Abstract
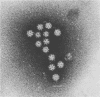
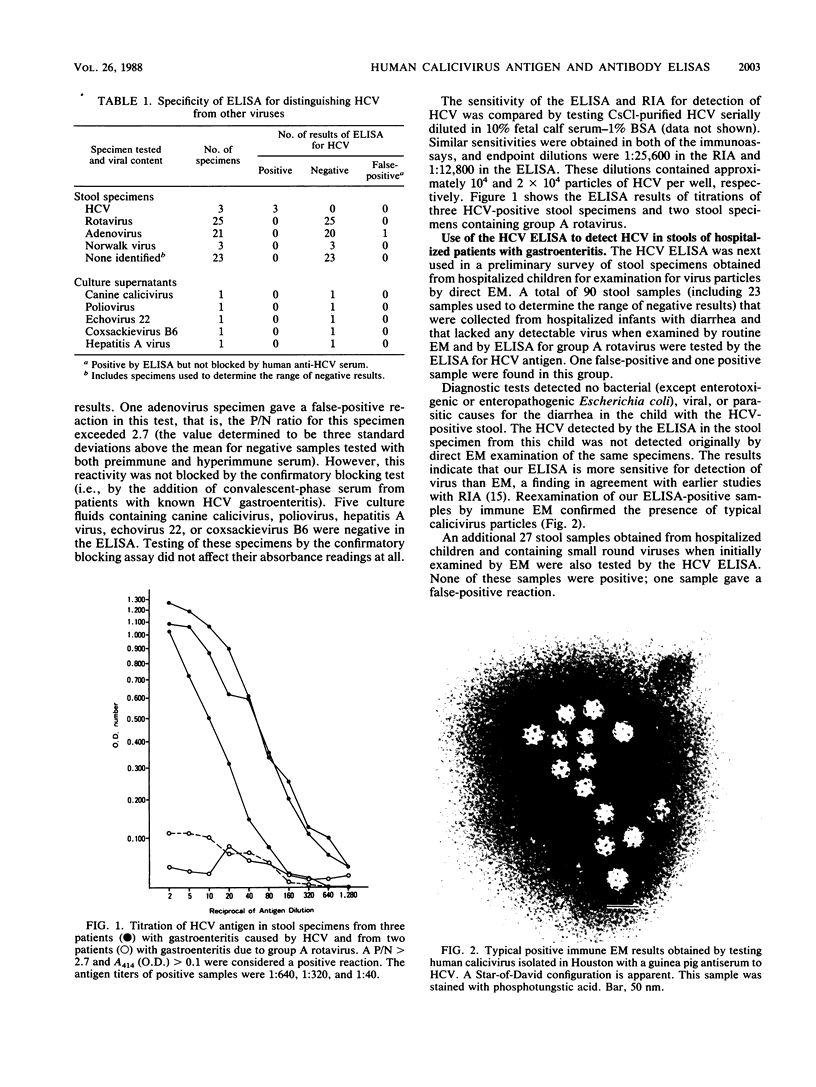
Enzyme-linked immunosorbent assays (ELISAs) were developed to detect human calicivirus (HCV) antigen and antibody to HCV. The ELISAs were specific for HCV and as sensitive as a previously developed radioimmunoassay. These ELISAs were used to search for evidence of HCV infection in the United States, where HCV gastroenteritis has rarely been reported. One hundred sixty-three stool samples collected from children hospitalized with diarrhea were examined; one sample was positive in the ELISA. Typical calicivirus particles were found in this stool sample, and these particles reacted with a hyperimmune guinea pig anti-HCV serum by immune electron microscopy. The age-related acquisition of antibody to HCV in hospitalized infants and children (from birth to 19 years old) without gastroenteritis and in healthy adults was also evaluated. The pattern of acquisition of antibody to HCV was similar to that for group A rotaviruses, namely, beginning in infancy and becoming 100% by the age of 4 years. These data suggest that HCV is associated with infantile gastroenteritis in the United States, that infections with HCV are common, and that many infections with HCV (Sapporo strain) may not require hospitalization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Nakata S., Nakamura I., Taniguchi K., Urasawa S., Fujinaga K., Nakao T. Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet. 1983 Oct 22;2(8356):954–957. doi: 10.1016/s0140-6736(83)90463-4. [DOI] [PubMed] [Google Scholar]
- Chiba S., Sakuma Y., Kogasaka R., Akihara M., Horino K., Nakao T., Fukui S. An outbreak of gastroenteritis associated with calicivirus in an infant home. J Med Virol. 1979;4(4):249–254. doi: 10.1002/jmv.1890040402. [DOI] [PubMed] [Google Scholar]
- Chiba S., Sakuma Y., Kogasaka R., Akihara M., Terashima H., Horino K., Nakao T. Fecal shedding of virus in relation to the days of illness in infantile gastroenteritis due to calicivirus. J Infect Dis. 1980 Aug;142(2):247–249. doi: 10.1093/infdis/142.2.247. [DOI] [PubMed] [Google Scholar]
- Conner M. E., Estes M. K., Graham D. Y. Rabbit model of rotavirus infection. J Virol. 1988 May;62(5):1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt W. D., Barrett A. D. Propagation of human candidate calicivirus in cell culture. J Gen Virol. 1984 Jun;65(Pt 6):1123–1126. doi: 10.1099/0022-1317-65-6-1123. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D., Blacklow N. R., Herrmann J. E., Nowak N. A., Nakata S., Chiba S. Antigenic relationships between human caliciviruses and Norwalk virus. J Infect Dis. 1987 Nov;156(5):806–814. doi: 10.1093/infdis/156.5.806. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D., McSwiggan D. A., Arstall S. An outbreak of calicivirus infection in a mother and baby unit. J Clin Pathol. 1980 Nov;33(11):1095–1098. doi: 10.1136/jcp.33.11.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt W. D., McSwiggan D. A., Moore W. Winter vomiting disease caused by calicivirus. J Clin Pathol. 1979 Aug;32(8):786–793. doi: 10.1136/jcp.32.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt W. D., McSwiggan D. A. Seroepidemiological survey of the prevalence of antibodies to a strain of human calicivirus. J Med Virol. 1987 Apr;21(4):361–368. doi: 10.1002/jmv.1890210408. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D., Pead P. J., Saeed A. A. A new serotype of calicivirus associated with an outbreak of gastroenteritis in a residential home for the elderly. J Clin Pathol. 1981 Aug;34(8):924–926. doi: 10.1136/jcp.34.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. H., Graham D. Y., Lopez J., Muchinik G., Velasco G., Stenback W. A., Estes M. K. RNA electropherotypes of human rotaviruses from North and South America. Bull World Health Organ. 1984;62(2):321–329. [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Chiba S., Terashima H., Nakao T. Prevalence of antibody to human calicivirus in Japan and Southeast Asia determined by radioimmunoassay. J Clin Microbiol. 1985 Oct;22(4):519–521. doi: 10.1128/jcm.22.4.519-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Chiba S., Terashima H., Sakuma Y., Kogasaka R., Nakao T. Microtiter solid-phase radioimmunoassay for detection of human calicivirus in stools. J Clin Microbiol. 1983 Feb;17(2):198–201. doi: 10.1128/jcm.17.2.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Chiba S., Terashima H., Yokoyama T., Nakao T. Humoral immunity in infants with gastroenteritis caused by human calicivirus. J Infect Dis. 1985 Aug;152(2):274–279. doi: 10.1093/infdis/152.2.274. [DOI] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Loosle R., Tao H., Wang S. H., Saif L. J., Melnick J. L. Antigenic characterization and ELISA detection of adult diarrhea rotaviruses. J Infect Dis. 1986 Sep;154(3):448–455. doi: 10.1093/infdis/154.3.448. [DOI] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Wang S. S., Gary G. W., Melnick J. L. Detection of antibody to group B adult diarrhea rotaviruses in humans. J Clin Microbiol. 1987 May;25(5):812–818. doi: 10.1128/jcm.25.5.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Saif L. J., Barrett H. J., Suzuki H., Ogra P. L. Potential spectrum of etiological agents of viral enteritis in hospitalized infants. J Clin Microbiol. 1983 Feb;17(2):352–356. doi: 10.1128/jcm.17.2.352-356.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y., Chiba S., Kogasaka R., Terashima H., Nakamura S., Horino K., Nakao T. Prevalence of antibody to human calicivirus in general population of northern Japan. J Med Virol. 1981;7(3):221–225. doi: 10.1002/jmv.1890070306. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Konno T., Kutsuzawa T., Imai A., Tazawa F., Ishida N., Katsushima N., Sakamoto M. The occurrence of calicivirus in infants with acute gastroenteritis. J Med Virol. 1979;4(4):321–326. doi: 10.1002/jmv.1890040410. [DOI] [PubMed] [Google Scholar]
- Terashima H., Chiba S., Sakuma Y., Kogasaka R., Nakata S., Minami R., Horino K., Nakao T. The polypeptide of a human calicivirus. Arch Virol. 1983;78(1-2):1–7. doi: 10.1007/BF01310853. [DOI] [PubMed] [Google Scholar]