Developmental dyslexia, the most common learning disorder in America, is defined as poor reading performance despite adequate intelligence, motivation and schooling [1]. Dyslexic readers (DRs) who are considered “recovered,” and are admitted to college, manifest sub-optimal performance in reading and reading-related tasks, proving that that early detection and treatment do not guarantee abiding recovery [2]. To identify novel biological interventions and paths to prevention, the underlying neural mechanisms of dyslexia need to be understood.
The neurological basis of dyslexia remains elusive. Classic, decades old, neuropathological evidence implicated cortical dysplasias as key [3]. However, a preponderance of recent studies point to abnormalities in brain connectivity [4-12]. However, the largest interhemispheric white matter tract, the corpus callosum (CC), has only been examined in terms of macrostructure, not microstructure. Interhemispheric connectivity is especially important in dyslexia, since functional imaging studies have suggested that DRs recruit right hemisphere areas to compensate for poor left hemisphere function during reading [13,14]. Macrostructure studies of the CC in dyslexia have inconsistent findings, but several studies point to a difference in posterior CC macrostructure, particularly the splenium [15,16]. Such findings are consistent with the fact that the splenium contains interhemispheric crossing for the white matter tracts connecting the temporal, parietal and occipital lobes, areas that demonstrate abnormal lateralization of activity in dyslexia [13,14].
Whole brain voxel-based methods have not found differences in the CC microstructure related to reading skill [8-11]. This is not surprising since such methods provide highly variable measurements in the CC area [17]. Using fiber tracking analysis, Dougherty et al. [17] found that the microstructure of temporal lobe interhemispheric pathways, measures 1cm on either side of midline, projecting through the CC splenium in children without dyslexia was related to the Phonological Awareness Composite (PA) of the Comprehensive Test of Phonological Processing (CTOPP).
In the current study we specifically measure CC splenium microstructure using our recently described and validated diffusion tensor imaging (DTI)-based segmentation algorithm [18,19] in young adult DRs and typical readers (TRs). Given the striking alteration in hemispheric cortical lateralization during reading in DRs into adulthood [13,14] and that CC microstructure is a sensitive indicator of hemispheric language lateralization [20], CC microstructure might be vital to understanding dyslexia. Since the CC continues to develop through adolescences [21] and into adulthood [22], developmental changes in DRs that extend into adulthood may be accompanied by neuroplastic changes in CC structure. Since abnormal lateralization of temporoparietal and occipital function may develop along two different trajectories, we predict that skills linked to each cortical area, specifically phonological awareness and orthographic skills, respectively, will demonstrate different relationships to CC microstructure.
Methods
Participants
Nine young adults DRs were matched on a 2:1 ratio to 18 TRs based on age and gender. Participants were given the Woodcock-Johnson III Letter-Word Identification (LWID), the CTOPP PA, Alternative Phonological Awareness (PAA), Rapid Naming (RN) and Alternative Rapid Naming (RNA) composites, the Tests of Variables of Attention (TOVA) and the Comprehensive Test of Non-Verbal Intelligence (CTONI; Table 1). DRs reading skills varied from normal to subnormal values and varied throughout the normal range for TRs. This prevented significant linear correlations from arising simply due to large interclass differences.
Table 1.
Participant Characteristics
| Typically Developing | Reading Disability | ||
|---|---|---|---|
| N (Male) | 18 (12) | 9 (6) | |
| Mean (SE) | Mean (SE) | t-test | |
| Age (years) | 24.3 (1.9) | 23.2 (0.9) | 0.65 |
| Intelligence | |||
| CTONI (std) | 111.6 (2.8) | 104.1 (4.0) | 1.57 |
| Attention | |||
| TOVA: Commissions (std) | 108.2 (2.6) | 108.5 (3.2) | 0.07 |
| TOVA: Omissions (std) | 101.8 (3.3) | 99.5 (3.5) | 0.45 |
| TOVA: Reaction Time (std) | 111.9 (2.6) | 100.9 (6.9) | 1.93 |
| TOVA: Reaction Time (ms) | 345.3 (10.9) | 389.5 (27.5) | |
| Language | |||
| Letter-Word Identification (std) | 104.7 (1.5) | 84.8 (6.3) | 4.27*** |
| Phonological Awareness (std) | 112 (1.1) | 93.2 (5.3) | 5.00**** |
| Rapid Naming (std) | 106.0 (4.4) | 82.8 (4.2) | 3.48** |
| Alt. Phonological Awareness (std) | 109.8 (3.3) | 89 (6.4) | 3.39** |
| Alt. Rapid Naming (std) | 103 (3.1) | 94.4 (4.8) | 1.48 |
p<0.05
p<0.01
p<0.001
p<0.0001
Participants were recruited through flyers and contacts in the Houston area as well as from a longitudinal child development study. DRs reported a history of reading disabilities during grade school and demonstrated specific weakness in reading related skills. Reading groups demonstrated equivalent intelligence and attention scores (Table 1). Phone interviews eliminated non-native English speakers, individuals with a abnormal neurologic, psychiatric or birth history, implanted ferromagnetic metal or device, claustrophobia or pregnancy. Right handedness was confirmed using the Edinburgh Handedness Inventory [23]. After description of the study to the participant, written informed consent was obtained in accordance with our institutional review board regulations for the protection of human subjects.
DTI Protocol
We utilized a high signal-to-noise ratio whole-brain DTI protocol at 3.0 T [18]. The CC was segmented on the midsagittal slice using the fractional anisotropy (FA) and axial diffusivity (LA). The feature space was obtained using region-of-interest (ROI) measurements placed on several locations on the midsagittal CC [18,19,22,24,25] (See Figure 1).
Figure 1.
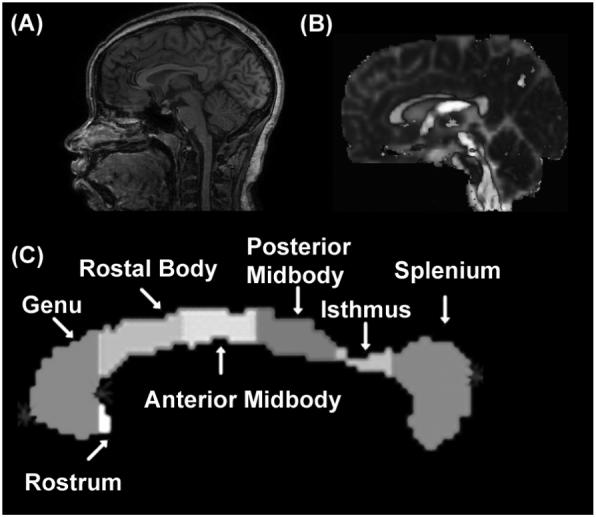
Illustration of the DTI-segmented midsagittal corpus callosum regional subdivisions based on the Witeson 7 segments for a representative subject. (a) midsagittal anatomical MRI View, (b) FA modulated principal vector (brightest gray = rostral-caudal direction, medium gray = anterior-posterior, lightest gray = medial-lateral), (c) segmented CC regions.
Statistical Analysis
Analysis-of-variance (ANOVA) compared microstructure values between reading group (RG). Linear regression was used to investigate the relationships between microstructure and reading skills. Linear regression was used to determine whether reading skills were related to microstructure and whether this relationship was different by RG. The initial linear regression model in the form ‘Reading Skill = Constant + Slope * Microstructure + RG*Constant + RG* Slope * Microstructure’ was fit to the data. The higher order interactions were hierarchically evaluated and dropped from the model if not significant and the model was recalculated. All models with an overall significance of 0.05 are presented below. The significance of each model coefficient is presented in Table 2.
Table 2.
F-values of Linear Regressions with Significant Effects for the Relationship Between Microstructure Indices and Language Skills for the CC Splenium
| Language Skill | Typical | Slope | Typical*Slope |
|---|---|---|---|
| Fractional Anisotropy | |||
| Letter Word Identification | 9.45** | ||
| Phonological Awareness Composite | 6.82* | 3.54 | 5.73* |
| Rapid Naming Composite | 6.94* | 0.66 | 4.54* |
| Axial Diffusivity | |||
| Letter Word Identification | 10.70** | ||
| Phonological Awareness Composite | 8.52** | 2.03 | 7.88* |
| Alternative Phonological Awareness Composite | 8.33** | 0.40 | 6.01* |
| Alternate Rapid Naming Composite | 5.68* | 1.00 | 4.44* |
p<0.05
p<0.01
Results
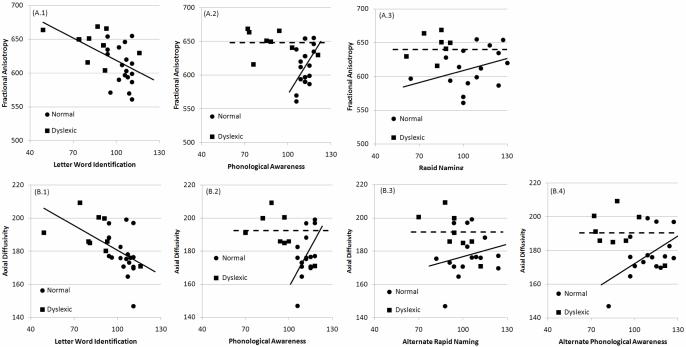
Splenium FA and LA were significantly higher for DRs as compared to TRs (Figure 2) [FA F(1,25)=9.34, p=0.005; LA F(1,25)=6.11, p=0.021]. LWID was negatively associated with FA and LA with these associations similar for both RGs (Figure 3A.1,B.1). PA and RN were positive associated with FA, and PA, PAA and RNA were positive associated with LA, for TRs but not DRs (Figure 3A.2-3,B.2-4). The coefficients in the regression analyses indicated that the average microstructure (FA,LA) values were significantly higher for DRs than TRs for the PA, RN, PAA and RNA regression models (Table 2).
Figure 2.
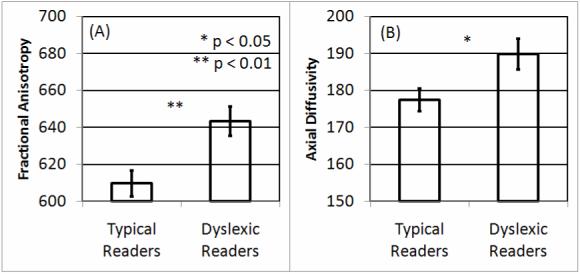
Differences in splenium microstructure between typical readers and dyslexic readers. Larger (A) fractional anisotropy and (B) axial diffusivity values were found for dyslexic readers as compared to typical readers.
Figure 3.
Relationship between reading skills and splenium microstructure. (A) fractional anisotropy and (B) axial diffusivity.
Discussion
This is the first study to specifically compare splenium microstructure between DRs and TRs. We found an increase in both FA and LA in the splenium of DRs as compare to TRs, as well as several relationships between reading skills and splenium microstructure. Studies in the past have examined the CC macrostructure but have provided inconsistent results. The findings from the current study could explain the reports of macrostructural differences in the posterior CC. Since increased FA is positively correlated with CCA [22], and the current study suggests that DRs, as a group, manifest increased FA, it is very possible that the FA in some groups of DRs may be high enough to be detectable in macrostructural measurements. In addition, since CCA and FA are dynamic, changing from childhood through adulthood, group differences in these indices may be very sensitive to age [21,22,24,25].
Skills associated with retrieval of orthographic information from the language lexicon (LWID) were negatively associated with indices of splenium white matter organization (Figure 3A.1,B.1). This decreased organization of splenium interhemispheric white matter tracts may represent decreased connectivity between the ventral occipital areas through occipital interhemispheric callosal fibers, and, thus, greater lateralization of orthographic processing. This would be consistent with the fact that TRs show left lateralized activation of the ventral occipital area, near the so-called visual word form area, while DRs show bilateral activation of this area, during reading [13].
White matter organization in the splenium was positively related to skills associated with phonological awareness (PA, PAA, RN, RNA; Figure 3A.2-3,B.2-4). This finding is consistent with the positive correlation between CC microstructure and hemispheric language lateralization found in previous studies [20]. It should be noted that although the microstructural indices of white matter organization for the DRs groups are not related to these specific reading skills in the splenium, these indices were, on average, higher for the DRs groups than the TRs group. The lack of correlation between phonological awareness skills and splenium microstructure for DRs could be due to the fact that these microstructure values are at a ceiling, possibly as part of a compensatory interhemispheric pathway. These data may also suggest that DRs use alternative pathways for phonologically awareness that are less reliant on interhemispheric fibers that cross at the splenium.
Conclusion
Overall, this study has demonstrated differences in the splenium microstructure between DRs and TRs. Two relationships with reading skills and CC microstructure were found. Skills associated with retrieval of orthographic information from the language lexicon were related to splenium microstructure with a similar relationship in both reading groups. Skills associated with phonological awareness were related to splenium microstructure differently in the two reading groups. While other studies have implicated only one specific white matter pathway related to reading skills, the current study suggests that two separate interhemispheric white matter pathways may be associated with different aspects of skills related to reading.
Acknowledgements
This study was supported by NS046565 to Dr. Richard E. Frye and NS052505-03 to Dr. Khader M. Hasan.
References
- [1].Shaywitz SE. Current concepts: Dyslexia. New England Journal of Medicine. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- [2].Snowling MJ, Gallagher A, Frith U. Family risk of dyslexia is continuous: individual differences in the precursors of reading skill. Child Development. 2003;73:358–373. doi: 10.1111/1467-8624.7402003. [DOI] [PubMed] [Google Scholar]
- [3].Humphreys P, Kaufmann WE, Galaburda AM. Developmental dyslexia in women: neuropathological findings in three patients. Annals of neurology. 1990;28:727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- [4].Jenner AR, Galaburda AM, Sherman GF. Connectivity of ectopic neurons in the molecular layer of the somatosensory cortex in autoimmune mice. Cereb Cortex. 2000;10:1005–1013. doi: 10.1093/cercor/10.10.1005. [DOI] [PubMed] [Google Scholar]
- [5].Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nature neuroscience. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- [6].Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, et al. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119(Pt 1):143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- [7].Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- [8].Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex; a journal devoted to the study of the nervous system and behavior. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- [9].Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, et al. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- [10].Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- [11].Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- [12].Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, et al. Age-related changes in reading systems of dyslexic children. Annals of neurology. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- [14].Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biological psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- [15].Duara R, Kushch A, Gross-Glenn K, Barker WW, Jallad B, Pascal S, et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Archives of neurology. 1991;48:410–416. doi: 10.1001/archneur.1991.00530160078018. [DOI] [PubMed] [Google Scholar]
- [16].Rumsey JM, Casanova M, Mannheim GB, Patronas N, De Vaughn N, Hamburger SD, et al. Corpus callosum morphology, as measured with MRI, in dyslexic men. Biological psychiatry. 1996;39:769–775. doi: 10.1016/0006-3223(95)00225-1. [DOI] [PubMed] [Google Scholar]
- [17].Dougherty RF, Ben-Shachar M, Deutsch G, Potanina P, Bammer R, Wandell BA. Occipital-callosal pathways in children: Validation and atlas development. Annals of the New York Academy of Sciences. 2005;1064:98–112. doi: 10.1196/annals.1340.017. [DOI] [PubMed] [Google Scholar]
- [18].Hasan KM, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, et al. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. NeuroImage. 2007;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Fractional Diffusion Tensor Anisotropy of the Seven Segments of the Normal Appearing White Matter of the Corpus Callosum in Healthy Adults and Relapsing Remitting Multiple Sclerosis. Journal of Magnetic Resonance Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- [20].Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, et al. The association of macro- and microstructure of the corpus callosum and language lateralisation. Brain and language. 2006;97:80–90. doi: 10.1016/j.bandl.2005.07.133. [DOI] [PubMed] [Google Scholar]
- [21].Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- [22].Hasan KM, Ewing-Cobbs L, Kramer L, Fletcher JM, Narayana PA. Diffusion Tensor Quantification of Macrostructure and Microstructure of the Human Midsagittal Corpus Callosum across the Lifespan. NMR in Biomedicine. 2008;21:1–8. doi: 10.1002/nbm.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [24].Hasan KM, Kamali A, Kramer LA, Papnicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain research. 2008 doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, et al. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]