Abstract
AIMS
(i) To classify antibacterial agents with QT liability on the basis of the available evidence, and (ii) to assess trends in their consumption over an 8-year period (1998–2005) in 14 European countries.
METHODS
Current published evidence on QT liability of antibiotics was retrieved through MEDLINE search and joined to official warnings from regulatory agencies. Each drug was classified according to an already proposed algorithm based on the strength of evidence: from group A (any evidence) to group E (clinical reports of torsades de pointes and warnings on QT liability). Consumption data were provided by the European Surveillance of Antibacterial Consumption (ESAC) project and were expressed as defined daily doses per 1000 inhabitants per day (DID).
RESULTS
Among 21 detected compounds, nine [six fluoroquinolones (FQs) and three macrolides (MACs)] belonged to group E. Use of group E drugs ranged from 1.3 (Sweden) to 4.1 DID (Italy) in 1998 and from 1.2 (Sweden) to 6.5 DID (Italy) in 2005. Significant exposure was observed in Italy and Spain (6.5 and 3.8 DID, respectively, in 2005). Only Denmark, Sweden and UK showed a slight decrease in use. Exposure to clarithromycin increased in 10 out of 14 countries, with a marked increment in Italy (3 DID in 2005).
CONCLUSIONS
Notwithstanding regulatory measures, in 2005 there was still significant exposure to antibacterials with strong evidence of QT liability and, in most countries, it was even increased. This warrants further investigation of appropriateness of use and suggests closer monitoring of group E drugs. Physicians should be aware when prescribing them to susceptible patients.
Keywords: antibacterial agents, drug exposure, QT liability, torsades de pointes
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Several noncardiovascular drugs with QT liability are currently on the market.
Previous epidemiological studies have shown significant exposure of the general population to drugs with QT liability with similar consumption in many European countries.
Several regulatory measures have concerned medicinal products carrying a pro-arrhythmic risk in humans.
WHAT THIS STUDY ADDS
The list of antibacterial agents with documented QT liability has grown over the last few years.
Notwithstanding stringent regulatory measures, population exposure to antibiotics with QT liability is still significant in several countries.
The magnitude of the problem is clearly heterogeneous, with remarkable diversity between Northern and Southern countries (lower and higher exposure, respectively).
Introduction
The explosion in the number of noncardiovascular drugs associated with ‘QT liability’ (i.e. capable to prolong the QT interval of the electrocardiogram) [1] is, at least in part, the result of the existing regulatory guidelines [International Conference on Harmonization (ICH) S7B [2] and ICH E14 [3], combining preclinical and clinical strategies to reveal the potential of drugs to delay ventricular repolarization, hence causing QT prolongation. This undesired effect is primarily caused by an intrinsic ability to block human ether a-go-go-related gene (hERG) K+ channels. Since QT prolongation may trigger the onset of potentially life-threatening arrhythmia, namely torsades de pointes (TdP) [4], the hERG K+ channel has become a primary antitarget in drug development [5–7].
Drug-induced TdP represents an important matter of concern for both clinicians and researchers, often culminating in regulatory interventions such as withdrawal (e.g. astemizole, grepafloxacin, cisapride, etc.) or restriction of use (e.g. terfenadine). A recent review [8] reported that QT prolongation (with or without TdP), together with hepatotoxicity, was responsible for >60% of drug withdrawals over the last 16 years. Moreover, estimates are that as many as 60% of new molecular entities are abandoned early during the drug development phase because they are positive on hERG assays, although their true torsadogenic potential is unknown. Therefore, QT liability of commonly prescribed drugs is a topic of particular interest, especially in patients susceptible to cardiac arrhythmias because of ‘reduced repolarization reserve’ (host- and drug-related risk factors) [9].
Previous investigations [10] have found significant exposure to non-antiarrhythmic drugs with QT-prolonging potential in the community: in 1998, approximately 2–3% of all drug prescriptions in the UK and Italy involved medications that may unintentionally cause the long QT syndrome. Moreover, an international drug utilization study, carried out in seven countries, highlighted that the total amount of QT-liability agents dispensed through community pharmacies in 1998 ranged from 13.1 to 19.6 defined daily doses (DDD) per 1000 inhabitants per day [11]. Thus, the question arises whether we are dealing with a class effect (i.e. shared by all agents of a given pharmacological class) or a specific effect of a few agents within a pharmacological class [12–16].
Among drugs with recognized QT liability, antibacterial agents were recently involved in regulatory interventions because of this risk (e.g. withdrawal of grepafloxacin and sparfloxacin from both US and European markets). The pro-arrhythmic potential of antibacterial agents deserves further investigation to define the risk–benefit profile of each drug.
The aim of this study was twofold: (i) to classify antibacterials with QT liability on the basis of the available evidence, and (ii) to estimate population exposure to these agents assessing consumption over an 8-year period (1998–2005) in 14 European countries.
Methods
Organizing evidence on QT liability of antibacterial agents
A MEDLINE search was performed to collect any preclinical and clinical evidence on QT liability of antibacterial agents up to December 2007. Search terms included ‘Anti-Bacterial Agents (Pharmacological Action) (MeSH)’, ‘Long QT Syndrome/chemically induced (MeSH)’, ‘Torsades de Pointes/chemically induced (MeSH)’, ‘HERG*’, ‘QT*’, ‘arrhythmia’, combined with each ‘compound name’. Review article and related reference sections from significant studies were also examined. Moreover, relevant official warnings from regulatory agencies (Food and Drug Administration, USA; European Medicines Agency, Europe; the Italian Pharmaceutical Agency, Italy; and the Medicines and Healthcare products Regulatory Agency, UK) were retrieved.
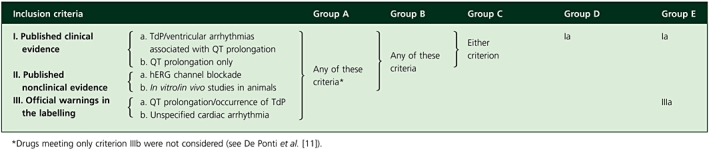
Drugs were first evaluated according to previous criteria suggested by De Ponti et al.[17]. Briefly, these criteria are based on available clinical and preclinical data as well as official warnings from regulatory agencies concerning QT prolongation, TdP/ventricular arrhythmias occurrence or hERG K+ channel blockade. Compounds were then divided into five categories according to the strength of evidence, in increasing order of clinical relevance for TdP risk: from group A, encompassing all agents with any evidence of QT liability, to group E, including only those compounds with the strongest evidence (i.e. clinical reports of TdP and warnings on QT prolongation). Table 1 summarizes the proposed algorithm to assign that risk to each drug. For more details on the method used to organize available data, see De Ponti et al.[11].
Table 1.
Criteria, based on the evidence available as of December 2007, used to group drugs according to the strength of evidence on QT liability
 |
Retrieval of consumption data
European consumption data of antibacterial agents were provided by the European Surveillance of Antibacterial Consumption (ESAC; http://www.esac.ua.ac.be) project, which is an international network of national surveillance systems officially launched during the European Conference on Antibiotic Use in Brussels in 2001. The start-up purpose was to collect harmonized and comparable data on antibacterial consumption (in and out of hospital setting) of the European community from publicly available sources, in order to assess the time trends in human exposure. The main long-term objectives of the ESAC project concern promotion of ‘Good Antibiotic Practice’. All European countries were invited to take part in this project and, in 2005, 34 of them participated, including all 25 EU countries, four applicant countries (Bulgaria, Croatia, Romania and Turkey) and three of the four members of the European Free Trade Association (Iceland, Norway and Switzerland). Before inclusion into the ESAC database, the validity of provided data was evaluated by means of a checklist including possible sources of bias (e.g. problems with population coverage, drug coverage and ambulatory care/hospital care mix).
As recommended by the World Health Organization (WHO), the Anatomical Therapeutic Chemical (ATC) code was assigned to each active substance (5th level of the ATC classification). The volume of consumption was expressed as DDD per 1000 inhabitants per day (DID). DDD represents the assumed average maintenance dose per day for a drug used in adults for its main indication (see also WHO Collaborating Centre for Drug Statistics Methodology Oslo, http://www.whocc.no/).
A sample of 14 countries (Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Italy, Luxembourg, the Netherlands, Slovenia, Spain, Sweden, UK) with consistent and bias-free data was chosen for a careful estimation of the European population exposure. Since Italian providers′ data were not able to share the ESAC project for 1998, we retrieved original data from the IMS Health database (their original source) and then converted them into DID in order to have comparable data. Results were presented both as absolute values and as fractions of overall antibacterial use. The drug utilization analysis of the 8-year period was performed according to the current published literature (as of December 2007).
A more comprehensive and thorough description of data providers, details on methodological approach and in-depth discussion are available in previous publications [18, 19].
Results
Literature data mining
Twenty-one antibacterial agents fulfilled at least one criterion (Table 2); nine of them were labelled to have the strongest evidence on QT liability (group E): six FQs (cipro-, gati-, grepa-, levo-, moxi- and sparfloxacin) and three MACs (azi-, clari- and erythromycin).
Table 2.
Antibacterial agents with documented QT liability (the references refer to information available through December 2007)
| Inclusion criteria | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Ia | Ib | IIa | IIb | IIIa | IIIb | 2001 | 2007 |
| Azitromycin | [34–37] | [38, 39] | [40]a | [40, 41]a | SPC1, PDR2, BNF3 | / | E | |
| Ciprofloxacin | [42–44] | [45–49]a | • | [50–52]a | PDR | SPC, BNF | / | E |
| Clarithromycin | • | • | • | • | • | E | E | |
| [53, 54] | [55, 56] | [21, 57] | [41] | SPC, PDR, BNF | ||||
| Clindamycin | • | D | D | |||||
| Erythromycin | • | • | [62–65][21, 57, 66] | • | • | • | E | E |
| [58–61] | [41, 62, 63, 67–69] | BNF, PDR | SPC | |||||
| Josamycin | [21] | / | B | |||||
| Gatifloxacin | [70–72] | [73] | • | • | • | B | E | |
| [74, 75] | PDR | |||||||
| Gemifloxacin | [76] | [77] | / | C | ||||
| Grepafloxacin | • | • | • | • | • | E | E | |
| Levofloxacin | • | [46, 48, 49, 84–86] | • | [50, 74] | SPC, PDR | BNF | D | E |
| [72, 78–83] | ||||||||
| Lomefloxacin | [87] | SPC | / | B | ||||
| Metronidazole | [88] | / | D | |||||
| Moxifloxacinb | [59, 79, 89, 90] | • | • | • | • | C | E | |
| [46, 48, 91, 92] | [40, 62, 63, 93–95] | [40, 50, 63, 94–97] | PDR, SPC, BNF | |||||
| Norfloxacin | [87] | PDR | / | B | ||||
| Ofloxacin | [72] | [50] | SPC, BNF | / | D | |||
| Prulifloxacin | [95] | [74, 95] | SPC | / | B | |||
| Roxithromycin | [98–100] | • | [21] | • | C | D | ||
| Sparfloxacin | [101, 102] | • | • | • | • | C | E | |
| [62, 103] | [68, 74, 75, 104, 105] | |||||||
| Spiramycin | • | D | D | |||||
| Cotrimoxazole | • | • | • | BNF | D | D | ||
| Telavancinc | [106]a | |||||||
| Telithromycin | [107–110] | [62, 63] | [63] | EMEA, SPC, PDR, BNF | / | C | ||
• Criteria met up to 2001[17].
Negative studies (i.e. reporting no effect) have been added for the sake of completeness.
Thorough QT Studies (TQTSs) submitted for regulatory purposes have not been included in the table.
Telavancin was not included in the analysis since no effect was reported for QT liability. Cases of TdP/QT prolongation caused by interactions have been included despite the causal association being doubtful.
Italian Summary of the Product Characteristics.
Physician Desk Reference or Dear Doctor Letters from the Food and Drug Administration (resulting in labelling changes).
British National Formulary 2004 edition.
The number of antimicrobials carrying a documented pro-arrhythmic potential has increased over recent years, as indicated by Table 2, where a synopsis of information available up to December 2001 and December 2007 is provided [17]. Notably, group A now includes 10 additional compounds, mainly due to novel published data for drugs already on the market (e.g. azithromycin) as well as marketing approval of new molecules (i.e. moxifloxacin, telithromycin, prulifloxacin, which is currently marketed only in Italy, and gemifloxacin, which received only a US market authorization in 2003). Moreover, for agents already known to affect cardiac repolarization (e.g. levofloxacin) new evidence on QT liability has become available. Thus, as shown in Table 2, several compounds (nine drugs in 2007) now belong to group E because of regulatory measures on the labelling or publication of new data.
Drug utilization data mining
Over the period of interest, the overall European consumption of antibacterials was substantially stable (266 DID in 1998 and 264 in 2005), with some notable exceptions: an increment was observed in Italy (6 DID), Denmark and Austria (2 DID, each). The overall use of antibiotics having any published evidence on QT liability ranged from 2.2 (Sweden) to 7.1 DID (Italy) in 1998 and from 2.0 (Sweden) to 8.4 DID (Italy) in 2005. Consumption increased over the years in half of countries: percentage increase ranged from 5% (Denmark) to 33% (Hungary). The remaining countries (Belgium, France, Luxembourg, Slovenia, Spain, Sweden and the UK) showed a decrease in use, with a maximum of 15% in Spain.
Focusing on drugs belonging to group E, their consumption increased during the period of interest (1998–2005) in most of countries (except for Denmark, Sweden and the UK). Specifically, their use ranged from 1.3 (Sweden and the Netherlands) to 4.1 DID (Italy) in 1998 and from 1.2 (Sweden) to 6.5 DID (Italy). Italy displayed the highest increase: in the 8-year period, community exposure increased to 1.6-fold (an increase of 2.4 DID). Significant exposure was observed also in Spain, Luxembourg (3.8 DID each), Hungary and Belgium (3.7 DID each). When the same data were expressed as a fraction of overall antibacterial use, most countries (except Denmark, Sweden and the UK) showed a positive time trend, with the highest increase in Hungary: from 11% (1998) to 19% (2005) (Figure 1).
Figure 1.
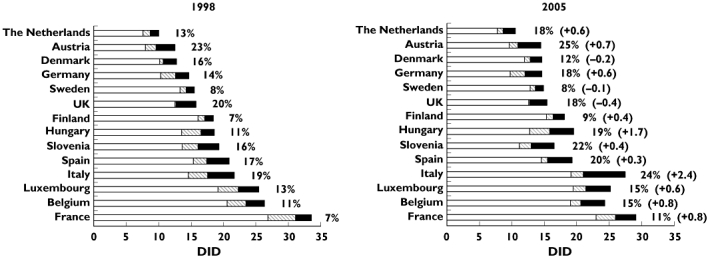
Consumption of antibacterial agents grouped by the strength of evidence on QT liability: comparison between 1998 and 2005 based on information available as of December 2007. Most countries showed an increase of population exposure both in terms of absolute value and as a fraction of overall antibacterial use. Countries were ranked by magnitude of total antibacterial consumption in 1998. Total consumption of antibacterials without evidence on QT-liability (□); Total consumption of antibacterials with QT-liability except for compounds listed in group E ( ); Group E, strongest level of evidence on QT-liability (▪). Percentage: antibacterial agents labeled as group E/total consumption of antibacterials. In parenthesis changes in use of group E compounds (absolute values); + = increment in consumption; − = decrease in consumption
); Group E, strongest level of evidence on QT-liability (▪). Percentage: antibacterial agents labeled as group E/total consumption of antibacterials. In parenthesis changes in use of group E compounds (absolute values); + = increment in consumption; − = decrease in consumption
All compounds with DID ≥0.1 were analysed in detail (Figure 2): FQs and MACs represented the most used drugs with documented QT liability within the antibacterial class. The use of FQs carrying a documented risk of TdP increased in all countries except in Slovenia (in which the decrease could probably be related to norfloxacin). This result may be partially explained by the current tendency to prescribe new-generation antibiotics (e.g. levofloxacin and moxifloxacin), albeit they are not first-line agents to treat outpatients with respiratory tract infections. As a matter of fact, their consumption increased in all countries except for levofloxacin in Denmark and moxifloxacin in the UK. By contrast, the overall use of MACs associated with QT-prolonging potential increased only in seven countries despite marketing introduction of telithromycin, which had significant use in France (0.4 DID in 2005), Belgium and Luxembourg (0.3 DID each in 2005).
Figure 2.
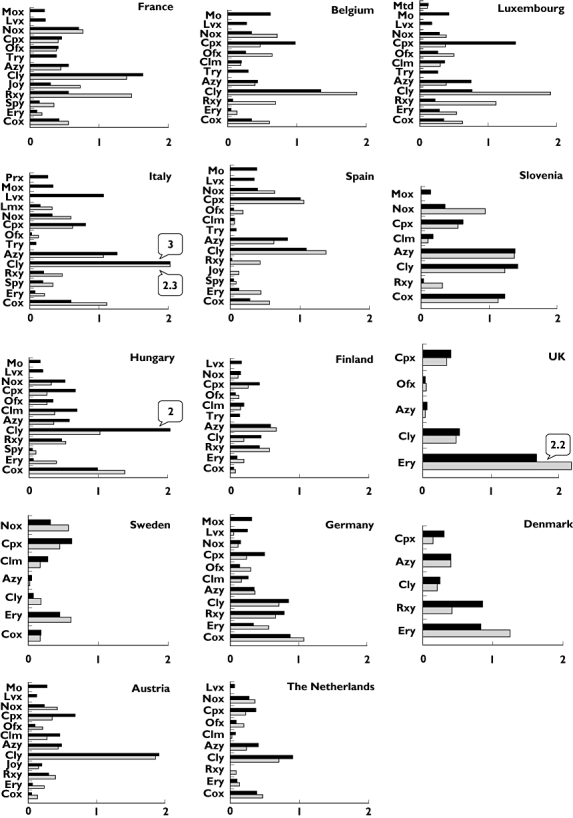
Consumption of each antibacterial drug with documented QT liability country by country: comparison between 1998 and 2005 [abscissa: defined daily doses per 1000 inhabitants per day (DID)]. Antibacterial agents with DID ≤0.1 were not included. Relevant consumption (≥2 DID) of some compounds have been outlined. Mtd, metronidazole; Mox, moxifloxacin; Prx, prulifloxacin; Lvx, levofloxacin; Lmx, lomefloxacin; Gpx, grepafloxacin; Nox, norfloxacin; Cpx, ciprofloxacin; Ofx, ofloxacin; Clim, clindamycin; Try, telithromycin; Azy, azithromycin; Cly, clarithromycin; Joy, josamycin; Rxy, roxithromycin; Ery, erythromycin; Cox, cotrimoxazole. 2005 (▪); 1998 ( )
)
Among MACs, exposure to clarithromycin increased in 10 out of 14 countries, with the highest increment in Hungary (up to twofold), where in 2005 it accounted for 2 DID (54% of total group E). Moreover, a peak of 3 DID was observed in Italy. By contrast, in Sweden it reached only 0.1 DID in 2005 (6% of total group E). Erythromycin use showed a decrease in all countries; however, it represented the most used drug in the UK (1.7 DID in 2005).
Discussion
Following an apparent ‘pharmaco-epidemic’ of antimicrobials with QT liability culminating in several regulatory interventions (e.g. withdrawals and warnings on FQs), the list of antibacterial agents with recognized risk of TdP onset has grown rapidly: 21 compounds were identified through literature data mining.
It should be pointed out that cardiac safety is not a novel topic of interest for antimicrobial agents, since most of them (e.g. MACs, ketolides, FQs, azoles, etc.) are known to affect in a significant way cardiac repolarization because of hERG blockade [15, 20, 21]. Moreover, existing guidelines recommended an integrated strategy based on clinical and preclinical studies to reveal the pro-arrhythmic potential of a new compound. Based on results of this integrated risk assessment, a molecule may obtain marketing approval despite being tested positive when assayed on QT liability because of a positive benefit–risk profile. This is the case with moxifloxacin, currently used as a positive control in thorough QT studies to check the sensitivity of clinical trials submitted for regulatory purpose [22, 23].
In clinical practice, TdP liability of individual drugs should be kept in mind, particularly in case of patients at risk because of ‘reduced repolarization reserve’[9] (e.g. inherited long QT syndrome), when the superimposition of a hERG-blocking drug may precipitate arrhythmia. Thus, this potentially fatal event, albeit rare, shared by drugs within the same therapeutic class makes choice of therapy very hard for physicians, especially in case of drugs used worldwide in general practice, such as antibacterial agents.
The present study has shown that, notwithstanding several regulatory measures, in 2005 there was still considerable use of antimicrobials associated with the strongest evidence on QT liability: 10 out of 14 countries showed an increase in consumption over the 8-year period. By contrast, Northern countries (i.e. Sweden, Denmark and Finland) displayed stable, low consumption. The use of group E agents, expressed as percentage of overall antimicrobial use, decrease only in Denmark and the UK in an 8-year period.
The overall trend towards increased consumption was of some concern in Italy, where in 2005 a peak of 6.5 DID was observed for compounds included in group E (an increase of 2.4 DID with respect to 1998). Exposure to clarithromycin accounted for 3 DID in Italy, with the highest increase in Hungary (1 DID) in the 8-year period (Figure 2). The reasons for this phenomenon are at present unclear: the fact that clarithromycin is used in several infective diseases (e.g. community-acquired pneumonia by atypical pathogens and eradication of Helicobacter pylori) may have contributed [24]. This remarkable use of clarithromycin should not be overlooked, not only because of its hERG-blocking properties, but also for its inhibitory effect on cytochrome P4503A4. Metabolic inhibition may indeed contribute to mixed pharmacokinetic–pharmacodynamic interactions with concomitant QT-prolonging agents: these interactions may lead to TdP, especially in patients with reduced repolarization reserve [25].
Differences among European countries in term of drug utilization may be due to several factors such as healthcare system, regulatory practice and prescription habits, as well as the number of marketed products, as recently suggested by Monnet [26]. The large variability in use of antibacterials across Europe has been discussed in recent papers [19, 27]. Based on 2005 consumption data of group E, we could divide countries into two main categories: Denmark, Finland, the Netherlands and Sweden can be considered low-exposure countries (<2 DID) and showed also the lowest pattern of overall consumption of antibacterials. The second group included countries with a higher exposure to group E drugs: Belgium, Luxemburg, Italy and France showed also high overall antibacterial consumption (>20 DID), whereas Germany, Austria, Slovenia, Spain and Hungary used lower amounts of antibacterials.
When considering community exposure, we should take into account both absolute and fractional values of antibacterial use: for example, in 2005 France and Austria had comparable use of antibiotics associated with the strongest evidence of QT liability (3.2 and 3.6 DID, respectively), although the same data represented 11 and 25%, respectively, of the overall use of antibacterials (29 and 14 DID). In order to decrease exposure to drugs with QT liability, one could tentatively consider shifting prescription habits from QT-prolonging drugs to safer agents. To this end, we should not overlook that current European guidelines do not recommend FQs and MACs as first-line therapy of respiratory infectious diseases; β-lactams are indeed preferred to treat nonsevere community-acquired pneumonia and have also no documented evidence on QT liability. By contrast, France may further consider reducing the overall use and proportionally those of QT-prolonging drugs. In fact, in order to decrease microbial resistance, France recently started the process of controlling antibacterial misuse and overuse in the community [28].
The clinical implications of this study should be viewed in the light of some intrinsic limitations of our approach, as already discussed elsewhere [11, 18]. Briefly, apart from potential bias affecting any method based on literature search, consumption data are only an indirect measure of population exposure and, more importantly, actual risk of TdP. To this end, pharmacovigilance databases may help to detect very rare adverse drug reactions such as TdP [29], although it should be acknowledged that over-consumption of an antibacterial with QT liability may not be associated with over-reporting of such a rare event, because of the known bias of spontaneous reporting systems [30–33]. For this reason, we carried this work to identify those antibacterials that warrant further investigation into pharmacovigilance databases because of their known QT liability and large population exposure.
Concerning the criteria that we adopted to classify drugs with QT liability, we acknowledge that they are not officially validated from a regulatory point of view, although they have already been used in previous publications [11, 17]. In any case, all antibacterials labelled as group E in our analysis are listed at http://www.torsades.org, a website where the University of Arizona Center for Education and Research on Therapeutics maintains an updated list of drugs with at least a conditional risk of TdP.
Furthermore, our approach labels as ‘alarming drugs’ (i.e. compounds classified in group E) many molecules regardless of large differences in terms of hERG blockade potency [20, 21] or consistency of clinical evidence. This is mainly caused by the regulatory concern often culminating in warnings, precautions and contraindications for drugs having doubtful evidence of QT liability (i.e. levofloxacin, norfloxacin and lomefloxacin; see Table 2). Thus, QT liability should not be viewed as a class effect of antibacterial agents because there seems to be large intraclass variability, as recently postulated [15, 16]. Therefore, additional studies are necessary to ascribe the individual risk of TdP to each compound: one resource can be data mining of spontaneous reporting systems.
In conclusion: (i) several antibacterial agents mostly belonging to macrolide and fluoroquinolone classes share torsadogenic potential; (ii) significant exposure is still present in the community, and in most countries consumption of antibacterial agents associated with the strongest evidence on QT liability has even increased, particularly in Italy and Hungary; and (iii) among European countries, remarkable differences emerged in the pattern of antimicrobial use carrying a documented risk of TdP. From a regulatory standpoint, this high antibacterial consumption in Southern European countries warrants further investigation of appropriateness of use and suggests closer monitoring of those agents associated with the strongest evidence on QT liability (group E drugs). From a clinical standpoint, these results should prompt careful risk–benefit assessment of each agent within antibacterial class, especially when a drug included in the list is prescribed to patients with known risk factors for TdP occurrence.
Acknowledgments
This work was supported by a grant from the University of Bologna (Progetto Strategico 2006).
Competing interests
None to declare.
REFERENCES
- 1.De Ponti F, Poluzzi E, Montanaro NQ. T-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol. 2000;56:1–18. doi: 10.1007/s002280050714. [DOI] [PubMed] [Google Scholar]
- 2.ICH Topic S 7 B – The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization QT Interval Prolongation) by Human Pharmaceuticals. London: EMEA; 2005. [PubMed] [Google Scholar]
- 3.ICH Topic E 14 – The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. London: EMEA; 2005. [Google Scholar]
- 4.Dessertenne F [Ventricular tachycardia with 2 variable opposing foci] Arch Mal Coeur Vaiss. 1966;59:263–72. [PubMed] [Google Scholar]
- 5.Recanatini M, Poluzzi E, Masetti M, Cavalli A, de Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005;25:133–66. doi: 10.1002/med.20019. [DOI] [PubMed] [Google Scholar]
- 6.De Ponti F. Pharmacological and regulatory aspects of QT prolongation. In: Vaz R, Klabunde T, editors. Antitargets. Prediction and Prevention of Drug Side Effects. I. Weinheim: Wiley-VCH; 2008. pp. 109–26. [Google Scholar]
- 7.Raschi E, Vasina V, Poluzzi E, de Ponti F. The hERG K(+) channel: target and antitarget strategies in drug development. Pharmacol Res. 2008;57:181–95. doi: 10.1016/j.phrs.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics. 2006;7:889–908. doi: 10.2217/14622416.7.6.889. [DOI] [PubMed] [Google Scholar]
- 9.Roden DM. Taking the ‘idio’ out of ‘idiosyncratic’: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–34. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 10.De Ponti F, Poluzzi E, Montanaro N, Ferguson JQ. Tc and psychotropic drugs. Lancet. 2000;356:75–6. doi: 10.1016/S0140-6736(05)73412-7. [DOI] [PubMed] [Google Scholar]
- 11.De Ponti F, Poluzzi E, Vaccheri A, Bergman U, Bjerrum L, Ferguson J, Frenz KJ, McManus P, Schubert I, Selke G, Terzis-Vaslamatzis G, Montanaro N. Non-antiarrhythmic drugs prolonging the QT interval: considerable use in seven countries. Br J Clin Pharmacol. 2002;54:171–7. doi: 10.1046/j.1365-2125.2002.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball P. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother. 2000;45:557–9. doi: 10.1093/jac/45.5.557. [DOI] [PubMed] [Google Scholar]
- 13.Iannini PB. Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J Antimicrob Chemother. 2001;47:893–4. doi: 10.1093/jac/47.6.893-a. [DOI] [PubMed] [Google Scholar]
- 14.Iannini PB, Doddamani S, Byazrova E, Curciumaru I, Kramer H. Risk of torsades de pointes with non-cardiac drugs. Prolongation of QT interval is probably a class effect of fluoroquinolones. BMJ. 2001;322:46–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Owens RC., Jr QT prolongation with antimicrobial agents: understanding the significance. Drugs. 2004;64:1091–124. doi: 10.2165/00003495-200464100-00005. [DOI] [PubMed] [Google Scholar]
- 16.Owens RC, Jr, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43:1603–11. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 17.De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of Torsades de Pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol. 2001;57:185–209. doi: 10.1007/s002280100290. [DOI] [PubMed] [Google Scholar]
- 18.Vander Stichele RH, Elseviers MM, Ferech M, Blot S, Goossens H. European surveillance of antimicrobial consumption (ESAC): data collection performance and methodological approach. Br J Clin Pharmacol. 2004;58:419–28. doi: 10.1111/j.1365-2125.2004.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother. 2006;58:401–7. doi: 10.1093/jac/dkl188. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol. 2001;59:122–6. doi: 10.1124/mol.59.1.122. [DOI] [PubMed] [Google Scholar]
- 21.Volberg WA, Koci BJ, Su W, Lin J, Zhou J. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther. 2002;302:320–7. doi: 10.1124/jpet.302.1.320. [DOI] [PubMed] [Google Scholar]
- 22.Shah RR. Drugs QTc interval prolongation and final ICH E14 guideline: an important milestone with challenges ahead. Drug Saf. 2005;28:1009–28. doi: 10.2165/00002018-200528110-00003. [DOI] [PubMed] [Google Scholar]
- 23.Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guideline. J Clin Pharmacol. 2006;46:498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- 24.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 25.Flockhart DA, Drici MD, Kerbusch T, Soukhova N, Richard E, Pearle PL, Mahal SK, Babb VJ. Studies on the mechanism of a fatal clarithromycin–pimozide interaction in a patient with Tourette syndrome. J Clin Psychopharmacol. 2000;20:317–24. doi: 10.1097/00004714-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Monnet DL, Ferech M, Frimodt-Moller N, Goossens H. The more antibacterial trade names, the more consumption of antibacterials: a European study. Clin Infect Dis. 2005;41:114–7. doi: 10.1086/430822. [DOI] [PubMed] [Google Scholar]
- 27.Elseviers MM, Ferech M, Vander Stichele RH, Goossens H. Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): trends, regional differences and seasonal fluctuations. Pharmacoepidemiol Drug Saf. 2007;16:115–23. doi: 10.1002/pds.1244. [DOI] [PubMed] [Google Scholar]
- 28.Goossens H, Guillemot D, Ferech M, Schlemmer B, Costers M, van Breda M, Baker LJ, Cars O, Davey PG. National campaigns to improve antibiotic use. Eur J Clin Pharmacol. 2006;62:373–9. doi: 10.1007/s00228-005-0094-7. [DOI] [PubMed] [Google Scholar]
- 29.Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280:1774–6. doi: 10.1001/jama.280.20.1774. [DOI] [PubMed] [Google Scholar]
- 30.Stephenson WP, Hauben M. Data mining for signals in spontaneous reporting databases: proceed with caution. Pharmacoepidemiol Drug Saf. 2007;16:359–65. doi: 10.1002/pds.1323. [DOI] [PubMed] [Google Scholar]
- 31.Hauben M, Horn S, Reich L. Potential use of data-mining algorithms for the detection of ‘surprise’ adverse drug reactions. Drug Saf. 2007;30:143–55. doi: 10.2165/00002018-200730020-00004. [DOI] [PubMed] [Google Scholar]
- 32.Shalviri G, Mohammad K, Majdzadeh R, Gholami K. Applying quantitative methods for detecting new drug safety signals in pharmacovigilance national database. Pharmacoepidemiol Drug Saf. 2007;16:1136–40. doi: 10.1002/pds.1459. [DOI] [PubMed] [Google Scholar]
- 33.Hauben M, Patadia V, Gerrits C, Walsh L, Reich L. Data mining in pharmacovigilance: the need for a balanced perspective. Drug Saf. 2005;28:835–42. doi: 10.2165/00002018-200528100-00001. [DOI] [PubMed] [Google Scholar]
- 34.Kezerashvili A, Khattak H, Barsky A, Nazari R, Fisher JD. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol. 2007;18:243–6. doi: 10.1007/s10840-007-9124-y. [DOI] [PubMed] [Google Scholar]
- 35.Huang BH, Wu CH, Hsia CP, Yin CC. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30:1579–82. doi: 10.1111/j.1540-8159.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 36.Tilelli JA, Smith KM, Pettignano R. Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy. 2006;26:147–50. doi: 10.1592/phco.2006.26.1.147. [DOI] [PubMed] [Google Scholar]
- 37.Kim MH, Berkowitz C, Trohman RG. Polymorphic ventricular tachycardia with a normal QT interval following azithromycin. Pacing Clin Electrophysiol. 2005;28:1221–2. doi: 10.1111/j.1540-8159.2005.50146.x. [DOI] [PubMed] [Google Scholar]
- 38.Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed. 2006;77:30–2. [PubMed] [Google Scholar]
- 39.Matsunaga N, Oki Y, Prigollini A. A case of QT-interval prolongation precipitated by azithromycin. NZ Med J. 2003;116:U666. [PubMed] [Google Scholar]
- 40.Thomsen MB, Beekman JD, Attevelt NJ, Takahara A, Sugiyama A, Chiba K, Vos MA. No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. Br J Pharmacol. 2006;149:1039–48. doi: 10.1038/sj.bjp.0706900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, Fleischer D, Kirchhof P, Fabritz L, Breithardt G, Haverkamp W. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002;303:218–25. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- 42.Kazmierczak J, Peregud-Pogorzelska M, Rzeuski R. QT Interval prolongation and torsades de pointes due to a coadministration of ciprofloxacin and azimilide in a patient with implantable cardioverter-defibrillator. Pacing Clin Electrophysiol. 2007;30:1043–6. doi: 10.1111/j.1540-8159.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 43.Flanagan MC, Mitchell ES, Haigney MC. Ciprofloxacin-induced torsade de pointes. Int J Cardiol. 2006;113:239–41. doi: 10.1016/j.ijcard.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 44.Daya SK, Gowda RM, Khan IA. Ciprofloxacin- and hypocalcemia-induced torsade de pointes triggered by hemodialysis. Am J Ther. 2004;11:77–9. doi: 10.1097/00045391-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Letsas KP, Sideris A, Kounas SP, Efremidis M, Korantzopoulos P, Kardaras F. Drug-induced QT interval prolongation after ciprofloxacin administration in a patient receiving olanzapine. Int J Cardiol. 2006;109:273–4. doi: 10.1016/j.ijcard.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Tsikouris JP, Peeters MJ, Cox CD, Meyerrose GE, Seifert CF. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol. 2006;11:52–6. doi: 10.1111/j.1542-474X.2006.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624–6. doi: 10.1016/j.hrthm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Noel GJ, Natarajan J, Chien S, Hunt TL, Goodman DB, Abels R. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther. 2003;73:292–303. doi: 10.1016/s0009-9236(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 49.Makaryus AN, Byrns K, Makaryus MN, Natarajan U, Singer C, Goldner B. Effect of ciprofloxacin and levofloxacin on the QT interval: is this a significant ‘clinical’ event? South Med J. 2006;99:52–6. doi: 10.1097/01.smj.0000197124.31174.7e. [DOI] [PubMed] [Google Scholar]
- 50.Milberg P, Hilker E, Ramtin S, Cakir Y, Stypmann J, Engelen MA, Monnig G, Osada N, Breithardt G, Haverkamp W, Eckardt L. Proarrhythmia as a class effect of quinolones: increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. J Cardiovasc Electrophysiol. 2007;18:647–54. doi: 10.1111/j.1540-8167.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki H, Watanabe H, Kitayama T, Nishida M, Nishi Y, Sekiya K, Suganami H, Yamamoto K. QT PRODACT sensitivity and specificity of the canine telemetry assay for detecting drug-induced QT interval prolongation. J Pharmacol Sci. 2005;99:523–9. doi: 10.1254/jphs.qt-c9. [DOI] [PubMed] [Google Scholar]
- 52.Toyoshima S, Kanno A, Kitayama T, Sekiya K, Nakai K, Haruna M, Mino T, Miyazaki H, Yano K, Yamamoto K. QT PRODACT in vivo QT assay in the conscious dog for assessing the potential for QT interval prolongation by human pharmaceuticals. J Pharmacol Sci. 2005;99:459–71. doi: 10.1254/jphs.qt-a2. [DOI] [PubMed] [Google Scholar]
- 53.Hensey C, Keane D. Clarithromycin induced torsade de pointes. Ir J Med Sci. 2008;177:67–8. doi: 10.1007/s11845-007-0057-3. [DOI] [PubMed] [Google Scholar]
- 54.Vallejo CN, Rodriguez PD, Sanchez HA, Tornos Mas MP, Ribera E, Soler SJ. Ventricular tachycardia and long QT associated with clarithromycin administration in a patient with HIV infection. Rev Esp Cardiol. 2002;55:878–81. doi: 10.1016/s0300-8932(02)76720-1. [DOI] [PubMed] [Google Scholar]
- 55.Germanakis I, Galanakis E, Parthenakis F, Vardas PE, Kalmanti M. Clarithromycin treatment and QT prolongation in childhood. Acta Paediatr. 2006;95:1694–6. doi: 10.1080/08035250600764800. [DOI] [PubMed] [Google Scholar]
- 56.Shi J, Chapel S, Montay G, Hardy P, Barrett JS, Sica D, Swan SK, Noveck R, Leroy B, Bhargava VO. Effect of ketoconazole on the pharmacokinetics and safety of telithromycin and clarithromycin in older subjects with renal impairment. Int J Clin Pharmacol Ther. 2005;43:123–33. doi: 10.5414/cpp43123. [DOI] [PubMed] [Google Scholar]
- 57.Stanat SJ, Carlton CG, Crumb WJ, Jr, Agrawal KC, Clarkson CW. Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol Cell Biochem. 2003;254:1–7. doi: 10.1023/a:1027309703313. [DOI] [PubMed] [Google Scholar]
- 58.Hinterseer M, Irlbeck M, Ney L, Beckmann BM, Pfeufer A, Steinbeck G, Kaab S. Acute respiratory distress syndrome with transiently impaired left ventricular function and Torsades de Pointes arrhythmia unmasking congenital long QT syndrome in a 25-year-old woman. Br J Anaesth. 2006;97:150–3. doi: 10.1093/bja/ael118. [DOI] [PubMed] [Google Scholar]
- 59.Flyer JL. Identifying patients at risk for QT interval prolongation: case studies. J Fam Pract. 2005;54(Suppl.):S18–9. [PubMed] [Google Scholar]
- 60.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–96. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 61.Kyrmizakis DE, Chimona TS, Kanoupakis EM, Papadakis CE, Velegrakis GA, Helidonis ES. QT prolongation and torsades de pointes associated with concurrent use of cisapride and erythromycin. Am J Otolaryngol. 2002;23:303–7. doi: 10.1053/ajot.2002.124543. [DOI] [PubMed] [Google Scholar]
- 62.Lu HR, Vlaminckx E, Van de Water A, Rohrbacher J, Hermans A, Gallacher DJ. In-vitro experimental models for the risk assessment of antibiotic-induced QT prolongation. Eur J Pharmacol. 2007;577:222–32. doi: 10.1016/j.ejphar.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 63.Wisialowski T, Crimin K, Engtrakul J, O'Donnell J, Fermini B, Fossa AA. Differentiation of arrhythmia risk of the antibacterials moxifloxacin, erythromycin, and telithromycin based on analysis of monophasic action potential duration alternans and cardiac instability. J Pharmacol Exp Ther. 2006;318:352–9. doi: 10.1124/jpet.106.101881. [DOI] [PubMed] [Google Scholar]
- 64.Duncan RS, Ridley JM, Dempsey CE, Leishman DJ, Leaney JL, Hancox JC, Witchel HJ. Erythromycin block of the HERG K+ channel: accessibility to F656 and Y652. Biochem Biophys Res Commun. 2006;341:500–6. doi: 10.1016/j.bbrc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Guo J, Zhan S, Lees-Miller JP, Teng G, Duff HJ. Exaggerated block of hERG (KCNH2) and prolongation of action potential duration by erythromycin at temperatures between 37 degrees C and 42 degrees C. Heart Rhythm. 2005;2:860–6. doi: 10.1016/j.hrthm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 66.Kirsch GE, Trepakova ES, Brimecombe JC, Sidach SS, Erickson HD, Kochan MC, Shyjka LM, Lacerda AE, Brown AM. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J Pharmacol Toxicol Methods. 2004;50:93–101. doi: 10.1016/j.vascn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Lu HR, Vlaminckx E, Gallacher DJ. Choice of cardiac tissue in vitro plays an important role in assessing the risk of drug-induced cardiac arrhythmias in human: beyond QT prolongation. J Pharmacol Toxicol Methods. 2008;57:1–8. doi: 10.1016/j.vascn.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Lu HR, Vlaminckx E, Teisman A, Gallacher DJ. Choice of cardiac tissue plays an important role in the evaluation of drug-induced prolongation of the QT interval in vitro in rabbit. J Pharmacol Toxicol Methods. 2005;52:90–105. doi: 10.1016/j.vascn.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Hanada E, Ohtani H, Hirota M, Uemura N, Nakaya H, Kotaki H, Sato H, Yamada Y, Iga T. Inhibitory effect of erythromycin on potassium currents in rat ventricular myocytes in comparison with disopyramide. J Pharm Pharmacol. 2003;55:995–1002. doi: 10.1211/0022357021459. [DOI] [PubMed] [Google Scholar]
- 70.Fteha A, Fteha E, Haq S, Kozer L, Saul B, Kassotis J. Gatifloxacin induced torsades de pointes. Pacing Clin Electrophysiol. 2004;27:1449–50. doi: 10.1111/j.1540-8159.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 71.Bertino JS, Jr, Owens RC, Jr, Carnes TD, Iannini PB. Gatifloxacin-associated corrected QT interval prolongation, torsades de pointes, and ventricular fibrillation in patients with known risk factors. Clin Infect Dis. 2002;34:861–3. doi: 10.1086/339075. [DOI] [PubMed] [Google Scholar]
- 72.Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy. 2001;21:1468–72. doi: 10.1592/phco.21.20.1468.34482. [DOI] [PubMed] [Google Scholar]
- 73.Ansari SR, Chopra N. Gatifloxacin and prolonged QT interval. Am J Med Sci. 2004;327:55–6. doi: 10.1097/00000441-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 74.Akita M, Shibazaki Y, Izumi M, Hiratsuka K, Sakai T, Kurosawa T, Shindo Y. Comparative assessment of prurifloxacin, sparfloxacin, gatifloxacin and levofloxacin in the rabbit model of proarrhythmia. J Toxicol Sci. 2004;29:63–71. doi: 10.2131/jts.29.63. [DOI] [PubMed] [Google Scholar]
- 75.Chiba K, Sugiyama A, Hagiwara T, Takahashi S, Takasuna K, Hashimoto K. In vivo experimental approach for the risk assessment of fluoroquinolone antibacterial agents-induced long QT syndrome. Eur J Pharmacol. 2004;486:189–200. doi: 10.1016/j.ejphar.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 76.Ball P, Mandell L, Patou G, Dankner W, Tillotson G. A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context. Int J Antimicrob Agents. 2004;23:421–9. doi: 10.1016/j.ijantimicag.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Seop KD, Kim KS, Hwan CK, Na H, Kim JI, Shin WH, Kim EJ. Electrophysiological safety of novel fluoroquinolone antibiotic agents gemifloxacin and balofloxacin. Drug Chem Toxicol. 2006;29:303–12. doi: 10.1080/01480540600652996. [DOI] [PubMed] [Google Scholar]
- 78.Maxa JL, Hebeler RF, Adeeko MA. Torsades de pointes following concurrent amiodarone and levofloxacin therapy. Proc (Bayl Univ Med Cent) 2006;19:345–6. doi: 10.1080/08998280.2006.11928199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morganroth J, Dimarco JP, Anzueto A, Niederman MS, Choudhri S. A randomized trial comparing the cardiac rhythm safety of moxifloxacin vs levofloxacin in elderly patients hospitalized with community-acquired pneumonia. Chest. 2005;128:3398–406. doi: 10.1378/chest.128.5.3398. [DOI] [PubMed] [Google Scholar]
- 80.Iannini PB, Doddamani S, Byazrova E, Curciumaru I, Kramer H. Risk of torsades de pointes with non-cardiac drugs. BMJ. 2001;322:46. [PMC free article] [PubMed] [Google Scholar]
- 81.Amankwa K, Krishnan SC, Tisdale JE. Torsades de pointes associated with fluoroquinolones: importance of concomitant risk factors. Clin Pharmacol Ther. 2004;75:242–7. doi: 10.1016/j.clpt.2003.11.376. [DOI] [PubMed] [Google Scholar]
- 82.Gandhi PJ, Menezes PA, Vu HT, Rivera AL, Ramaswamy K. Fluconazole- and levofloxacin-induced torsades de pointes in an intensive care unit patient. Am J Health Syst Pharm. 2003;60:2479–83. doi: 10.1093/ajhp/60.23.2479. [DOI] [PubMed] [Google Scholar]
- 83.Paltoo B, O'Donoghue S, Mousavi MS. Levofloxacin induced polymorphic ventricular tachycardia with normal QT interval. Pacing Clin Electrophysiol. 2001;24:895–7. doi: 10.1046/j.1460-9592.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 84.Nykamp DL, Blackmon CL, Schmidt PE, Roberson AGQ. Tc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 2005;39:543–6. doi: 10.1345/aph.1E513. [DOI] [PubMed] [Google Scholar]
- 85.Basyigit I, Kahraman G, Ilgazli A, Yildiz F, Boyaci H. The effects of levofloxacin on ECG parameters and late potentials. Am J Ther. 2005;12:407–10. doi: 10.1097/01.mjt.0000127358.38755.c5. [DOI] [PubMed] [Google Scholar]
- 86.Noel GJ, Goodman DB, Chien S, Solanki B, Padmanabhan M, Natarajan J. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol. 2004;44:464–73. doi: 10.1177/0091270004264643. [DOI] [PubMed] [Google Scholar]
- 87.Zunkler BJ, Claassen S, Wos-Maganga M, Rustenbeck I, Holzgrabe U. Effects of fluoroquinolones on HERG channels and on pancreatic beta-cell ATP-sensitive K+ channels. Toxicology. 2006;228:239–48. doi: 10.1016/j.tox.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Kounas SP, Letsas KP, Sideris A, Efraimidis M, Kardaras F. QT interval prolongation and torsades de pointes due to a coadministration of metronidazole and amiodarone. Pacing Clin Electrophysiol. 2005;28:472–3. doi: 10.1111/j.1540-8159.2005.09348.x. [DOI] [PubMed] [Google Scholar]
- 89.Altin T, Ozcan O, Turhan S, Ongun OA, Akyurek O, Karaoguz R, Guldal M. Torsade de pointes associated with moxifloxacin: a rare but potentially fatal adverse event. Can J Cardiol. 2007;23:907–8. doi: 10.1016/s0828-282x(07)70850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dale KM, Lertsburapa K, Kluger J, White CM. Moxifloxacin and torsade de pointes. Ann Pharmacother. 2007;41:336–40. doi: 10.1345/aph.1H474. [DOI] [PubMed] [Google Scholar]
- 91.Balfour JA, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs. 2000;59:115–39. doi: 10.2165/00003495-200059010-00010. [DOI] [PubMed] [Google Scholar]
- 92.Andriole VT, Haverstock DC, Choudhri SH. Retrospective analysis of the safety profile of oral moxifloxacin in elderly patients enrolled in clinical trials. Drug Saf. 2005;28:443–52. doi: 10.2165/00002018-200528050-00007. [DOI] [PubMed] [Google Scholar]
- 93.Alexandrou AJ, Duncan RS, Sullivan A, Hancox JC, Leishman DJ, Witchel HJ, Leaney JL. Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br J Pharmacol. 2006;147:905–16. doi: 10.1038/sj.bjp.0706678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X, Cass JD, Bradley JA, Dahm CM, Sun Z, Kadyszewski E, Engwall MJ, Zhou J. QT prolongation and proarrhythmia by moxifloxacin: concordance of preclinical models in relation to clinical outcome. Br J Pharmacol. 2005;146:792–9. doi: 10.1038/sj.bjp.0706389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lacroix P, Crumb WJ, Durando L, Ciottoli GB. Prulifloxacin: in vitro (HERG current) and in vivo (conscious dog) assessment of cardiac risk. Eur J Pharmacol. 2003;477:69–72. doi: 10.1016/s0014-2999(03)02180-0. [DOI] [PubMed] [Google Scholar]
- 96.Chaves AA, Zingaro GJ, Yordy MA, Bustard KA, O'Sullivan S, Galijatovic-Idrizbegovic A, Schuck H, Christian DB, Hoe CM, Briscoe RJ. A highly sensitive canine telemetry model for detection of QT interval prolongation: studies with moxifloxacin, haloperidol and MK-499. J Pharmacol Toxicol Methods. 2007;56:103–14. doi: 10.1016/j.vascn.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Holzgrefe HH, Cavero I, Buchanan LV, Gill MW, Durham SK. Application of a probabilistic method for the determination of drug-induced QT prolongation in telemetered cynomolgus monkeys: effects of moxifloxacin. J Pharmacol Toxicol Methods. 2007;55:227–37. doi: 10.1016/j.vascn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Keskin S, Sayali E, Temeloglu E, Gurol T, Ekizoglu I. QT prolongation and ventricular tachycardia due to roxithromycin. Anadolu Kardiyol Derg. 2005;5:319–21. [PubMed] [Google Scholar]
- 99.Justo D, Mardi T, Zeltser D. Roxithromycin-induced torsades de pointes. Eur J Intern Med. 2004;15:326–7. doi: 10.1016/j.ejim.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Promphan W, Khongphatthanayothin A, Horchaiprasit K, Benjacholamas V. Roxithromycin induced torsade de pointes in a patient with complex congenital heart disease and complete atrioventricular block. Pacing Clin Electrophysiol. 2003;26:1424–6. doi: 10.1046/j.1460-9592.2003.t01-1-00204.x. [DOI] [PubMed] [Google Scholar]
- 101.Ahluwalia G. Torsade de pointes probably induced by sparfloxain. J Assoc Physicians India. 2003;51:835. [PubMed] [Google Scholar]
- 102.Kakar A, Byotra SP. Torsade de pointes probably induced by sparfloxacin. J Assoc Physicians India. 2002;50:1077–8. [PubMed] [Google Scholar]
- 103.Zunkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Ther. 2006;112:12–37. doi: 10.1016/j.pharmthera.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Terrar DA, Wilson CM, Graham SG, Bryant SM, Heath BM. Comparison of guinea-pig ventricular myocytes and dog Purkinje fibres for in vitro assessment of drug-induced delayed repolarization. J Pharmacol Toxicol Methods. 2007;56:171–85. doi: 10.1016/j.vascn.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Chiba K, Sugiyama A, Watanabe K, Takasuna K, Hashimoto K. Acute hypokalemia may not be an effective way to sensitize the in situ canine heart for sparfloxacin-induced long QT syndrome. J Pharmacol Sci. 2006;100:88–92. doi: 10.1254/jphs.scj05008x. [DOI] [PubMed] [Google Scholar]
- 106.Barriere S, Genter F, Spencer E, Kitt M, Hoelscher D, Morganroth J. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J Clin Pharmacol. 2004;44:689–95. doi: 10.1177/0091270004266620. [DOI] [PubMed] [Google Scholar]
- 107.Shi J, Montay G, Bhargava VO. Clinical pharmacokinetics of telithromycin, the first ketolide antibacterial. Clin Pharmacokinet. 2005;44:915–34. doi: 10.2165/00003088-200544090-00003. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen M, Chung EP. Telithromycin: the first ketolide antimicrobial. Clin Ther. 2005;27:1144–63. doi: 10.1016/j.clinthera.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Demolis JL, Strabach S, Vacheron F, Funck-Brentano C. Assessment of the effect of a single oral dose of telithromycin on sotalol-induced qt interval prolongation in healthy women. Br J Clin Pharmacol. 2005;60:120–7. doi: 10.1111/j.1365-2125.2005.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Demolis JL, Vacheron F, Cardus S, Funck-Brentano C. Effect of single and repeated oral doses of telithromycin on cardiac QT interval in healthy subjects. Clin Pharmacol Ther. 2003;73:242–52. doi: 10.1067/mcp.2003.4. [DOI] [PubMed] [Google Scholar]


