Abstract
AIMS
QTc interval prolongation and torsades de pointes have been reported in HIV-infected patients. Protease inhibitors (PIs) are suspected to contribute to this adverse reaction. However, many factors can prolong QTc interval. We examined factors influencing QTc duration in HIV-infected patients.
METHODS
Unselected HIV-infected patients (n = 978) were enrolled in this prospective, single-centre cross-sectional study. Variables related to infection and treatments were collected. A digital electrocardiographic record was recorded in each patient and QT interval duration was measured and corrected using both Bazett's (QTcB) and Fridericia's (QTcF) formula. Results were analysed with a multivariable linear model.
RESULTS
After excluding arrhythmias and complete bundle branch blocks, QT interval was measured in 956 patients. The mean (SD) QTcB was 418 ms (23) and QTcF was 405 ms (20). QTc was found prolonged (>450 ms in women and >440 ms in men) in 129 [13.5%; 95% confidence interval (CI) 11.5, 15.8] and 38 (4%; 95% CI 2.9, 5.4) patients using Bazett and Fridericia corrections, respectively. On multivariable analysis, incomplete bundle branch block, ventricular hypertrophy, signs of ischaemic cardiopathy, female gender, White ethnic origin and age were significantly associated with QTc prolongation. The only HIV variable independently associated with QTc prolongation was the duration of infection (P = 0.023). After adjustment, anti-HIV treatment, in particular PI (P = 0.99), was not associated with QTc prolongation.
CONCLUSIONS
Although PIs block in vitro hERG current, they are not independently associated with QTc interval prolongation. Prolonged QTc interval in HIV-infected patients is primarily associated with factors commonly known to prolong QT and with the duration of HIV infection.
Keywords: electrocardiography, HIV infection, HIV protease inhibitors, long QT syndrome
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The risks of torsade de pointes and QTc prolongation in HIV patients have been reported.
Authors have shown that four protease inhibitors (PIs) blocked human ether-a-go-go-related gene (hERG), the current that underlies QTc prolongation and drug-induced proarrhythmia, and have also reported cases of torsades and suggested that PIs were responsible for these adverse events.
This earlier paper has had a major impact on the perception of the arrhythmogenic risk associated with PIs, and regulatory agencies in Europe and the USA have modified the labelling of PIs accordingly.
WHAT THIS STUDY ADDS
The present study provides alternative explanations for QTc prolongation in a cohort of HIV patients and reports ECG abnormalities found in such patients.
It does not confirm that PIs play a significant role in QTc interval prolongation in HIV patients.
In contrast, it shows that QTc prolongation is related to common causes and to the duration of HIV infection rather than to anti-HIV treatments.
Introduction
Protease inhibitors (PI) are widely prescribed in the treatment of HIV-infected patients. Recently, Anson et al. reported 14 cases of QT/QTc interval prolongation and/or arrhythmia in patients treated with PI [1]. They showed in vitro inhibition of human ether-a-go-go-related gene (hERG) current (underlying QT prolongation) with the four PIs tested (lopinavir, nelfinavir, ritonavir and saquinavir) and recommended QT interval monitoring in patients treated with PI. However, no human study has unequivocally reported PI-induced QT interval prolongation. A recent prospective study found a nonstatistically significant 3.2-ms QTc interval prolongation 2 h after initiation of atazanavir therapy and no prolongation at steady state, casting doubt on the proarrhythmic potential of PI [2].
QT prolongation has many causes [3] and is associated with chronic diseases such as diabetes, hypertension and cirrhosis [4–6]. QT interval prolongation in HIV-infected patients has been reported with a prevalence as high as 65% in patients with autonomic neuropathy [7–9]. Although these studies were not designed to address this issue formally, no relationship with anti-HIV drugs has been found. With increasing care and available treatments, HIV infection has become a chronic disease with potential cardiac consequences [10]. Thus, other HIV-related and non-HIV-related effects on cardiac repolarization cannot be excluded and might explain QTc prolongation in PI-treated patients. PIs and HIV infection may also have cumulative effects on cardiac repolarization.
We conducted a large prospective study to assess the effects of HIV treatments, in particular PI, on QTc interval duration in HIV-infected patients.
Materials and methods
Patients were enrolled in this prospective single-centre study after giving their informed consent. The protocol was approved by the Ethics Committee of Saint-Antoine Hospital (Paris, France). The study was conducted among patients of the Department of Infectious Disease of Saint-Antoine Hospital (Paris, France), which has an active file of 1900 HIV-infected patients. From July to December 2005, all ambulatory HIV-infected patients who came to the hospital for a routine visit were invited to have an electrocardiographic recording (ECG) at the Clinical Investigation Centre. In volunteer patients, a 1-min 12-lead digital electrocardiographic recording (Cardioplug; Cardionics SA, Brussels, Belgium) was made after 5 min of rest in the supine position and an automatic brachial blood pressure measurement (Omnicare; Hewlett Packard, Houston, TX, USA) was performed. Blood pressure and heart rate were reassessed after 1 min in the standing position. During the study period, all HIV-infected patients hospitalized in the Infectious Disease Unit were also included, regardless of the reason for hospitalization. In these patients, a 10-s digital electrocardiogram was performed (Cartouch; Cardionics SA) in the resting position.
Data collection
For each patient, information on drug treatment (HIV and non-HIV) was obtained, including name, dosage, date and hour of the last intake. In addition, a questionnaire was performed in search of dysautonomic symptoms. This included six items (each item scoring one point): symptoms of orthostatic hypotension (i.e. dizziness …), urological symptoms, sweating, diarrhoea, erectile dysfunction and limb paraesthesia. Patients’ general characteristics and medical history were extracted from a database used in the routine care of these patients (DIAMM/G software; Micro6, Villers les Nancy, France) including date of birth, gender, ethnic group, height, weight and viral hepatitis status. Duration of HIV infection was estimated from the date of first known positive HIV serology to the day of ECG recording. Duration of AIDS was estimated from the date of C status diagnosis of the Centers for Disease Control classification to the date of ECG recording.
Biological data
A blood sample was drawn on the same day as the ECG recording in 152 patients. Plasma potassium level was measured in these samples using the routine biochemical laboratory of Saint-Antoine Hospital.
Electrocardiographic analysis
In outpatients, a 10-s portion of the 1-min ECG was selected for evaluations based on heart rate stability. QT interval was measured semimanually by a single observer (A.R.) using an averaged cardiac cycle over 10 s with Cardionics software (Cardionics SA). The QT interval was corrected (QTc) using the averaged heart rate over the 10 s using both Bazett's and Fridericia's correction formula. Whichever formula was used, QTc was defined as prolonged when >440 ms in men and >450 ms in women. One hundred randomly chosen ECGs were re-measured 4 months later by the same observer and by another observer (B.C.) to assess reproducibility. The intra- and interobserver coefficients of variation were 1.5 and 1.7%, respectively. All ECGs were also assessed by two senior cardiologists (C.F-B., F.B.) for detection of electrocardiographic diagnoses and morphological changes.
Statistical analysis
It was calculated that 800 patients were necessary to show a QTc prolongation of ≥5 ms associated with PI use, assuming a standard deviation of 25 ms and 50% overall PI exposure. We arbitrarily increased the size to 1000 to gain power in the multivariable analysis. Quantitative variables are presented as mean (1 SD) or median (25th percentile; 75th percentile), where appropriate. Percentages are presented with 95% confidence intervals (CIs). A linear multiple regression with a backwards selection procedure (all variables associated at P < 0.2 were first introduced in the model and eliminated one at a time until all remaining variables were associated at the 0.05 level) was performed to identify independent predictors of QTc interval prolongation. Quantitative variables showing nonlinear association with QTc were introduced in the model as four quartiles. In order to increase the sensitivity of our analysis to detect drug-induced QTc prolongation around peak plasma concentrations, we also considered the time lag between drug intake and ECG recording. Peak effect was considered for each treatment when the time lag between drug intake and ECG recording was >30 min and <480 min for drugs administered once daily and <240 min for other dosing regimens. All statistical analyses were performed with SAS 9.1 software (SAS Institute, Cary, NC, USA).
Results
One thousand and eighteen electrocardiographic records were available; 941 (92%) were performed in outpatients. Forty ECGs (4%) were not included in the analysis for the following reasons: six patients not HIV infected, 33 duplicate recordings (in patients who already had an ECG for the present study), and one ECG not readable (Figure 1). Therefore, 978 ECGs from different HIV-infected patients were eligible for ECG analysis. Their clinical characteristics are shown in Table 1.
Figure 1.
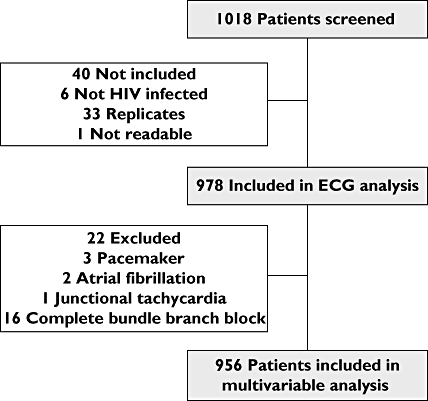
Flow chart
Table 1.
Demographical characteristics
| General characteristics (n = 978) | |
| Age (years) | 45 (10) |
| Body mass index (kg m−2) | 22.9 (3.6) |
| Sex (male/female) | 762/216 (78/22%) |
| Ethnic origin | |
| White | 754 (77%) |
| African | 166 (17%) |
| Other | 42 (4%) |
| Missing | 16 (2%) |
| Systolic/diastolic blood pressure (mmHg) | 127 (15)/70 (11) |
| Hepatitis C infection (n, %) | 146 (15%) |
| Disease | |
| CDC status | A: 52%; B: 15%; C: 32%; missing: 1% |
| Duration of HIV infection (years) | 11.3 (4.3; 16.5) |
| Duration of AIDS (years); n = 314 | 4.5 (1.5; 8.7) |
| HIV drug therapy | |
| Nucleoside reverse transcriptase inhibitors | 719 (74%) |
| Lamivudine | 391 (54%) |
| Tenofovir | 340 (47%) |
| Abacavir | 162 (23%) |
| Protease inhibitors | 455 (47%) |
| Ritonavir | 245 (54%) |
| Lopinavir | 213 (47%) |
| Atazanavir | 137 (30%) |
| Non-nucleoside reverse transcriptase inhibitors | 294 (30%) |
| Efavirenz | 201 (68%) |
| Nevirapine | 100 (34%) |
| No anti-HIV treatment | 223 (23%) |
Percentage of anti-HIV treatment given in italics are calculated within each treatment category (for the three most used drugs) and therefore can be >100%, reflecting drug combinations. Ritonavir always given as a low-dose pharmacological booster. CDC, Centers for Disease Control.
Electrocardiographic analysis
ECG diagnoses are shown in Table 2. At least one abnormality was found in 213 ECGs (22%). Conduction defects were found in 122 patients (12.5%; 95% CI 10.5, 14.7), and signs of ischaemia were found in 39 (4.0%; 95% CI 2.9, 5.4). Atrial fibrillation, complete bundle branch block or pacemaker ventricular stimulation were found in 22 patients. They were excluded from the final analysis (Figure 1).
Table 2.
ECG morphological analysis
| ECG abnormality | n (total = 978) |
|---|---|
| Conduction | |
| Complete bundle branch block | 16 (1.6%; 0.9, 2.6) |
| Incomplete right bundle branch block | 43 (4.4%; 3.2, 5.9) |
| Left anterior bundle branch block | 20 (2.0%; 1.3, 3.1) |
| Left posterior bundle branch block | 8 (0.8%; 0.4, 1.6) |
| 1st degree atrioventricular block | 43 (4.4%; 3.2, 5.9) |
| Rhythm | |
| Atrial fibrillation | 2 (0.2%; 0.02, 0.74) |
| Pacemaker | 3 (0.3%; 0.06, 0.89) |
| Ischaemic abnormalities | |
| Q wave | 16 (1.6%; 0.9, 2.6) |
| Ischaemic T wave | 25 (2.6%; 1.7, 3.7) |
| Others | |
| Atrial hypertrophy | 24 (2.5%; 1.6, 3.6) |
| Left ventricular hypertrophy | 61 (6.2%; 4.8, 7.9) |
| No morphological abnormality | 765 (78%; 75, 81) |
Number of subjects is given with prevalence and 95% confidence intervals. Total abnormalities exceed the 213 noted in the Results section because of multiple abnormalities in some patients.
Heart rate and QT interval were measured in the remaining 956 ECGs. Mean heart rate was 73 beats min−1 (13) and QT interval was 383 ± 30 ms, resulting in a mean QTc interval of 418 (23) and 405 ms (20) using Bazett's and Fridericia's formula, respectively. QTc interval was found prolonged in 129 patients (13.5%; 95% CI 11.5, 15.8) and 38 patients (4.0%; 95% CI 2.9, 5.4) using Bazett's and Fridericia's correction formula, respectively. The slope of the linear regression for the relationship between corrected QT interval and RR interval (60/heart rate) was −79 ms s−1 (4) (P < 0.0001 vs. zero) using Bazett and 3 ms s−1 (4) (P = 0.48 vs. zero) using Fridericia. Thus, in contrast to Bazett's formula, Fridericia's correction appropriately corrected QT interval [11]. It was therefore used for further analysis.
Factors influencing QTc interval
Results of univariate and multivariable analyses of factors related to QTc values are shown in Table 3. Eight factors remained significant predictors in the multivariable model, namely demographical variables: sex, age and ethnic origin; morphological ECG abnormalities: incomplete right bundle branch block, left anterior bundle branch block, left ventricular hypertrophy and ischaemic abnormalities. The only significant parameter related to HIV was the duration of infection, with significant QTc prolongation after the year 4 of infection.
Table 3.
Univariate and multivariable analysis of factors influencing QTc duration
| Level (reference) | n | Average QTc difference from reference level (univariate analysis) | P | Average QTc difference from reference level (multivariable analysis) n = 939 | P | |
|---|---|---|---|---|---|---|
| Demography | ||||||
| Age (years) | (per additional year) | 956 | 0.5 (0.4;0.6) | <0.001 | 0.4 (0.3;0.6) | <0.001 |
| Sex | Female | 214 | 6.7 (3.8;9.6) | <0.001 | 12.6 (9.7;15.5) | <0.001 |
| Male (ref.) | 742 | 0 | 0 | |||
| Ethnic origin | White | 734 | 6.1 (2.9;9.3) | <0.001 | 8.2 (4.8;11.5) | <0.001 |
| Other | 42 | 1 (−5.5;7.5) | 4 (−2;10) | |||
| African (ref.) | 164 | 0 | 0 | |||
| Clinical characteristics | ||||||
| Heart rate (beats min−1) | (per additional beat) | 956 | 0 (−0.1;0) | 0.35 | ||
| Body mass index (kg m−2) | (per additional unit) | 934 | −0.3 (−0.6;0.1) | 0.13 | ||
| Orthostatic hypotension | Yes | 26 | 7.3 (−0.2;14.7) | 0.05 | ||
| No (ref.) | 922 | 0 | ||||
| Dysautonomic symptoms | ≥2 | 266 | 3.4 (.5;6.3) | 0.06 | ||
| 1 | 231 | 0.3 (−2.7;3.4) | ||||
| 0 (ref.) | 459 | 0 | ||||
| ECG abnormalities | ||||||
| Left anterior bundle branch block | Yes | 20 | 13.6 (5.1;22.2) | 0.002 | 10 (2.5;18) | 0.001 |
| No (ref.) | 936 | 0 | 0 | |||
| Incomplete right bundle branch block | Yes | 43 | 14 (8.1;19.8) | <0.001 | 14 (8.7;19.4) | <0.001 |
| No (ref.) | 913 | 0 | 0 | |||
| Ischaemic abnormalities | Yes | 37 | 13.8 (7.5;20.1) | <0.001 | 9.5 (3.6;15.4) | 0.002 |
| No (ref.) | 919 | 0 | 0 | |||
| Left ventricular hypertrophy | Yes | 60 | 5.1 (0.1;10.2) | 0.047 | 6.4 (1.8;11) | 0.006 |
| No (ref.) | 896 | 0 | 0 | |||
| HIV disease | ||||||
| Duration of AIDS (years) | (per additional year) | 309 | 0.4 (0.1;0.8) | 0.02 | ||
| Duration of HIV infection (years) | ≥16 years | 265 | 10.5 (7.1;13.8) | <0.001 | 4.1 (0.7;7.5) | 0.02 |
| ≥11 and <16 years | 219 | 8.2 (4.6;11.7) | 4.5 (1.1;7.9) | |||
| ≥4 and <11 years | 246 | 7.4 (3.9;10.8) | 4.5 (1.3;7.7) | |||
| <4 years (ref.) | 225 | 0 | ||||
| Other diseases | ||||||
| C hepatitis | Yes | 138 | 4.5 (1;8) | 0.01 | ||
| No (ref.) | 791 | 0 | ||||
| Diabetes | Yes | 24 | −2.2 (−10;5.7) | 0.59 | ||
| No (ref.) | 932 | 0 | ||||
| Non-HIV-related drugs* | ||||||
| QT-prolonging drug | Yes | 33 | 3.6 (−3.1;10.4) | 0.29 | ||
| No (ref.) | 923 | 0 | ||||
| Nonpotassium sparing diuretics | Yes | 26 | 8.7 (1.4;16) | 0.02 | ||
| No (ref.) | 930 | 0 | ||||
| Potassium concentration (mmol l−1) | 151 | 2.2 (−7.3;11.7) | 0.65 | |||
HIV-related drugs are shown in Table 4.
Effects of anti-HIV drugs on QTc interval duration are shown in Table 4. No anti-HIV drug was associated with QTc prolongation after standardization of potential confounders. In particular, PI treatment was not associated with QTc lengthening. This remained the case when the analysis took into account peak and nonpeak data. For example, with PI, the averaged QTc difference in the adjusted analysis was −0.5 ms (−3.4; 2.5) (n = 186) when PI were considered at peak level and 0.3 ms (−2.3; 2.9) (n = 254) otherwise; P = 0.89 vs. no PI. Known QT-prolonging drugs were co-prescribed in 33 patients: methadone (n = 15), chloroquine (n = 3), haloperidol (n = 2), domperidone (n = 10) and moxifloxacine (n = 5). These drugs were not significantly associated with QTc prolongation on univariate analysis.
Table 4.
Univariate and adjusted analysis of anti-HIV drugs in relation with QTc interval duration
| Anti-HIV drugs | n | Univariate analysis Average difference in QT duration from reference level | P-value | Adjusted analysis* Average difference in QT duration from reference level | P-value | |
|---|---|---|---|---|---|---|
| Protease inhibitors | Yes | 440 | 1 (−1.4;3.5) | 0.41 | 0 (−2.3;2.2) | 0.99 |
| No (ref.) | 516 | 0 | 0 | |||
| NRTI | Yes | 701 | 5.7 (2.8;8.6) | <0.001 | 2.8 (−0.1;5.7) | 0.12 |
| No more NRTI | 35 | 5 (−1.9;11.8) | 0.1 (−6.2;6.5) | |||
| No (ref.) | 220 | 0 | 0 | |||
| Non-nucleoside reverse transcriptase inhibitors | Yes | 289 | 2.2 (−0.5;4.9) | 0.1 | −0.4 (−2.9;2.1) | 0.75 |
| No (ref.) | 667 | 0 | 0 |
Adjusted on: age, sex, ethnic origin, left anterior bundle branch block, incomplete right bundle branch block, ischaemic abnormality, left ventricular hypertrophy and HIV duration. NRTI, nucleoside reverse transcriptase inhibitor.
The number of symptoms of dysautonomia was ≥2 in 28% of patients. The most frequent were paraesthesia in 272 (28%) patients, followed by sweating in 205 (21%) and erectile dysfunction in 131 (13%). However, only 2.7% of patients had proven (measured) orthostatic hypotension. Neither objective nor subjective factors were significantly associated with QTc prolongation. Hepatitis C serology status was not related to QTc values on multivariable analysis. In patients in whom potassium plasma concentration was assessed, eight had a potassium level <3.5 mEq l−1. No correlation between QTc and plasma potassium values was found (n = 152; P = 0.65).
All the statistical analyses were also performed using Bazett's QT correction formula. Factors associated with QTc interval prolongation in the multivariable analysis were unchanged except for heart rate, which was significantly associated with QTc (Bazett) lengthening (P < 0.0001).
Discussion
Besides factors commonly known to be associated with prolonged QTc interval duration, such as advanced age [12, 13], female gender [14] or White origin [15], a significant relationship was found between duration of HIV infection and QTc interval, but no significant influence of antiretroviral drugs, in particular PI, on QTc. The association between duration of HIV infection and QTc interval prolongation has never been reported. The reason for this association is unclear, but could reflect the influence of chronic infection or cumulative exposure to anti-HIV drugs on the heart and the autonomic nervous system. Villa et al. [7]. have reported greater QTc interval duration in HIV-infected patients with asymptomatic autonomic neuropathy than in HIV-infected patients without neurological disorder. Asymptomatic alterations of the sympatho-vagal balance occur at the early stage of the disease [16, 17]. These autonomic alterations are also suspected to be due to antiretroviral treatments associated with the adipose redistribution syndrome in HIV-infected patients [18]. We did not find a relationship between QTc interval and symptoms of dysautonomia. However, our method of assessment of dysautonomia was not sensitive.
We used conventional threshold limits for QTc prolongation in this study (>440 ms in men and >450 ms in women) [3, 19]. Although the present study was not designed to compare the prevalence of QTc prolongation in HIV- and non-HIV-infected patients, the proportion of patients with a prolonged QTc interval was within or slightly above the expected prevalence in the general population [20, 21], and lower than previously reported [8, 9]. In the study by Nordin et al[9], 20% of 816 HIV-infected patients had QTc >470 ms, whereas Kocheril et al. [8] found 28% of 42 hospitalized patients with HIV disease with QTc >440 ms. However, both studies included patients with more advanced HIV disease than those included in the present study.
Also, Nordin et al. [9] found that hepatitis C infection was associated with QTc prolongation, a result that was not confirmed in our study. We voluntarily chose to include unselected patients to obtain the most representative group of HIV-infected patients treated in a teaching hospital. Our results may not be extrapolated to patients with more severe HIV infection. Moreover, we did not systematically collect information about the existence of HIV cardiomyopathy that might be a confounding factor regarding the proarrhythmic risk.
Although PIs inhibit hERG current in vitro[1] and are therefore expected to prolong the duration of ventricular repolarization, we did not find a relationship between PI intake and QTc prolongation. This discrepancy between in vitro and in vivo data is well known and has led international agencies to require clinical QTc assessment even in the absence of a positive signal in experimental models of prolonged repolarization [22]. Among all the PIs assessed by Anson et al. [1], lopinavir had the greatest hERG inhibitory effect, with an IC50 of 8.6 µM [1]. In HIV-infected patients, the mean maximal total plasma concentration of lopinavir after once-daily oral administration of 800 mg (combined with ritonavir 200 mg) is 12.8 mg l−1 (∼20 µM) [23]. Lopinavir, like other PIs, is extensively bound to plasma protein, leading to an unbound fraction of <2% [24]. Assuming a free concentration of 0.4 µM of lopinavir at peak, and based on the in vitro hERG data of Anson et al. [1], one would expect <5% inhibition of hERG current at peak plasma concentration. Theoretical models suggest that such a degree of hERG blockade is expected to produce a prolongation of QTc interval of <5 ms [25]. This could explain the lack of a significant influence of PI on QTc prolongation we have observed, a result that is consistent with recent data showing the absence of effect of atazanavir on ventricular repolarization during repeated administration [2]. To increase our sensitivity to detect an effect of PI on QTc interval duration, we examined the influence of high (near peak) plasma concentrations of HIV treatments on QTc interval duration. This analysis did not alter our results. Although our study was powered to detect a QTc prolongation of at least 5 ms, a threshold in accordance with current guidelines [22], this epidemiological approach cannot definitely exclude an effect of small amplitude in some patients. It indicates, however, that anti-HIV drugs are not a major factor in QTc interval prolongation in HIV-infected patients.
In HIV-infected patients, QTc interval prolongation is related to common, drug-independent factors and appears to be relatively infrequent. In this population, longer duration of infection, not anti-HIV therapy, is associated with QTc prolongation.
Acknowledgments
This study was supported by a grant in aid from the Assistance Publique#x2013;Hôpitaux de Paris and the Institut National de la Santé et de la Recherche Médicale at the Clinical Investigation Centre of Saint-Antoine University Hospital (Paris, France). These institutions were not involved in the design or analysis of the study. The authors thank the physicians involved in this study: Herve Bideault, Pauline Campa, Laurent Fonquernie, Jean-Luc Meynard, Zineb Ouazene, Philippe Roussard, Marie-Caroline Meyohas, and the nurses: Catherine Gachenot, Annie Joachim, Marinette Perrin, Monique Dubousquet. We thank Catherine Lupin for her help in the use of the clinical database, the staff of the Saint-Antoine Clinical Investigation Centre, and Sofia Meurisse and Paul Merckx for their help in statistical analysis.
Competing interests
None declared.
REFERENCES
- 1.Anson BD, Weaver JG, Ackerman MJ, Akinsete O, Henry K, January CT, Badley AD. Blockade of HERG channels by HIV protease inhibitors. Lancet. 2005;365:682–6. doi: 10.1016/S0140-6736(05)17950-1. [DOI] [PubMed] [Google Scholar]
- 2.Busti AJ, Tsikouris JP, Peeters MJ, Das SR, Canham RM, Abdullah SM, Margolis DM. A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med. 2006;7:317–22. doi: 10.1111/j.1468-1293.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 3.Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, Schepis F, Mandini M, Simoni P, Contin M, Raimondo G. QT interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 5.Schillaci G, Pirro M, Ronti T, Gemelli F, Pucci G, Innocente S, Porcellati C, Mannarino E. Prognostic impact of prolonged ventricular repolarization in hypertension. Arch Intern Med. 2006;166:909–13. doi: 10.1001/archinte.166.8.909. [DOI] [PubMed] [Google Scholar]
- 6.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV. Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the strong heart study. Diabetes. 2004;53:434–40. doi: 10.2337/diabetes.53.2.434. [DOI] [PubMed] [Google Scholar]
- 7.Villa A, Foresti V, Confalonieri F. Autonomic neuropathy and prolongation of QT interval in human immunodeficiency virus infection. Clin Auton Res. 1995;5:48–52. doi: 10.1007/BF01845498. [DOI] [PubMed] [Google Scholar]
- 8.Kocheril AG, Bokhari SA, Batsford WP, Sinusas AJ. Long QTc and torsades de pointes in human immunodeficiency virus disease. Pacing Clin Electrophysiol. 1997;20:2810–6. doi: 10.1111/j.1540-8159.1997.tb05439.x. [DOI] [PubMed] [Google Scholar]
- 9.Nordin C, Kohli A, Beca S, Zaharia V, Grant T, Leider J, Marantz P. Importance of hepatitis C coinfection in the development of QT prolongation in HIV-infected patients. J Electrocardiol. 2006;39:199–205. doi: 10.1016/j.jelectrocard.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–55. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–20. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 12.Grandinetti A, Seifried S, Mor J, Chang HK, Theriault AG. Prevalence and risk factors for prolonged QTc in a multiethnic cohort in rural Hawaii. Clin Biochem. 2005;38:116–22. doi: 10.1016/j.clinbiochem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil. 2005;12:363–8. doi: 10.1097/01.hjr.0000173110.21851.a9. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–6. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 15.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Cardiol. 1998;81:453–9. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 16.Becker K, Gorlach I, Frieling T, Haussinger D. Characterization and natural course of cardiac autonomic nervous dysfunction in HIV-infected patients. AIDS. 1997;11:751–7. doi: 10.1097/00002030-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Mittal CM, Wig N, Mishra S, Deepak KK. Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol. 2004;94:1–6. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Fliers E, Sauerwein HP, Romijn JA, Reiss P, van der Valk M, Kalsbeek A, Kreier F, Buijs RM. HIV-associated adipose redistribution syndrome as a selective autonomic neuropathy. Lancet. 2003;362:1758–60. doi: 10.1016/s0140-6736(03)14858-1. [DOI] [PubMed] [Google Scholar]
- 19.Robbins J, Nelson JC, Rautaharju PM, Gottdiener JS. The association between the length of the QT interval and mortality in the Cardiovascular Health Study. Am J Med. 2003;115:689–94. doi: 10.1016/j.amjmed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RJ, Bengtson J, Chen Z, Anderson KM, Locati E, Levy D. Duration of the QT Interval and Total and Cardiovascular Mortality in Healthy Persons (the Framingham Heart Study Experience) Am J Cardiol. 1991;67:55–8. doi: 10.1016/0002-9149(91)90099-7. [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen J, Reunanen A, Ristola P, Viitasalo M. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77:543–8. doi: 10.1136/hrt.77.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee for Medicinal Products for Human Use. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. [26 June 2008];2005 CHMP/ICH/2/04 November Available at http://www.emea.europa.eu/pdfs/human/ich/000204en.pdf.
- 23.van Heeswijk RP, Bourbeau M, Seguin I, Giguere P, Garber GE, Cameron DW. Absence of circadian variation in the pharmacokinetics of lopinavir/ritonavir given as a once daily dosing regimen in HIV-1-infected patients. Br J Clin Pharmacol. 2005;59:398–404. doi: 10.1111/j.1365-2125.2005.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boffito M, Hoggard PG, Lindup WE, Bonora S, Sinicco A, Khoo SH, Di Perri G, Back DJ. Lopinavir protein binding in vivo through the 12-hour dosing interval. Ther Drug Monit. 2004;26:35–9. doi: 10.1097/00007691-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Jonker DM, Kenna LA, Leishman D, Wallis R, Milligan PA, Jonsson EN. A pharmacokinetic–pharmacodynamic model for the quantitative prediction of dofetilide clinical QT prolongation from human ether-a-go-go-related gene current inhibition data. Clin Pharmacol Ther. 2005;77:572–82. doi: 10.1016/j.clpt.2005.02.004. [DOI] [PubMed] [Google Scholar]


