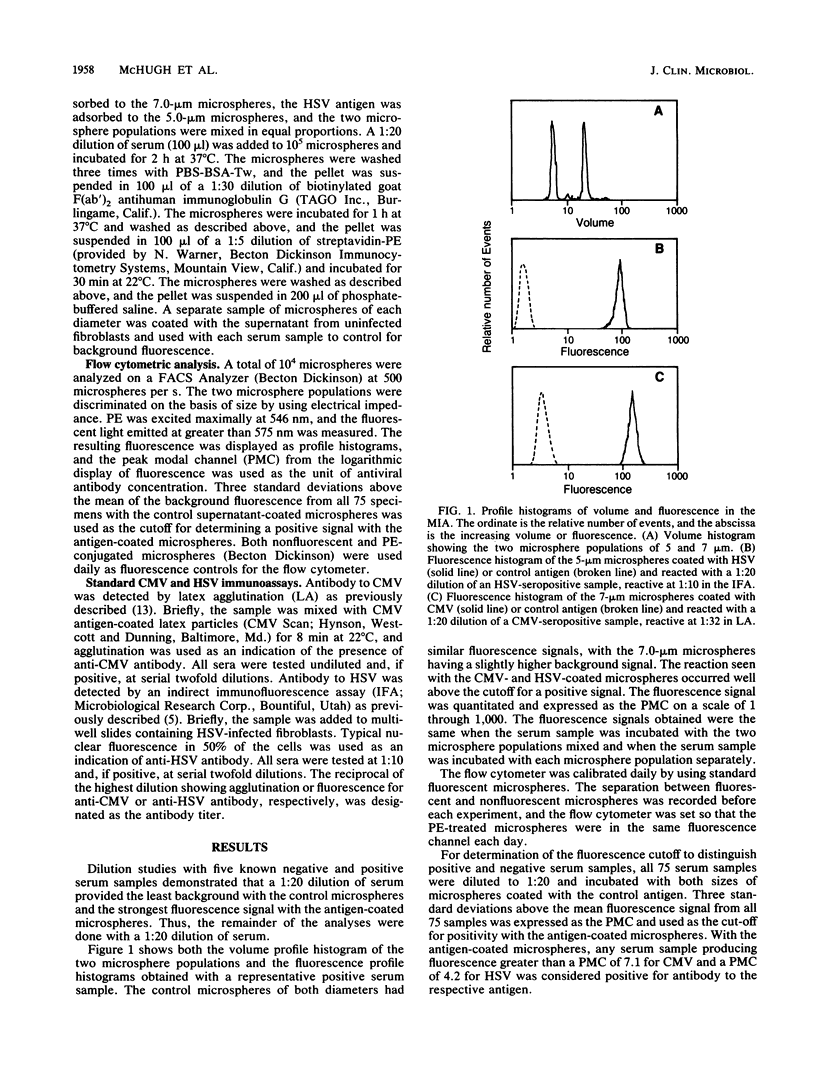
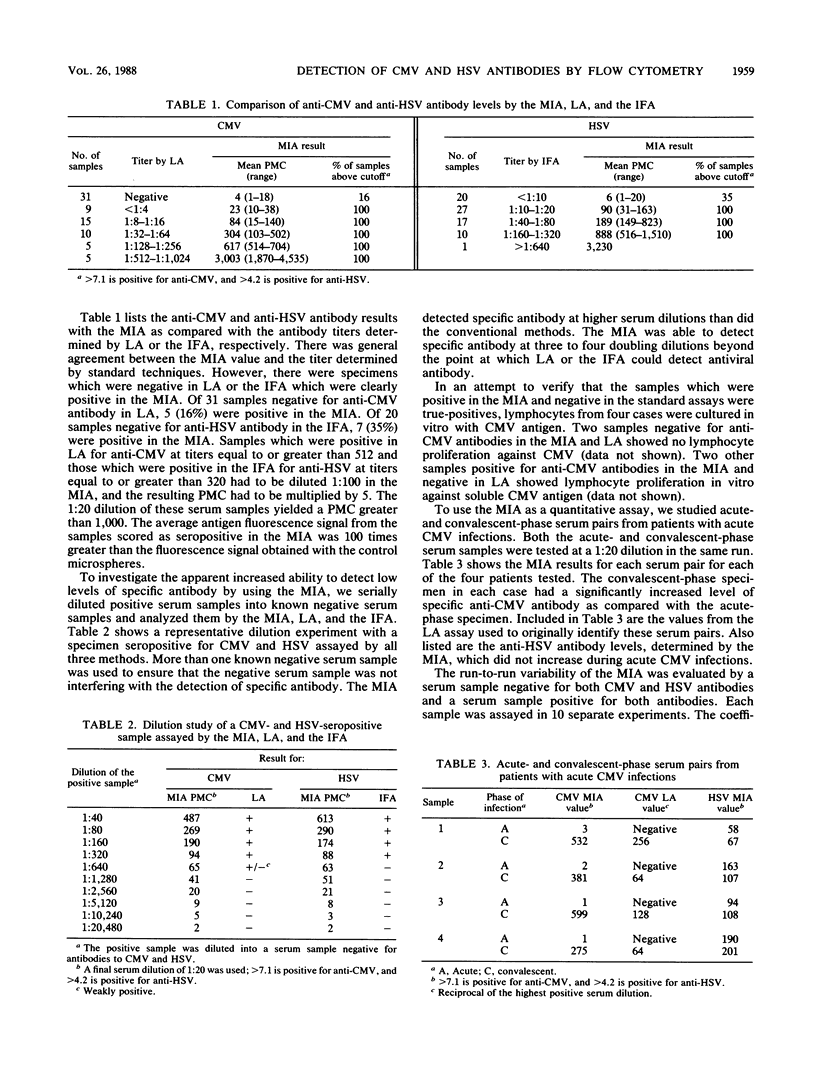
Abstract
A sensitive assay for the simultaneous detection of anti-cytomegalovirus and anti-herpes simplex virus antibodies was developed. Two different sizes of polystyrene microspheres were coated with purified viral antigens. Human antiviral antibodies were detected with a biotin-streptavidin amplification procedure with phycoerythrin as the fluorescent label. Microsphere-associated fluorescence was quantitated with a flow cytometer. Sixteen percent of samples initially scored as seronegative for cytomegalovirus and 35% of samples initially scored as seronegative for herpes simplex virus by conventional assays were clearly found positive by the microsphere technique. This flow cytometric assay can simultaneously detect several specific antibodies at levels which are below the sensitivity of standard assays. The dynamic range of this assay is at least sixfold greater than that of enzyme immunoassays. This technique is amenable to numerous serologic assays and could greatly expand the clinical laboratory applications of flow cytometry.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Lawrence L. T., Baggett J., Biro V., Sharp D. E. Prevention of transfusion-associated cytomegalovirus infection in very low-birthweight infants using frozen blood and donors seronegative for cytomegalovirus. Transfusion. 1984 Jul-Aug;24(4):333–335. doi: 10.1046/j.1537-2995.1984.24484275576.x. [DOI] [PubMed] [Google Scholar]
- Ashley R., Mertz G. J., Corey L. Detection of asymptomatic herpes simplex virus infections after vaccination. J Virol. 1987 Feb;61(2):264–268. doi: 10.1128/jvi.61.2.264-268.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Oba D. E., Hutt-Fletcher L. M. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J Virol. 1987 Apr;61(4):1125–1135. doi: 10.1128/jvi.61.4.1125-1135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry N. J., Grundy J. E., Griffiths P. D. Radioimmunoassay for the detection of IgG antibodies to herpes simplex virus and its use as a prognostic indicator of HSV excretion in transplant recipients. J Med Virol. 1987 Feb;21(2):147–154. doi: 10.1002/jmv.1890210206. [DOI] [PubMed] [Google Scholar]
- Fraser C. E., Melendez L. V., Simeone T. Specificity differentiation of herpes simplex virus types 1 and 2 by indirect immunofluorescence. J Infect Dis. 1974 Jul;130(1):63–66. doi: 10.1093/infdis/130.1.63. [DOI] [PubMed] [Google Scholar]
- Gilljam G., Sundqvist V. A., Linde A., Pihlstedt P., Eklund A. E., Wahren B. Sensitive analytic ELISAs for subclass herpes virus IgG. J Virol Methods. 1985 Mar;10(3):203–214. doi: 10.1016/0166-0934(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Wheeless L. L., Jr Quantitative single cell analysis and sorting. Science. 1977 Oct 14;198(4313):149–157. doi: 10.1126/science.905822. [DOI] [PubMed] [Google Scholar]
- Jacobsen N., Keiding N., Ryder L., Ringdén O., Lönnqvist B., Gahrton G., Rajantie J., Siimes M., Volin L., Ruutu T. Graft-versus-leukaemia activity associated with cytomegalovirus antibody positive bone marrow donors in acute myeloid leukaemia. Lancet. 1987 Feb 21;1(8530):456–457. doi: 10.1016/s0140-6736(87)90166-8. [DOI] [PubMed] [Google Scholar]
- Kahlon J., Chatterjee S., Lakeman F. D., Lee F., Nahmias A. J., Whitley R. J. Detection of antibodies to herpes simplex virus in the cerebrospinal fluid of patients with herpes simplex encephalitis. J Infect Dis. 1987 Jan;155(1):38–44. doi: 10.1093/infdis/155.1.38. [DOI] [PubMed] [Google Scholar]
- Kohl S., Cox P. A., Loo L. S. Defective production of antibody to herpes simplex virus in neonates: defective production of T helper lymphokine and induction of suppression. J Infect Dis. 1987 Jun;155(6):1179–1187. doi: 10.1093/infdis/155.6.1179. [DOI] [PubMed] [Google Scholar]
- Kronick M. N. The use of phycobiliproteins as fluorescent labels in immunoassay. J Immunol Methods. 1986 Aug 21;92(1):1–13. doi: 10.1016/0022-1759(86)90496-5. [DOI] [PubMed] [Google Scholar]
- Krowka J., Stites D., Mills J., Hollander H., McHugh T., Busch M., Wilhelm L., Blackwood L. Effects of interleukin 2 and large envelope glycoprotein (gp 120) of human immunodeficiency virus (HIV) on lymphocyte proliferative responses to cytomegalovirus. Clin Exp Immunol. 1988 May;72(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- McHugh T. M., Casavant C. H., Wilber J. C., Stites D. P. Comparison of six methods for the detection of antibody to cytomegalovirus. J Clin Microbiol. 1985 Dec;22(6):1014–1019. doi: 10.1128/jcm.22.6.1014-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh T. M., Reid M. E., Stites D. P., Chase E. S., Casavant C. H. Detection of the human erythrocyte surface antigen gerbich by flow cytometry using human antibodies and phycoerythrin for extreme immunofluorescence sensitivity. Vox Sang. 1987;53(4):231–234. doi: 10.1111/j.1423-0410.1987.tb05072.x. [DOI] [PubMed] [Google Scholar]
- McHugh T. M., Stites D. P., Casavant C. H., Fulwyler M. J. Flow cytometric detection and quantitation of immune complexes using human C1q-coated microspheres. J Immunol Methods. 1986 Dec 4;95(1):57–61. doi: 10.1016/0022-1759(86)90317-0. [DOI] [PubMed] [Google Scholar]
- Mirolo G., Baldassarri B., Ripalti A., Re M. C., Clementi M., Manzin A., Landini M. P. Antibody response to individual cytomegalovirus structural proteins in different groups of subjects. Eur J Clin Microbiol. 1987 Apr;6(2):207–210. doi: 10.1007/BF02018217. [DOI] [PubMed] [Google Scholar]
- Saunders G. C., Jett J. H., Martin J. C. Amplified flow-cytometric separation-free fluorescence immunoassays. Clin Chem. 1985 Dec;31(12):2020–2023. [PubMed] [Google Scholar]
- Wilson M. R., Wotherspoon J. S. A new microsphere-based immunofluorescence assay using flow cytometry. J Immunol Methods. 1988 Mar 16;107(2):225–230. doi: 10.1016/0022-1759(88)90222-0. [DOI] [PubMed] [Google Scholar]


