Abstract
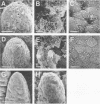
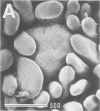
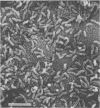
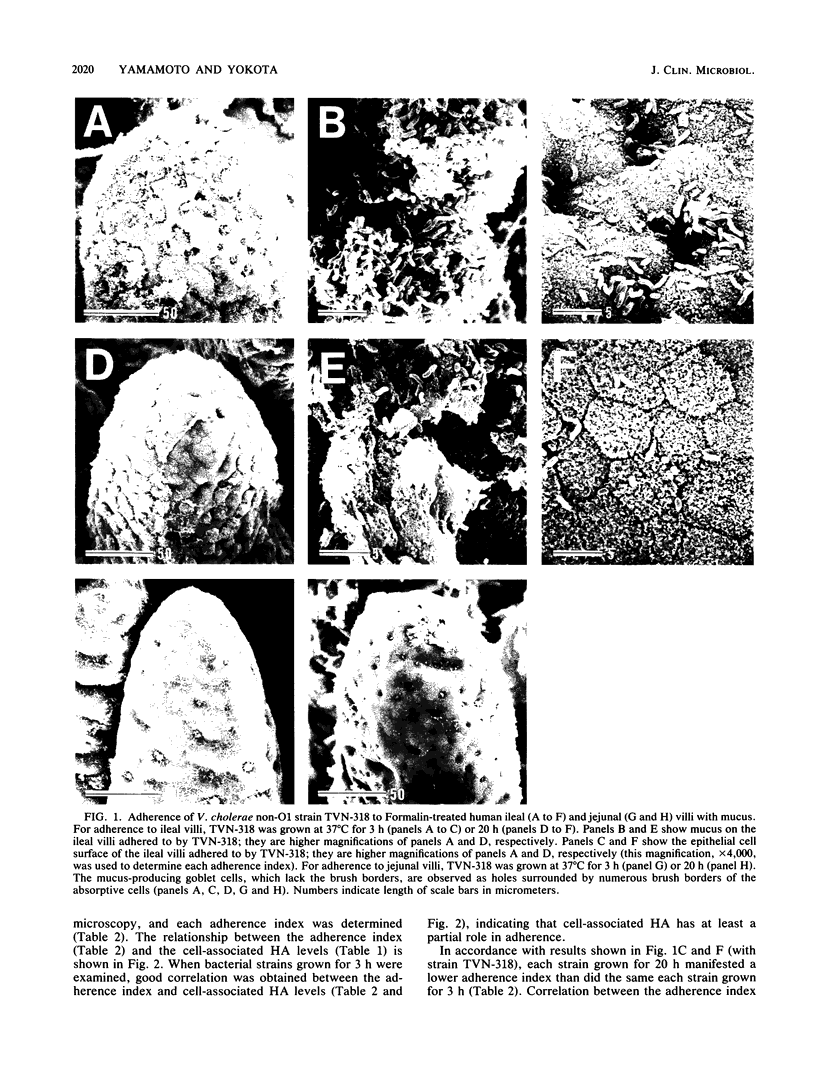
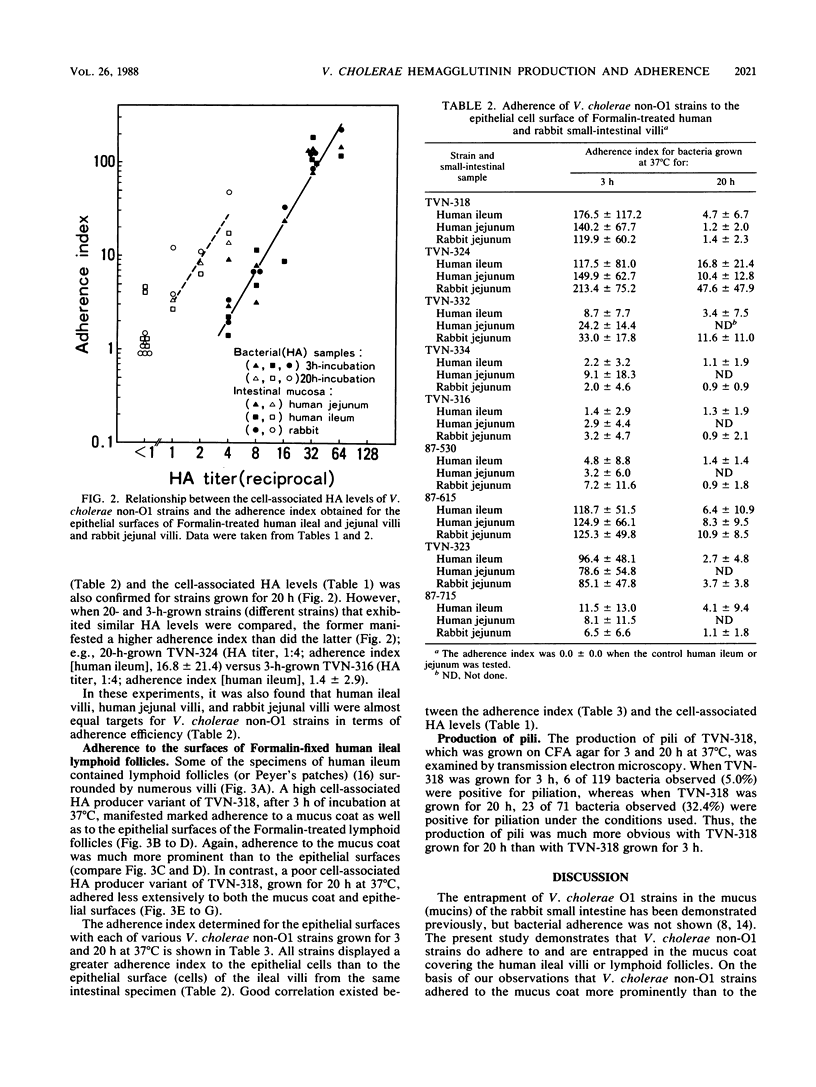
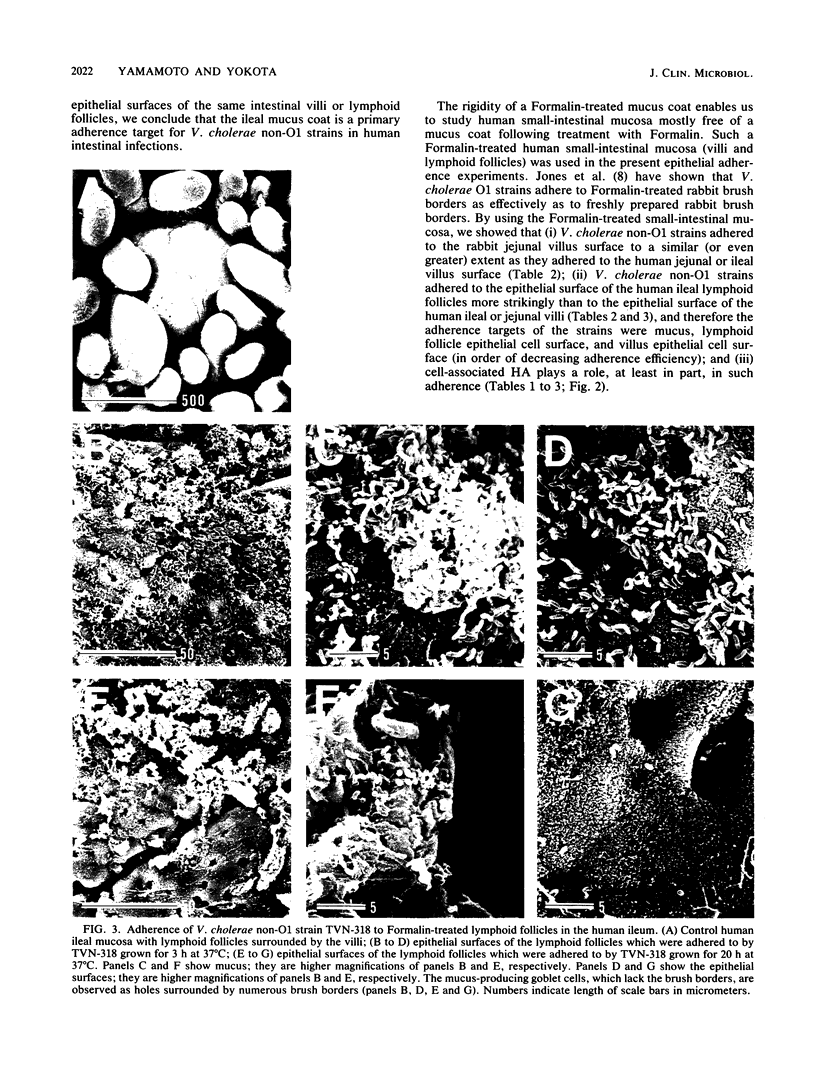
Clinically isolated Vibrio cholerae non-O1 strains produced more cell-associated hemagglutinins (HAs) on colonization factor antigen agar after ca. 3 h than after ca. 20 h of incubation at 37 degrees C. A high cell-associated HA producer variant of strain TVN-318, grown for 3 h at 37 degrees C, was entrapped in a native mucus coat covering the human ileal mucosa and displayed a striking ability to adhere to the surface of a Formalin-treated mucus coat, in contrast to a poor cell-associated HA producer variant of TVN-318, grown for 20 h at 37 degrees C. Adherence to the Formalin-treated human mucus coat was confirmed with all of the strains tested. V. cholerae non-O1 strains also possessed the ability to adhere to the epithelial surfaces of Formalin-treated human and rabbit ileal or jejunal villi, as well as human lymphoid follicles, in proportion to cell-associated HA levels. The epithelial surface of the lymphoid follicles provided most of the adherence sites for V. cholerae non-O1 strains under the test conditions. We conclude that a mucus coat covering the human small intestinal mucosa is a primary adherence target for V. cholerae non-O1 strains in human intestinal infections and that cell-associated HAs have at least a partial role in the adherence of V. cholerae non-O1 strains to the human small intestine, suggesting a potential role for V. cholerae non-O1 strains in an oral live vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arita M., Takeda T., Honda T., Miwatani T. Purification and characterization of Vibrio cholerae non-O1 heat-stable enterotoxin. Infect Immun. 1986 Apr;52(1):45–49. doi: 10.1128/iai.52.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Finkelstein R. A. Presence of hemagglutinin/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J Infect Dis. 1986 Jul;154(1):183–186. doi: 10.1093/infdis/154.1.183. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Comparative study of Vibrio cholerae non-O1 protease and soluble hemagglutinin with those of Vibrio cholerae O1. Infect Immun. 1987 Feb;55(2):451–454. doi: 10.1128/iai.55.2.451-454.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose Y., Yamamoto K., Nakasone N., Tanabe M. J., Takeda T., Miwatani T., Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987 May;55(5):1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. Cholera and other vibrioses in the United States. N Engl J Med. 1985 Feb 7;312(6):343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- Nakasone N., Iwanaga M. Quantitative evaluation of colonizing ability of Vibrio cholerae O1. Microbiol Immunol. 1987;31(8):753–761. doi: 10.1111/j.1348-0421.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J., Rollins D. M., Joseph S. W. Vibrio factors cause rapid fluid accumulation in suckling mice. Infect Immun. 1983 Jun;40(3):1083–1091. doi: 10.1128/iai.40.3.1083-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takeda Y., Miwatani T., Craig J. P. Purification and some properties of a non-o1 Vibrio cholerae enterotoxin that is identical to cholera enterotoxin. Infect Immun. 1983 Mar;39(3):1128–1135. doi: 10.1128/iai.39.3.1128-1135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh M., Honda T., Miwatani T. Purification and partial characterization of a non-O1 Vibrio cholerae hemolysin that cross-reacts with thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1986 Apr;52(1):319–322. doi: 10.1128/iai.52.1.319-322.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]