Abstract
AIMS
The oxysterol 4β-hydroxycholesterol has been suggested as a marker for CYP3A4/5 activity. We have previously shown that plasma 4β-hydroxycholesterol continues to increase for several weeks after maximal induction of CYP3A4/5 by carbamazepine at the dose given. In the present study we aimed to determine the time course of the decrease in plasma 4β-hydroxycholesterol after termination of induction of CYP3A4/5 by rifampicin. An additional aim was to determine the variation in plasma level of 4β-hydroxycholesterol with time in 12 untreated healthy volunteers.
METHODS
Twenty-four healthy subjects were allocated into three study groups of equal sizes. The volunteers were treated with rifampicin (either 20 mg day–1, 100 mg day–1 or 500 mg day–1) for 2 weeks. Blood samples were taken before, during and after rifampicin treatment. In another group of 12 untreated volunteers blood samples were collected at different time points in order to determine the intraindividual variations in plasma 4β-hydroxycholesterol concentrations. Plasma levels of 4β-hydroxycholesterol were determined by isotope-dilution gas chromatography–mass spectrometry.
RESULTS
Rifampicin treatment increased plasma 4β-hydroxycholesterol levels. After termination of rifampicin treatment plasma levels of 4β-hydroxycholesterol decreased slowly with an apparent half-life of 17 days. The intraindividual variation in plasma levels of 4β-hydroxycholesterol in untreated subjects was low, with coefficients of variation of between 4.8 and 13.2% over a period of 3 months.
CONCLUSIONS
After termination of induction of CYP3A4/5, plasma 4β-hydroxycholesterol levels decreased slowly during 8 weeks. The half-life of elimination (17 days) resembled that of cholesterol rather than other oxysterols. The long half-life results in stable plasma concentrations with time.
Keywords: 4β-hydroxycholesterol, CYP3A4, CYP3A5, rifampicin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
We have suggested that 4β-hydroxycholesterol may be used as an endogenous marker of CYP3A activity.
Recently, we found unexpectedly that the plasma concentration of 4β-hydroxycholesterol continued to increase for several weeks after complete induction of CYP3A4/5 by carbamazepine.
In the present study we investigated the time course of elimination of 4β-hydroxycholesterol from the circulation following CYP3A induction with rifampicin.
WHAT THIS STUDY ADDS
4β-Hydroxycholesterol is eliminated very slowly from the circulation with an apparent half-life of 17 days.
The long half-life results in a low variation in plasma concentration with time, but excludes 4β-hydroxycholesterol as a marker for rapid changes in CYP3A activity.
Introduction
Cytochome P450 3A4 (CYP3A4) and CYP3A5 are important drug-metabolizing enzymes that exhibit a large variation in hepatic expression and biological activity between different individuals [1]. It would therefore be valuable to have a marker for this activity for proper dose adjustments and for screening of induction potential of new drug candidates.
The endogenous oxysterol 4β-hydroxycholesterol is formed by CYP3A4 and CYP3A5, and has been suggested as a new marker for CYP3A4/5 activity [2, 3]. Patients treated with drugs known to be strong inducers of CYP3A4/5 have highly elevated levels of 4β-hydroxycholesterol in the circulation [2, 4]. Patients treated with ursodeoxycholic acid, a weak inducer of CYP3A4/5, had slightly elevated levels of 4β-hydroxycholesterol in plasma [2, 4]. Also, during antiretroviral drug treatment inducers increased and inhibitors of CYP3A decreased plasma levels of 4β-hydroxycholesterol [5]. These data taken together support the hypothesis that 4β-hydroxycholesterol may be a suitable marker for CYP3A4/5 activity. When we recently investigated the time course of the increase in plasma 4β-hydroxycholesterol concentration during treatment of paediatric patients with the CYP3A- inducer carbamazepine, it was unexpectedly found that the increase in 4β-hydroxycholesterol concentration continued for several weeks after the completion of induction of CYP3A4/5 [6]. In the present investigation, we have studied the time course of elimination of 4β-hydroxycholesterol from the circulation following termination of treatment with a strong CYP3A4/5 inducer, rifampicin. In addition, we determined the variation with time of plasma 4β-hydroxycholesterol in healthy untreated volunteers.
Materials and methods
Study subjects
The 12 healthy volunteers who were investigated for intraindividual variation in 4α- and 4β-hydroxycholesterol concentrations were the same as those described previously [7]. Unused frozen (−70°C) duplicate samples from the previous study were utilized for this study.
The 24 healthy volunteers treated with rifampicin were those described recently [8].
Analysis of 4α- and 4β-hydroxycholesterol
The two oxysterols 4α- and 4β-hydroxycholesterol in plasma were determined by isotope dilution gas chromatography–mass spectrometry using deuterium-labelled internal standard as described [2]. 4β-Hydroxycholesterol 1 ng ml–1 corresponds to 2.5 nmol l–1.
Twelve aliquots of a pooled plasma sample were analysed during 1 day to determine the within-day coefficient of variation (CV). The CV for 4α-hydroxycholesterol was 6.9% (at 5.5 ng ml–1) and the CV for 4β-hydroxycholesterol was 3.7% (at 27.0 ng ml–1). This pooled material was also analysed repeatedly during 1 year. The between-day CV for 4α-hydroxycholesterol was 30.4% (n = 44) and the corresponding figure for 4β-hydroxycholesterol was 8.2% (n = 44).
Calculation of the half-life of elimination of 4β-hydroxycholesterol
When the half-lives were calculated, the basal concentration of 4β-hydroxycholesterol, measured before rifampicin treatment, was subtracted from the 4β-hydroxycholesterol concentrations at the different time points.
All study subjects gave written, informed consent to participate, and the study was approved by the local research ethics committee at Karolinska Institutet. Blood sampling was originally planned to continue until 2 weeks after termination of rifampicin treatment. When it was found that 4β-hydroxycholesterol levels were still higher at this time point than before treatment, we applied for and obtained an additional ethical permit to take blood samples also at 4 and 8 weeks after termination of rifampicin treatment.
Results
Variations in plasma 4α- and 4β-hydroxycholesterol concentration with time
The intraindividual variations in plasma 4α- and 4β-hydroxycholesterol over time are shown in Figure 1. Both oxysterols showed remarkably stable plasma concentrations, and the CVs for 4α-hydroxycholesterol for the 12 subjects ranged from 6.2 to 16.0% with an average CV of 8.75% at an average concentration of 7.1 ng ml–1. The CVs for 4β-hydroxycholesterol ranged from 4.8 to 13.2% with an average CV of 7.1% at an average concentration of 30.8 ng ml–1. It should be noted that it is not the same subject that drops in concentration of 4α- and 4β-hydroxycholesterol at the time point 3 months in Figure 1.
Figure 1.
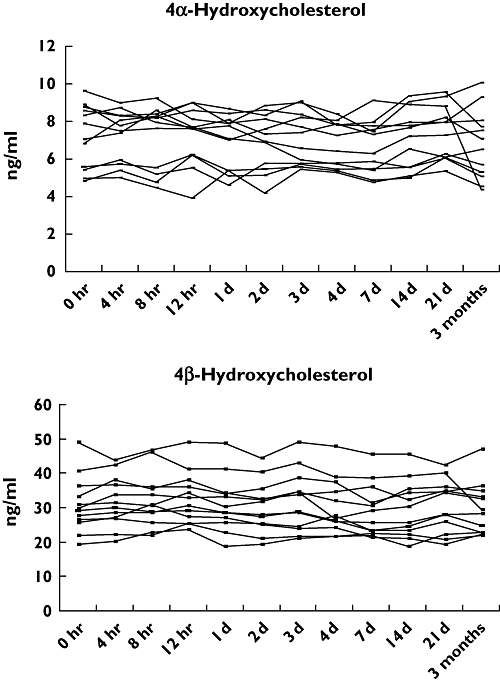
Plasma concentrations (ng ml−1) of 4α- and 4β-hydroxycholesterol in 12 different occasions during a 3-month period
Rifampicin treatment of 24 healthy volunteers
The plasma concentrations of 4β-hydroxycholesterol for the 24 volunteers treated with either 20, 100 or 500 mg day–1 of rifampicin are shown in Figure 2. We have recently reported [8] that there is a dose-dependent increase in 4β-hydroxycholesterol in plasma after 2 weeks of rifampicin treatment. The rifampicin treatment was terminated on day 15, and the plasma concentration of 4β-hydroxycholesterol was determined 1 and 2 weeks thereafter for all subjects (days 22 and 29) and, in addition, after 4 and 8 weeks for some subjects (days 43 and 71). The highest dose, 500 mg day–1, caused a significant increase in 4β-hydroxycholesterol already after 1 week of treatment. The average concentration rose from 38 to 105 ng ml–1 (P < 0.001). The concentration continued to increase, although at a lower pace, during the second week of treatment and reached 143 ng ml–1 after 2 weeks (P = 0.001).
Figure 2.
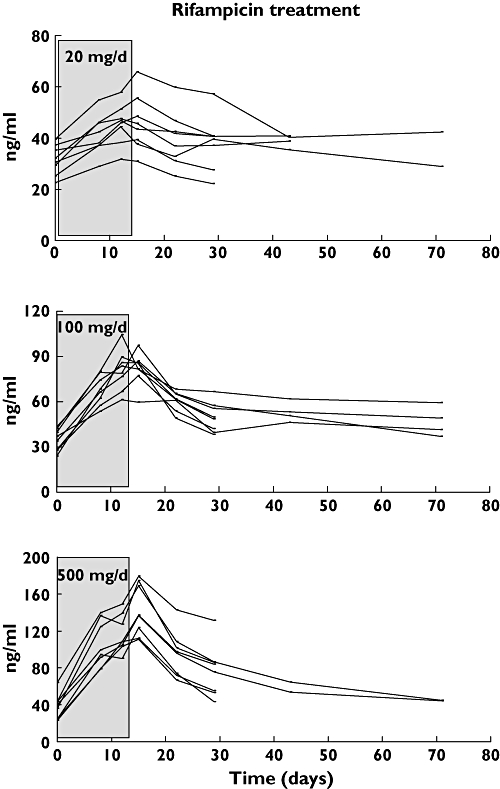
Plasma concentrations (ng ml–1) of 4β-hydroxycholesterol in 24 healthy volunteers before, during and after administration of different doses of rifampicin. Three groups of eight volunteers received 20, 100 or 500 mg day−1 of rifampicin during 2 weeks
Administration of rifampicin at 100 or 20 mg day–1 resulted also in statistically significant increases in plasma 4β-hydroxycholesterol. One week of treatment increased 4β-hydroxycholesterol by 97 and 31%, respectively (P < 0001), and 2 weeks of treatment caused increases of 138 and 146%, respectively (P < 0.001).
In addition to 4β-hydroxycholesterol, the isomer 4α-hydroycholesterol was measured in all subjects. The concentration of 4α-hydroxycholesterol was not influenced by rifampicin treatment, as illustrated for one representative volunteer in Figure 3, indicating that it is not a product of CYP3A4/5-catalysed metabolism.
Figure 3.
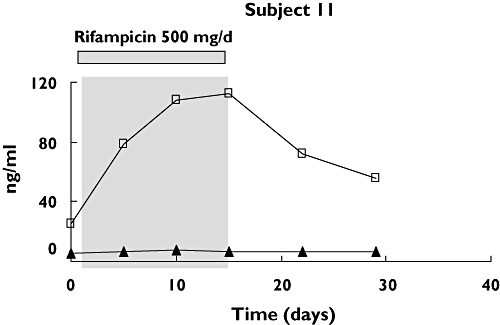
Plasma concentrations (ng ml–1) of 4α-hydroxycholesterol (4α-OH) and 4β-hydroxycholesterol (4β-OH) in subject 11 treated with rifampicin (500 mg day−1) during 2 weeks
Termination of rifampicin treatment resulted in a significant decrease in 4β-hydroxycholesterol concentration. One week after termination the average concentration in the group receiving 500 mg day–1 was 95 ng ml–1 (P < 0.001), and after 2 weeks the average concentration was 77 ng ml–1 (P < 0.001). On average the concentration of 4β-hydroxycholesterol was 58% higher 2 weeks after termination of treatment compared with pretreatment values. When we found that 4β-hydroxycholesterol had not returned to the pre-rifampicin value 2 weeks after the last dose of rifampicin, we analysed 4β-hydroxycholesterol at 4 and 8 weeks after the last dose in 10 and eight subjects, respectively. At 4 weeks after termination of treatment the concentration of 4β-hydroxycholesterol was on average still 31% higher than before treatment, whereas after 8 weeks the concentrations were on average 14% higher.
Estimation of the half-life in plasma for 4β-hydroxycholesterol
In two of the volunteers receiving the highest dose of rifampicin (500 mg day–1) the concentration of 4β-hydroxycholesterol was determined 1, 2, 4 and 8 weeks after termination of treatment. The elimination curves for 4β-hydroxycholesterol from the circulation for these two volunteers are shown in Figure 4. The elimination rate was not constant, but decreased during the experiment. Calculation of the elimination rate from the two last time points resulted in a half-life of about 17 days (Figure 4).
Figure 4.
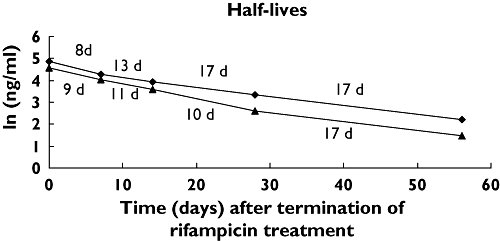
Elimination curves for plasma 4β-hydroxycholesterol in two healthy volunteers treated with rifampicin (500 mg day−1) for 2 weeks. Time 0 corresponds to the day after termination of rifampicin treatment. The different half-lives are displayed in the figure
Discussion
We have recently reported on the time course of increase in plasma 4β-hydroxycholesterol during carbamazepine treatment [6]. In the present investigation, we studied the time course of elimination of 4β-hydroxycholesterol from the circulation after termination of treatment with the strong CYP3A4/5 inducer rifampicin. Rifampicin administration resulted in a rapid increase in plasma 4β-hydroxycholesterol concentration during the first week, analogous to what we have observed previously using carbamazepine as an enzyme inducer [6]. The increase continued during the second week of treatment, but at a lower rate (Figure 2). Originally, we collected blood samples 1 and 2 weeks after the end of treatment. However, when we analysed the plasma samples from the first 11 volunteers for 4β-hydroxycholesterol, it was clear that in only one of the subjects had the plasma level returned to the pretreatment level 2 weeks after termination of treatment. The remaining volunteers were therefore followed until 8 weeks after termination of treatment, and blood samples were drawn at two additional occasions (at 4 and 8 weeks after termination of treatment). Not all the volunteers had the possibility of extending the study, but additional samples were collected from most of the remaining volunteers. As shown in Figure 2, there was a very slow elimination of 4β-hydroxycholesterol from the plasma. Four weeks after termination of treatment, the average level was still 30% higher than the pretreatment level, and even after 8 weeks slightly increased levels of 4β-hydroxycholesterol were observed. It has been reported that the induction of CYP3A4 by carbamazepine disappeared rapidly after termination of treatment [9], and the same has been shown for induction by rifampicin, where the induction dissipates within 2 weeks following termination of treatment [10]. When we calculated the half-life of elimination of 4β-hydroxycholesterol from the circulation, we did not find a constant elimination rate using either linear (Figure 2) or logarithmic concentration scales (Figure 4). Instead, a decreased elimination rate with time was found. This is analogous to the elimination of cholesterol when administered in deuterium- or tritium-labelled form, where a series of exponential rates, rather than a constant rate, was seen during the first 50 days after administration, and only at later time points was a single exponential rate observed [11, 12].
To explain this slow elimination of labelled cholesterol from the circulation a three-compartment model for cholesterol turnover has been proposed. The various tissue compartments of exchangeable cholesterol are thus categorized in three groups in terms of the rates at which they equilibrate with circulating cholesterol [13, 14]. When the half-life of 4β-hydroxycholesterol was approximated from the two last time points a value of 17 days was obtained. It should be pointed out that the limited number of sampling points does not permit precise determination of the half-life of 4β-hydroxycholesterol.The calculated half-life of 17 days is therefore a relatively rough estimate, but it shows, however, that 4β-hydroxycholesterol can not be used to monitor rapid changes in CYP3A activity. This long half-life is not too dissimilar from that reported for cholesterol (72 days [11]). There are no data available on the content and exchangeability of 4β-hydroxycholesterol in different tissues, so we have to assume a similar situation as for cholesterol to explain the slow elimination of 4β-hydroxycholesterol [11].
We have shown earlier that deuterium-labelled 4β-hydroxycholesterol injected intravenously into healthy volunteers disappears from the circulation with a half-life of approximately 60 h [3]. This half-life probably represents a distribution phase rather than true elimination by metabolism and/or excretion. The present study suggests that 4β-hydroxycholesterol resembles cholesterol rather than other oxysterols regarding the rate of elimination from the circulation. Cholesterol-like behaviour of 4β-hydroxycholesterol was also seen during studies of oxysterol transport from red cell membranes to lipoprotein acceptors. Whereas high rates of transfer were obtained for 24-hydroxycholesterol and 27-hydroxycholesterol, the rates for 4β-hydroxycholesterol and cholesterol were hardly possible to measure [15]. The rate of transfer of 25-hydroxycholesterol from erythrocytes to plasma has been reported to be >1000 times faster than for cholesterol [16]. Therefore, the position of the extra hydroxyl group in the cholesterol molecule is important for its properties.
The long half-life of 4β-hydroxycholesterol should be reflected by small variations in plasma concentration with time. This was verified in 12 healthy volunteers, where blood samples were taken at different time points up to 3 months. As shown in Figure 1, very small variations in plasma concentrations were observed for both 4α- and 4β-hydroxycholesterol within each individual. The variations were smaller than for other major oxysterols in the circulation [7].
Both the present study using rifampicin as an inducing agent and our previous study in paediatric patients treated with carbamazepine [6] indicate that the induction of CYP3A4/5 by these drugs may be monitored by 4β-hydroxycholesterol. We have also found that 4β-hydroxycholesterol and the exogenous CYP3A4 marker quinine give similar results in both untreated [17] and rifampicin-treated volunteers [8], suggesting that 4β-hydroxycholesterol may be a useful endogenous marker of CYP3A4/5.
In conclusion, an unexpectedly long half-life of elimination was found for 4β-hydroxycholesterol. This long half-life results in small variations within subjects in plasma concentration and is an advantage when the oxysterol is used as a marker for CYP3A activity during steady-state conditions. In contrast, the long half-life excludes 4β-hydroxycholesterol as a marker for rapid changes in CYP3A activity.
Acknowledgments
This study was supported by grants from AstraZeneca, Torsten och Ragnar Söderbergs Stiftelser, ALF (SLL 560177), The Swedish Research Council, Medicine (3902) and EDCTP (European and Developing Countries Clinical Trials Partnership, CGct 05-32030-001).
Competing interests
None declared.
REFERENCES
- 1.Eichelbaum M, Burk O. Cyp3a genetics in drug metabolism. Nat Med. 2001;7:285–7. doi: 10.1038/85417. [DOI] [PubMed] [Google Scholar]
- 2.Bodin K, Bretillon L, Aden Y, Bertilsson L, Broomé U, Einarsson C, Diczfalusy U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. Evidence for involvement of cytochrome P450 3A4. J Biol Chem. 2001;276:38685–9. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- 3.Bodin K, Andersson U, Rystedt E, Ellis E, Norlin M, Pikuleva I, Eggertsen G, Björkhem I, Diczfalusy U. Metabolism of 4β-hydroxycholesterol in humans. J Biol Chem. 2002;277:31534–40. doi: 10.1074/jbc.M201712200. [DOI] [PubMed] [Google Scholar]
- 4.Marschall H-U, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–85. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Josephson F, Bertilsson L, Böttiger Y, Flamholc L, Gisslén M, Ormaasen V, Sönnerborg A, Diczfalusy U. CYP3A induction and inhibition by different antoretroviral regimens reflected by changes in plasama 4β-hydroxycholesterol. Eur J Clin Pharmacol. 2008;64:775–81. doi: 10.1007/s00228-008-0492-8. [DOI] [PubMed] [Google Scholar]
- 6.Wide K, Larsson H, Bertilsson L, Diczfalusy U. Time course of the increase in 4β-hydroxycholesterol concentration during carbamazepine treatment of pediatric patients with epilepsy. Br J Clin Pharmacol. 2008;65:708–15. doi: 10.1111/j.1365-2125.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson H, Böttiger Y, Iuliano L, Diczfalusy U. In vivo interconversion of 7β-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free Rad Biol Med. 2007;43:695–701. doi: 10.1016/j.freeradbiomed.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Kanebratt KP, Diczfalusy U, Bäckström T, Sparve E, Bredberg E, Böttiger Y, Andersson TB, Bertilsson L. Cytochrome P450 induction by rifampicin in healthy subjects: determination by the Karolinska cocktail and the endogenous CYP3A4 marker 4β-hydroxycholesterol. Clin Pharm Ther. 2008 doi: 10.1038/clpt.2008.132. Advance online publication 23 July. doi:10.1038/clpt. 2008.132. [DOI] [PubMed] [Google Scholar]
- 9.Bertilsson L, Tomson T, Tybring G. Pharmacokinetics: time-dependent changes – autoinduction of carbamazepine epoxidation. J Clin Pharmacol. 1986;26:459–62. doi: 10.1002/j.1552-4604.1986.tb03558.x. [DOI] [PubMed] [Google Scholar]
- 10.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Burrows BA, Hollander W. Body cholesterol metabolism in man. II. Measurement of the body cholesterol miscible pool and turnover rate. J Clin Invest. 1962;41:1738–44. doi: 10.1172/JCI104632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meaney S, Hassan M, Sakinis A, Lütjohann D, von Bergmann K, Wennmalm Å, Diczfalusy U, Björkhem I. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J Lipid Res. 2001;42:70–8. [PubMed] [Google Scholar]
- 13.Goodman DS, Smith FR, Sepowitz AH, Ramakrishnan R, Dell RB. Prediction of the parameters of whole body cholesterol metabolism in humans. J Lipid Res. 1980;21:699–713. [PubMed] [Google Scholar]
- 14.Ostlund RE., Jr. A minimal model for human whole body cholesterol metabolism. Am J Physiol. 1993;265:E513–20. doi: 10.1152/ajpendo.1993.265.3.E513. [DOI] [PubMed] [Google Scholar]
- 15.Meaney S, Bodin K, Diczfalusy U, Björkhem I. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res. 2002;43:2130–5. doi: 10.1194/jlr.m200293-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Lange Y, Ye J, Strebel F. Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J Lipid Res. 1995;36:1092–7. [PubMed] [Google Scholar]
- 17.Diczfalusy U, Miura J, Roh H-K, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allqvist A, Jande M, Kim J-W, Aklillu E, Gustafsson LL, Bertilsson L. The concentration of 4β-hydroxycholesterol in plasma is an endogenous marker of CYP3A4 enzyme activity – relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics. 2008;18:201–8. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]


