Abstract
Male infertility, a common barrier that prevents successful conception, is a reproductive difficulty affecting 15% of couples. Heritable forms of nonsyndromic male infertility can arise from single-gene defects as well as chromosomal abnormalities. Although no CATSPER gene has been identified as causative for human male infertility, male mice deficient for members of the CatSper gene family are infertile. In this study, we used routine semen analysis to identify two consanguineous Iranian families segregating autosomal-recessive male infertility. Autozygosity by descent was demonstrated in both families for a ∼11 cM region on chromosome 11q13.1, flanked by markers D11S1765 and D11S4139. This region contains the human CATSPER1 gene. Denaturing high-performance liquid chromatography (DHPLC) and bidirectional sequence analysis of CATSPER1 in affected family members revealed two separate insertion mutations (c.539-540insT and c.948-949insATGGC) that are predicted to lead to frameshifts and premature stop codons (p.Lys180LysfsX8 and p.Asp317MetfsX18). CATSPER1 is one of four members of the sperm-specific CATSPER voltage-gated calcium channel family known to be essential for normal male fertility in mice. These results suggest that CATSPER1 is also essential for normal male fertility in humans.
Main Text
In approximately half of the 15% of couples who cannot conceive, the cause is ascribed to male infertility.1,2 The underlying pathogenetic mechanisms can reflect the effect of environmental toxins, such as pesticides, glycol ethers, and heavy metals; systemic disorders, like hypothalamic-pituitary disease, testicular cancers, and germ cell aplasia; and genetic factors, including aneuploidies and single-gene mutations.3
Of the aneuploidies associated with male infertility, the classic example is Klinefelter's syndrome.4,5 The presence of an extra X chromosome in these 47, XXY males leads to destruction of the seminiferous epithelium and azoospermia (absence of sperm). Single-gene defects associated with azoospermia and asthenozoospermia (abnormal sperm motility) include mutations in the cyclic-dependent protein kinases AKAP3 (MIM 604689) and AKAP4 (MIM 300185). In both humans and mice, deletions in these two genes cause sperm immotility secondary to dysplasia of the fibrous sheath surrounding the axoneme.6–8 Mutations in the dynein genes that encode proteins of the axonemal dynein cluster (DNAH1 [MIM 603332], DNAH5 [MIM 603335], and DNAH11 [MIM 603339]) have been identified in persons with Kartagener's syndrome (MIM 244400) and also cause asthenozoospermia.9,10
Two four-generation consanguineous Iranian families segregating nonsyndromic autosomal-recessive male infertility were the subject of this study. In consenting persons, 10 ml of whole blood was obtained as a source of genomic DNA. Human Research Institutional Review Boards at the Welfare Science and Rehabilitation University and the Iran University of Medical Sciences, Tehran, Iran and at the University of Iowa, Iowa City, USA approved all procedures. In both families, affected males of first-cousin marriages who were themselves married and undergoing fertility evaluation were identified as being infertile (Family L-1025, IV:2 and IV:4; Family L-968, IV:4) (Figure 1). Clinical analysis of semen from individuals IV:2 (L-1025) and IV:4 (L-968) showed sperm defects and reduced fertility (Table 1). This routine analysis included measurement of volume, pH, sperm count, sperm motility and sperm form (structure). Although the pH of the semen from both individuals was in the normal range, examination of all other parameters revealed abnormalities (Table 1). Both affected males demonstrated nonmotile sperm or sperm motility below the normal threshold, low sperm count, increased abnormally structured spermatozoa, and reduced semen volume.
Figure 1.
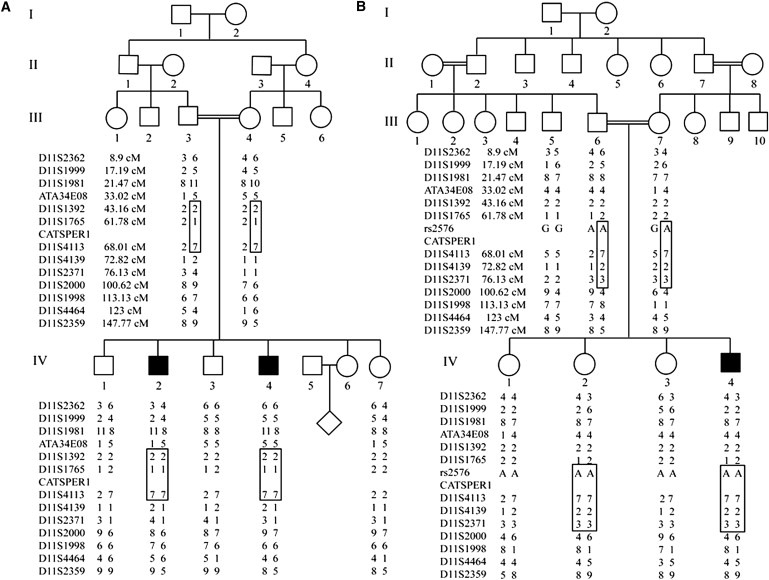
Linkage Mapping of an Infertility Locus Shared by Two Iranian Families
Genotyping of STRPs and a SNP on chromosome 11 in (A) family L-1025 and (B) family L-968. The region of autozygosity by descent, which includes CATSPER1, is boxed.
Table 1.
Semen Analysis of an Affected Male from Each Family
| Test | IV:2 (L-1025) | IV:4 (L-968) | Normal Range |
|---|---|---|---|
| Volume (mL) | 1.0 | 0.4 | 1.5–7 |
| pH | 8.0 | 7.5 | 7.2–8.0 |
| Sperm count (million) | 12 | 10.4 | 20–160 |
| Motility (% motile) | 50 | 0 | >70 |
| Form (% normal) | 65 | 20 | >75 |
Because neither family alone was sufficiently large to allow for generation of a LOD score > 3.0, short tandem repeat polymorphisms (STRPs) flanking the four CATSPER genes were screened for allelic homozygosity with the use of previously reported PCR conditions.11 The CATSPER genes were selected as candidates because their deficiency had previously been linked to male infertility in mice.12,13 Regions of homozygosity were expanded with the use of additional STRPs and single-nucleotide polymorphisms (SNPs), showing that affected males in both families demonstrated autozygosity by descent over a common interval, located on chromosome 11, that spans ∼11 cM and includes the testis-specific CATSPER1 gene (MIM 606389) (Figure 1). The combined LOD score at this locus, with the use of only males from these two families, was 3.13, suggesting that mutations in CATSPER1 could be causally related to the infertility in these men. Regions of autozygosity by descent were not present at the other three CATSPER loci (data not shown).
Genomic DNA from persons in both families was amplified, and all 12 coding exons and splice sites of CATPSER1 were analyzed by denaturing high-performance liquid chromatography (DHPLC) and bidirectional sequencing with the use of gene-specific primers and previously reported conditions (Table S1, available online).11 Both methods independently confirmed the presence of sequence variations; the specific mutations at the nucleotide level were resolved by bidirectional sequencing. Sequence chromatograms were compared to the human CATSPER1 cDNA sequence (uc001ogt.1; NM_053054).
In family L-1025, an insertion mutation (c.539-540insT) that segregated in homozygous fashion in the two infertile males IV:2 and IV:4 was identified in exon 1 (Figure 2A). The insertion of the single thymine nucleotide is predicted to disrupt the open reading frame (ORF) of CATSPER1 and introduce eight new amino acids followed by a premature stop codon (p.Lys180LysfsX8). If the aberrant transcript is not subject to nonsense-mediated decay (NMD), a severely truncated 188 aa protein will be translated (Figure 3A). In family L-968, a second insertion mutation (c.948-949insATGGC) was discovered in exon 1 (Figure 2B). This mutation introduces five nucleotides into the CATSPER1 ORF and is homozygous in one affected male (IV:4) and one female (IV:2) family member. The frameshift caused by this insertion is predicted to substitute the D317 residue for a methionine and introduce 18 new residues before a premature stop codon (p.Asp317MetfsX18). In the absence of NMD, the predicted product would be a truncated 335 residue protein (Figure 3B). For exclusion of the possibility that the two insertion mutations identified in these families are polymorphisms, 576 Iranian control individuals (1152 chromosomes) were screened. All control individuals were negative for both of these mutations.
Figure 2.
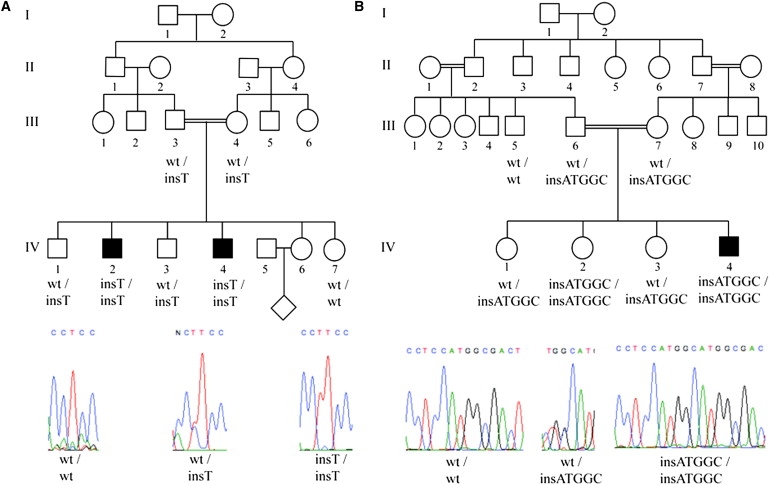
Genetic Analyses of Affected Family Members Reveals CATSPER1 Mutations Segregating with Infertility
Insertion mutations were identified in exon 1 of CATSPER1 in both families.
(A) Family L-1025 segregates a c.539-540insT mutation, family L-968 a c.948-949insATGGC mutation. Sequences were aligned to the human CATSPER1 cDNA sequence (uc001ogt.1; NM_053054). The forward DNA strand and CATSPER1 genotypes for screened family members are shown.
Figure 3.
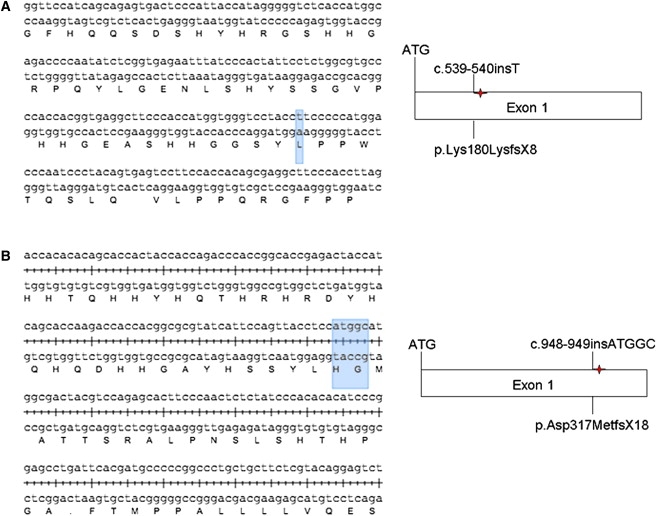
Predicted Effect of the Mutations on the CATSPER1 Protein
The predicted alterations of CATSPER1 are severely truncated proteins if the transcripts are not subject to NMD ([A] family L-1025, [B] family L-968). Forward and reverse DNA strands, as well as the corresponding amino acid sequence, are shown.
The CATSPER1 protein belongs to a family of four sperm-specific tetrameric voltage-gated cation channels (CATSPER1,2,3,4) that are highly conserved in humans and mice.13,14 CATSPER proteins consist of a single, six-transmembrane-spanning repeat with a P loop between transmembrane domains S5 and S6 that shows homology to four-repeat calcium (Cav) channels.13 The mutant CATSPER1 proteins predicted for families L-1025 (p.Lys180LysfsX8) and L-968 (p.Asp317MetfsX18) lack all six transmembrane domains and the P loop. Thus, even if a truncated protein is made, CATSPER1 channel activity would be abolished in homozygous carriers of either of these mutations. It is important to note that these effects have been predicted on the basis of the identified DNA alterations at the genomic level and that additional studies are required for confirmation of these effects at the transcriptional or translational level.
The expression of CatSper1 is restricted to the plasma membrane of spermatozoa above the fibrous sheath in the principal piece of the sperm tail (Figure 4A).13 Its functionality was confirmed by Carlson and colleagues and Ren and colleagues in experiments that demonstrated the requirement of CatSper1 for Ca2+ entry into the flagellum and for Ca2+-mediated hyperactivated sperm motility.13,15
Figure 4.
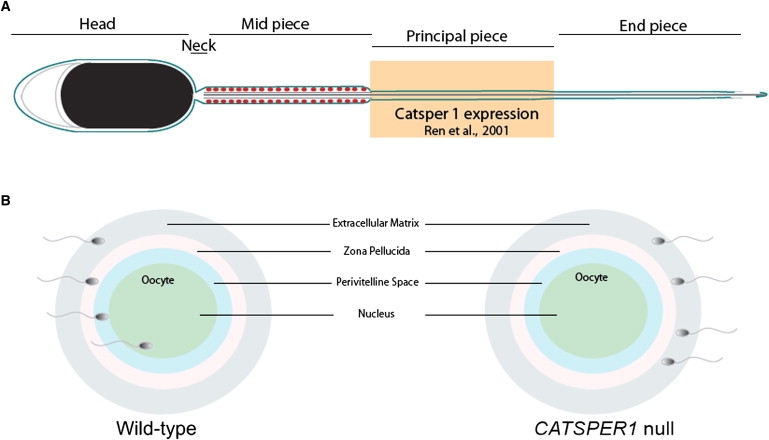
The Role of CatSper1 in Capacitation and Fertilization
Murine studies have shown that CatSper1 is essential for normal capacitation and fertilization.
(A) CatSper1 expression in the mouse sperm tail.
(B) CatSper1 null sperm cannot penetrate the zona pellucida to fertilize the oocyte.
Once sperm enter the female reproductive tract, they become hyperactive, in a process termed “capacitation” that involves three Ca2+-mediated events: initial activation, hyperactivation of motility, and engagement of a protein kinase cascade.15 Hyperactivated motility occurs at the site of fertilization to facilitate penetration into the oocyte (Figure 4B) and is characterized by a high-amplitude, asymmetric waveform. Absence of CatSper1 impairs this phase of motility.15
Definitive evidence that CatSper1 functions as a voltage-gated calcium channel to facilitate hyperactivated sperm motility came from whole-cell patch-clamp measurements of spermatozoa.16 CatSper1 channel currents were potentiated in response to intracellular alkalinization, increasing the concentration of intraflagellar calcium and thereby inducing hyperactivity. Although those studies were murine studies, the high degree of conservation of CatSper1 between mouse and human suggests that a similar mechanism might be responsible for the infertility in the males that we report in this study.
Clinical evaluation of fertility in males is commonly limited to routine semen analysis that assesses the ejaculate for abnormalities of sperm number, morphology, and motility, as well as for indicators of the function of the genital tract, including semen volume and pH.17 These measures are relatively rudimentary, and although semen analysis is effective for determination of azoospermia, changes in sperm morphology and motility can be missed. Despite the fact that a significant number of gene products have now been associated with infertility in men (reviewed in8), many of these etiologies may go undiagnosed in the absence of a more rigorous clinical examination that includes measurement of sperm-motility parameters, such as path velocity, progressive velocity, and track speed. These parameters were all markedly impaired in CatSper1−/− mouse sperm as compared to wild-type sperm. CatSper1−/− sperm were also sluggish, displayed less directed movement, and lacked vigorous beating and bending in the tail region.13 Unfortunately, because of technological limitations, we were unable to examine these parameters in the sperm of the Iranian male family members described here.
In this study, we have described a rare example of Mendelian inheritance of nonsyndromic male infertility. The rarity of similar reports might reflect difficulties in diagnosing and characterizing male infertility. The insertion mutations that we identified in CATSPER1 suggest that mutations in the CATSPER family of calcium channel subunits should be considered candidate genes in cases of male infertility. As a corollary, our findings also suggest that therapeutic strategies designed to block CATSPER1 activity could be useful as a male contraceptive.18
Acknowledgments
The authors sincerely thank the families for their participation in this study. R.J.H.S. is the Sterba Hearing Research Professor, University of Iowa College of Medicine. M.S.H. is supported by an Australian National Health and Medical Research (NHMRC) Overseas Biomedical Fellowship.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
UCSC Genome Browser, http://genome.ucsc.edu/
References
- 1.Templeton A., Fraser C., Thompson B. The epidemiology of infertility in Aberdeen. BMJ. 1990;301:148–152. doi: 10.1136/bmj.301.6744.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosher W., Pratt W. National Center for Health Statistics; Hyattsville: 1990. Fecundity and Infertility in the United States, 1965–1988. Advanced Data from Vital and Health Statistics. [PubMed] [Google Scholar]
- 3.Skakkebaek N.E., Giwercman A., de Kretser D. Pathogenesis and management of male infertility. Lancet. 1994;343:1473–1479. doi: 10.1016/s0140-6736(94)92586-0. [DOI] [PubMed] [Google Scholar]
- 4.Arce B., Padron S. Spermatogenesis in Klinefelter's syndrome. Reproduccion. 1980;4:177–184. [PubMed] [Google Scholar]
- 5.Grabski J., Pusch H., Schirren C., Passarge E., Held K., Bartsch W., Wernicke I. Andrologia. 1979;11:182–196. [PubMed] [Google Scholar]
- 6.Turner R.M., Musse M.P., Mandal A., Klotz K., Jayes F.C., Herr J.C., Gerton G.L., Moss S.B., Chemes H.E. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J. Androl. 2001;22:302–315. [PubMed] [Google Scholar]
- 7.Chemes H.E., Brugo S., Zanchetti F., Carrere C., Lavieri J.C. Dysplasia of the fibrous sheath: An ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil. Steril. 1987;48:664–669. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 8.Matzuk M.M., Lamb D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. Epub 2008 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuccarello D., Ferlin A., Cazzadore C., Pepe A., Garolla A., Moretti A., Cordeschi G., Francavilla S., Foresta C. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum. Reprod. 2008;23:1957–1962. doi: 10.1093/humrep/den193. Epub 2008 May 20. [DOI] [PubMed] [Google Scholar]
- 10.Schwabe G.C., Hoffmann K., Loges N.T., Birker D., Rossier C., de Santi M.M., Olbrich H., Fliegauf M., Failly M., Liebers U. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008;29:289–298. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S., Kolln K.A., Cucci R.A., Trembath R.C., Van Camp G., Smith R.J. Pendred syndrome and DFNB4-mutation screening of SLC26A4 by denaturing high-performance liquid chromatography and the identification of eleven novel mutations. Am. J. Med. Genet. A. 2004;124A:1–9. doi: 10.1002/ajmg.a.20272. [DOI] [PubMed] [Google Scholar]
- 12.Qi H., Moran M.M., Navarro B., Chong J.A., Krapivinsky G., Krapivinsky L., Kirichok Y., Ramsey I.S., Quill T.A., Clapham D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. Epub 2007 Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren D., Navarro B., Perez G., Jackson A.C., Hsu S., Shi Q., Tilly J.L., Clapham D.E. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobley A., Pierron V., Reynolds L., Allen L., Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: Characterisation of a common interaction domain and evidence for expression in testis. Reprod. Biol. Endocrinol. 2003;1:53. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson A.E., Westenbroek R.E., Quill T., Ren D., Clapham D.E., Hille B., Garbers D.L., Babcock D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. Epub 2003 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirichok Y., Navarro B., Clapham D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Cambridge University Press; Cambridge: 1999. World Health Organization Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. [Google Scholar]
- 18.Li H., Ding X., Guan H., Xiong C. Inhibition of human sperm function and mouse fertilization in vitro by an antibody against cation channel of sperm 1: The contraceptive potential of its transmembrane domains and pore region. Fertil. Steril. 2008 doi: 10.1016/j.fertnstert.2008.07.1751. in press. Published online October 31, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


