Abstract
Human diploid foreskin fibroblast cells grown in 24-well plates were inoculated with clinical specimens by centrifugation at 1,000 X g for 45 min. Cultures were incubated at 37 degrees C overnight, fixed, and stained with peroxidase-labeled monoclonal antibodies against herpes simplex virus types 1 and 2. Stained plaques of infected cells were large enough to be detected with the naked eye, and microscopic examination did not reveal any further positive specimens. The method was compared with standard isolation in human fibroblasts grown in shell vials and inoculated by centrifugation at 4,000 X g, observed microscopically for the occurrence of typical cytopathogenic effect three times a week for 10 days, and then typed by enzyme immunoassay. Of the 289 specimens tested, 105 were positive and 174 were negative by both methods. Six specimens were positive by standard isolation only, two of them containing varicella-zoster virus, and two specimens were stored frozen before being tested by immunoperoxidase staining. Two specimens found negative by standard isolation were positive by immunoperoxidase staining. For two specimens negative by immunoperoxidase staining, the standard isolation cultures were lost due to microbial contamination. Forty-two specimens found positive by standard isolation were clearly positive when stained only 8 h after inoculation. By standard isolation, positive results were reported on the average 3 to 4 days after inoculation, whereas by immunoperoxidase staining the result was available within less than 24 h. Immunoperoxidase staining of infected cells is a sensitive method for rapid laboratory diagnosis of herpes simplex virus infections, and 24-well plates are convenient for the handling of a large number of specimens.
Full text
PDF
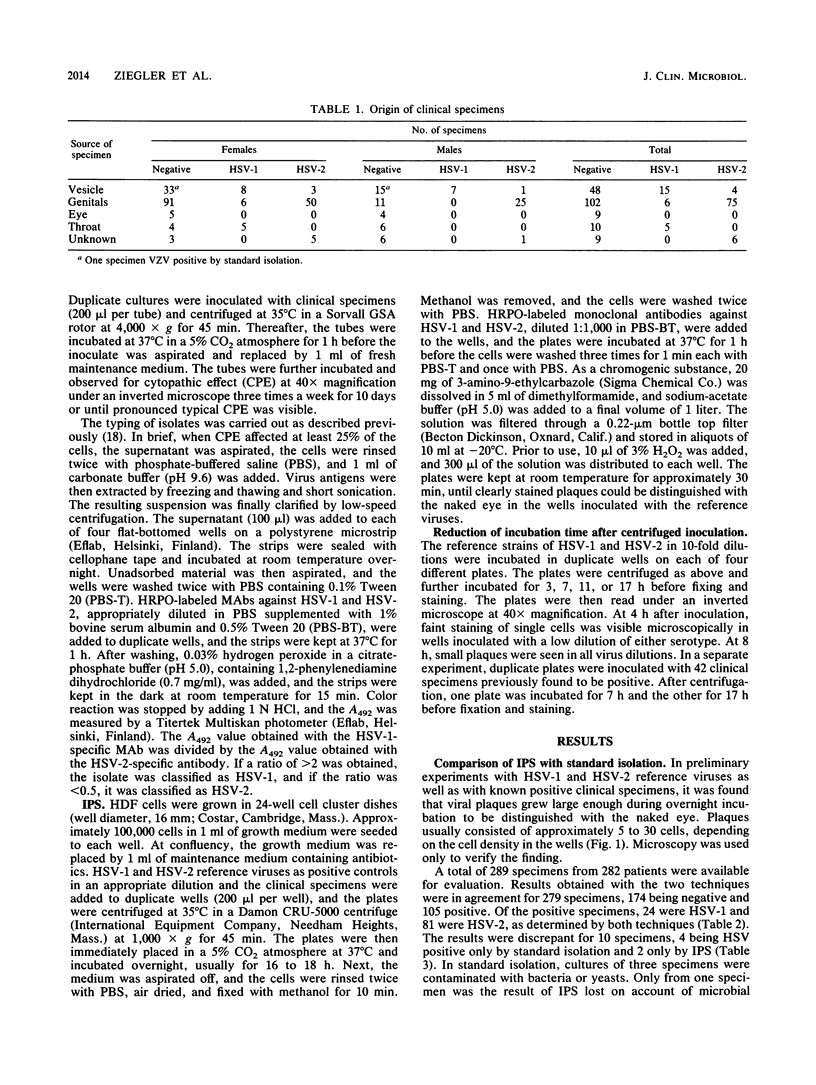
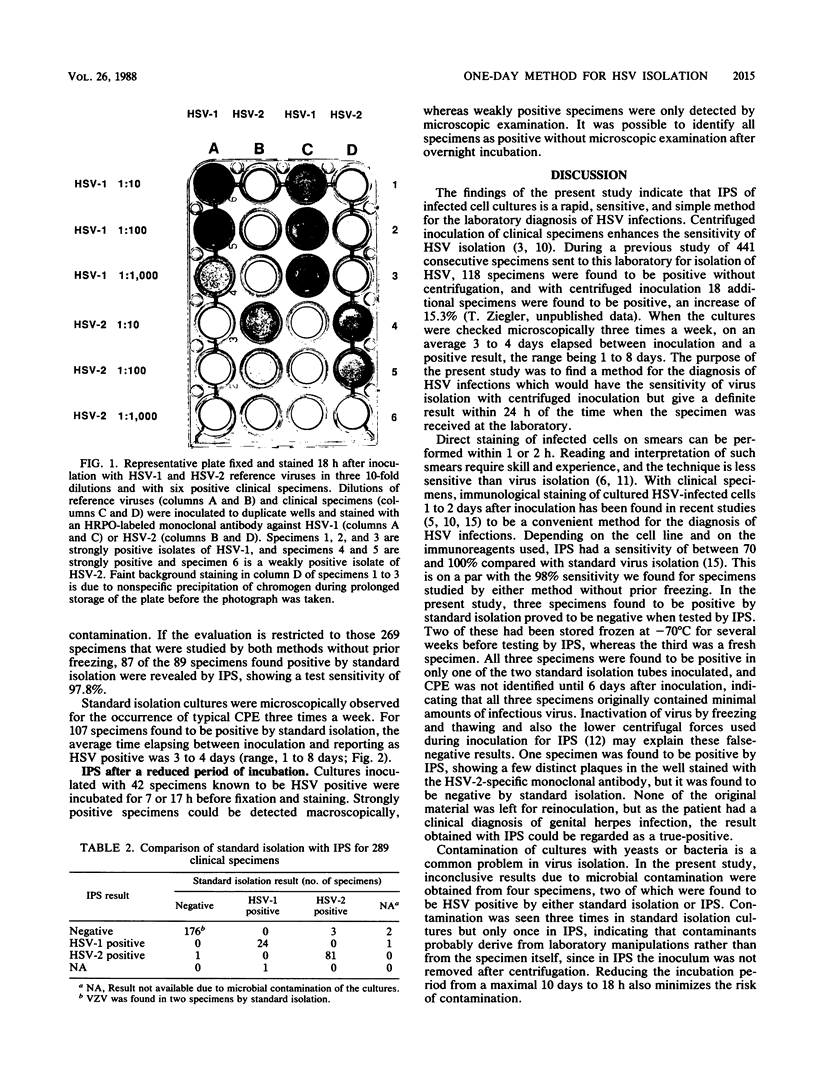
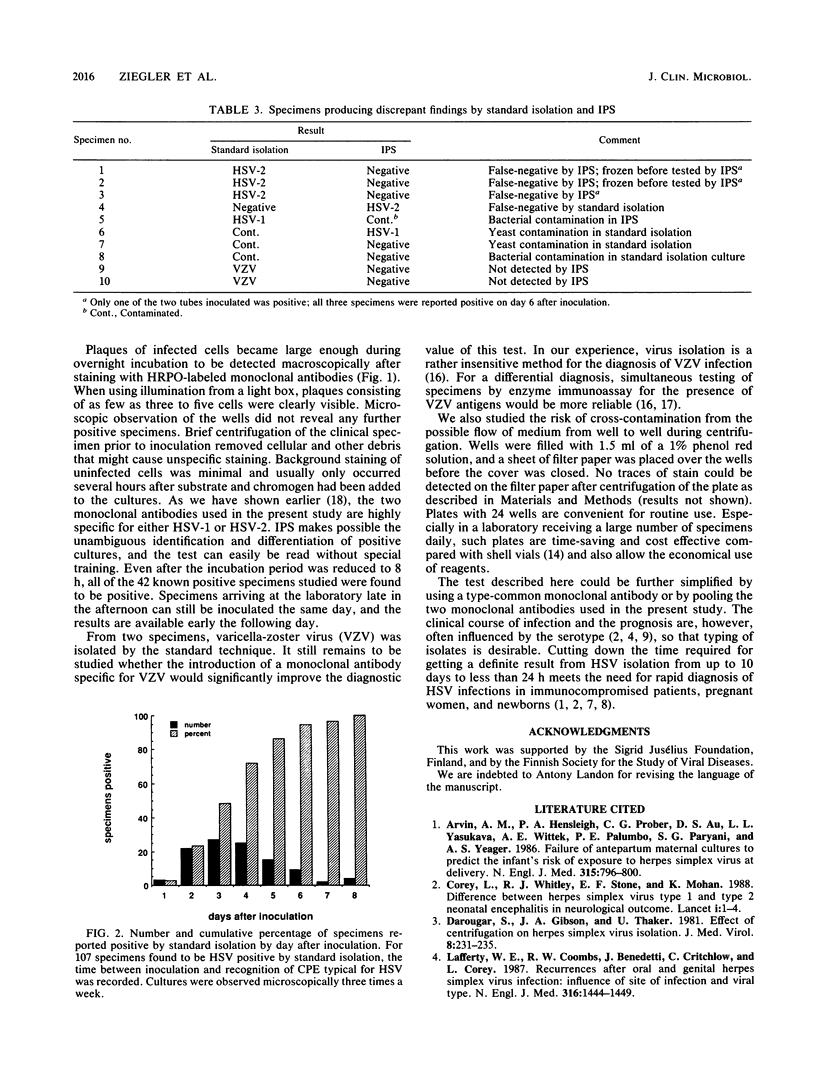

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Hensleigh P. A., Prober C. G., Au D. S., Yasukawa L. L., Wittek A. E., Palumbo P. E., Paryani S. G., Yeager A. S. Failure of antepartum maternal cultures to predict the infant's risk of exposure to herpes simplex virus at delivery. N Engl J Med. 1986 Sep 25;315(13):796–800. doi: 10.1056/NEJM198609253151303. [DOI] [PubMed] [Google Scholar]
- Corey L., Whitley R. J., Stone E. F., Mohan K. Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet. 1988 Jan 2;1(8575-6):1–4. doi: 10.1016/s0140-6736(88)90997-x. [DOI] [PubMed] [Google Scholar]
- Darougar S., Gibson J. A., Thaker U. Effect of centrifugation on herpes simplex virus isolation. J Med Virol. 1981;8(4):231–235. doi: 10.1002/jmv.1890080403. [DOI] [PubMed] [Google Scholar]
- Lafferty W. E., Coombs R. W., Benedetti J., Critchlow C., Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med. 1987 Jun 4;316(23):1444–1449. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- Mayo D. R., Brennan T., Egbertson S. H., Moore D. F. Rapid herpes simplex virus detection in clinical samples submitted to a state virology laboratory. J Clin Microbiol. 1985 May;21(5):768–771. doi: 10.1128/jcm.21.5.768-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R. C., Corey L., Benjamin D., Winter C., Remington M. L. Comparison of viral isolation, direct immunofluorescence, and indirect immunoperoxidase techniques for detection of genital herpes simplex virus infection. J Clin Microbiol. 1981 May;13(5):913–918. doi: 10.1128/jcm.13.5.913-918.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober C. G., Hensleigh P. A., Boucher F. D., Yasukawa L. L., Au D. S., Arvin A. M. Use of routine viral cultures at delivery to identify neonates exposed to herpes simplex virus. N Engl J Med. 1988 Apr 7;318(14):887–891. doi: 10.1056/NEJM198804073181404. [DOI] [PubMed] [Google Scholar]
- Prober C. G., Sullender W. M., Yasukawa L. L., Au D. S., Yeager A. S., Arvin A. M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987 Jan 29;316(5):240–244. doi: 10.1056/NEJM198701293160503. [DOI] [PubMed] [Google Scholar]
- Reeves W. C., Corey L., Adams H. G., Vontver L. A., Holmes K. K. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981 Aug 6;305(6):315–319. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- Salmon V. C., Turner R. B., Speranza M. J., Overall J. C., Jr Rapid detection of herpes simplex virus in clinical specimens by centrifugation and immunoperoxidase staining. J Clin Microbiol. 1986 Apr;23(4):683–686. doi: 10.1128/jcm.23.4.683-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Devlin V., Gallo D., Mills J. Comparison of direct immunofluorescence and direct immunoperoxidase procedures for detection of herpes simplex virus antigen in lesion specimens. J Clin Microbiol. 1983 Aug;18(2):445–448. doi: 10.1128/jcm.18.2.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B. Ultracentrifugal inoculation of herpes simplex virus. Infect Immun. 1978 Jul;21(1):281–285. doi: 10.1128/iai.21.1.281-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Young A., Johnson A., Thiele G. M. Detection of cytomegalovirus by 24-well plate centrifugation assay using a monoclonal antibody to an early nuclear antigen and by conventional cell culture. J Virol Methods. 1987 Dec;18(4):207–213. doi: 10.1016/0166-0934(87)90082-6. [DOI] [PubMed] [Google Scholar]
- Zhao L. S., Landry M. L., Balkovic E. S., Hsiung G. D. Impact of cell culture sensitivity and virus concentration on rapid detection of herpes simplex virus by cytopathic effects and immunoperoxidase staining. J Clin Microbiol. 1987 Aug;25(8):1401–1405. doi: 10.1128/jcm.25.8.1401-1405.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T. Detection of varicella-zoster viral antigens in clinical specimens by solid-phase enzyme immunoassay. J Infect Dis. 1984 Jul;150(1):149–154. doi: 10.1093/infdis/150.1.149. [DOI] [PubMed] [Google Scholar]
- Ziegler T., Halonen P. E. Rapid detection of herpes simplex- and varicella-zoster virus antigens from clinical specimens by enzyme immunoassay. Antiviral Res. 1985;Suppl 1:107–110. doi: 10.1016/s0166-3542(85)80016-4. [DOI] [PubMed] [Google Scholar]
- Ziegler T., Hukkanen V., Arstila P., Auvinen P., Jalava A., Hyypiä T. Typing of herpes simplex virus isolates with monoclonal antibodies and by nucleic acid spot hybridization. J Virol Methods. 1985 Oct;12(1-2):169–177. doi: 10.1016/0166-0934(85)90017-5. [DOI] [PubMed] [Google Scholar]