5-Fluorouracil (5-FU) is a cornerstone in the treatment of many cancers, including colorectal, head and neck, stomach, and breast carcinomas. Recently, 5-FU oral prodrugs, such as capecitabine, have been successfully introduced in cancer therapy, and their clinical use is rapidly and constantly growing. The approach to the prediction of severe toxicities due to 5-FU (and its oral prodrugs) has been matter of debate for many years and still remains a hot topic in oncology. The efforts in this field are almost entirely focused on the analysis of dihydropyrimidine dehydrogenase (DPD) gene mutations and of peripheral blood mononuclear cell (PBMC) DPD activity. For these reasons, in this letter we would like to suggest a novel, rational diagnostic algorithm that, integrating the analysis already available, is centred on the use of 5-FU and 5-fluoro-5,6-dihydrouracil (5-FDHU) pharmacokinetics as a tool to prevent severe and life-threatening 5-FU toxicities in patients with impaired 5-FU metabolism.
DPD plays a pivotal role in the metabolism of 5-FU [1] and, as such, a deficiency of DPD has been recognized as an important risk factor, predisposing patients to the development of severe toxicity. Numerous genetic [2, 3] and phenotypic (i.e. DPD activity, breath test or plasma dihydrouracil/uracil ratio) [4–6] approaches have been proposed to prevent life-threatening toxicities, and, in our opinion, the time has come to develop an integrated approach to this clinically relevant issue.
The screening of DPYD mutations [3] and of peripheral blood mononuclear cell DPD activity [7] in patient candidates for 5-FU treatment has been proposed as a routine approach to identify severely reduced DPD activity. However, both the genotype and phenotype options have some advantages but also limitations, as comprehensively pointed out by Ezzeldin and Diasio [8]. In order to identify all patients with impaired 5-FU clearance, no matter which type of DPYD mutations or DPD activity in PBMC compartment they have, in the last decade some authors have suggested a phenotypic approach of screening, looking at the systemic 5-FU catabolism and measuring the pharmacokinetic parameters of the parental drug and of its principal metabolite 5-FDHU [9–11].
As an example, our group has recently proposed the administration of a 5-FU test dose in patient candidates for 5-FU chemotherapy to calculate the pharmacokinetic parameters, such as 5-FU clearance and t1/2β or 5-FDHU Cmax, Tmax and t1/2β, that could be profoundly altered in the presence of an impaired systemic clearance [12]. Indeed, our study revealed markedly impaired 5-FU and 5-FDHU kinetics in some patients, as previously shown for patients with severe toxicities in other published studies [13], suggesting a possible profound alteration of 5-FU metabolism; owing to the detection of this abnormality, these subjects were not given a potentially life-threatening standard dose of 5-FU. Moreover, based on our experimental data, statistically significant relationships have been demonstrated between 5-FDHU pharmacokinetic parameters and moderate to severe degrees of three major dose-limiting toxicities related to 5-FU treatments (stomatitis, diarrhoea and neutropenia) after the first cycle of 5-FU standard therapy. In particular, a prolonged 5-FDHU t1/2β and a 5-FDHU Tmax higher than the median value of our population (30 min) could help identify patients at risk of developing moderate to severe neutropenia or diarrhoea [12]. The 5-FU test dose can be easily performed in a hospital setting with a clinical pharmacology unit such as those usually present in medium- or large-sized university hospitals of Western countries. In particular, an organized regional network among major clinical oncology units may allow screening patients within 2–3 days. The analysis can be available in a few days and the patients can be ready to start the 5-FU therapy at standard or reduced dose in the following week when 5-FU and 5-FDHU kinetic parameters are available.
Based on this clinical experience, we detail the principles that should guide the decision-making process regarding the prevention of 5-FU severe toxicity and propose a diagnostic algorithm (Figure 1) in order to screen candidate patients to fluoropyrimidine therapy. In the suggested diagnostic algorithm (Figure 1), the predictive 5-FU test dose could be regarded as a triage test, allowing detection of the fraction of patients with normal, impaired or absent fluoropyrimidine metabolism. Other analyses, such as DPD genotyping or even DPD PBMC activity, could be used later as add-on tests and, limited to the still undiagnosed subgroup, to detect those degrees of enzyme activity impairment suitable for possible reduction of 5-FU dose or different treatments. Overall, the published data strongly suggest the use of a diagnostic algorithm based on the sequential application of a 5-FU pharmacokinetic test followed by DPD genotyping and activity in order to make a highly probable diagnosis of altered 5-FU metabolism. Moreover, the application of this model could result in a consistent reduction of costs and morbidity, by limiting genotyping and PBMC DPD activity analysis to only selected subgroups of patients.
Figure 1.
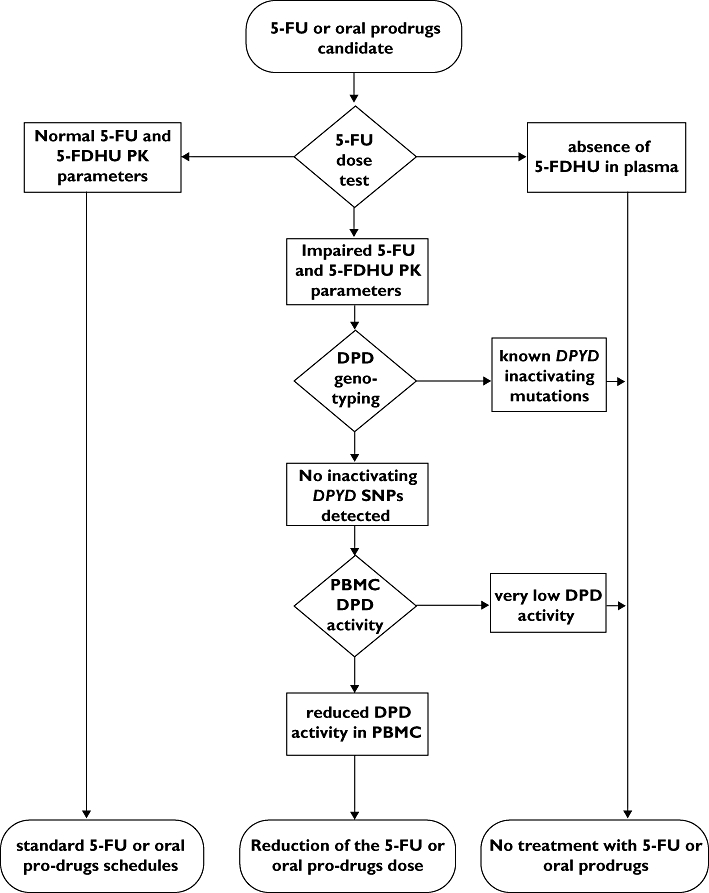
Suggested diagnostic flow chart for the detection of patients with impaired fluoropyrimidine metabolism. 5-FU, 5-fluorouracil; 5-FDHU, 5-fluoro-5,6-dihydrouracil; DPD, dihydropyrimidine dehydrogenase; PBMC, peripheral blood mononuclear cell; PK, pharmacokinetics; SNP, single nucleotide polymorphism
In conclusion, the published clinical experience suggests that an integrated approach based on pharmacokinetic analysis combined with DPD genotyping and/or phenotyping seems to be a safer strategy for optimizing the administration of 5-FU and its oral prodrugs, which remain major drugs used extensively in clinical oncology.
Competing interests
None declared.
REFERENCES
- 1.Mercier C, Ciccolini J. Profiling dihydropyrimidine dehydrogenase deficiency in patients with cancer undergoing 5-fluorouracil/capecitabine therapy. Clin Colorectal Cancer. 2006;6:288–96. doi: 10.3816/CCC.2006.n.047. [DOI] [PubMed] [Google Scholar]
- 2.Ezzeldin HH, Lee AM, Mattison LK, Diasio RB. Methylation of the DPYD promoter: an alternative mechanism for dihydropyrimidine dehydrogenase deficiency in cancer patients. Clin Cancer Res. 2005;11:8699–705. doi: 10.1158/1078-0432.CCR-05-1520. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J, Schwab M, Eichelbaum M, Zanger UM. Mutational analysis of the human dihydropyrimidine dehydrogenase gene by denaturing high-performance liquid chromatography. Genet Test. 2003;7:97–105. doi: 10.1089/109065703322146777. [DOI] [PubMed] [Google Scholar]
- 4.Etienne MC, Lagrange JL, Dassonville O, Fleming R, Thyss A, Renee N, Schneider M, Demard F, Milano G. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol. 1994;12:2248–53. doi: 10.1200/JCO.1994.12.11.2248. [DOI] [PubMed] [Google Scholar]
- 5.Mattison LK, Ezzeldin H, Carpenter M, Modak A, Johnson MR, Diasio RB. Rapid identification of dihydropyrimidine dehydrogenase deficiency by using a novel 2–13C-uracil breath test. Clin Cancer Res. 2004;10:2652–8. doi: 10.1158/1078-0432.ccr-03-0374. [DOI] [PubMed] [Google Scholar]
- 6.Garg MB, Sevester JC, Sakoff JA, Ackland SP. Simple liquid chromatographic method for the determination of uracil and dihydrouracil plasma levels: a potential pretreatment predictor of 5-fluorouracil toxicity. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:223–30. doi: 10.1016/s1570-0232(02)00239-8. [DOI] [PubMed] [Google Scholar]
- 7.Ogura K, Ohnuma T, Minamide Y, Mizuno A, Nishiyama T, Nagashima S, Kanamaru M, Hiratsuka A, Watabe T, Uematsu T. Dihydropyrimidine dehydrogenase activity in 150 healthy Japanese volunteers and identification of novel mutations. Clin Cancer Res. 2005;11:5104–11. doi: 10.1158/1078-0432.CCR-05-0217. [DOI] [PubMed] [Google Scholar]
- 8.Ezzeldin HH, Diasio RB. Predicting fluorouracil toxicity: can we finally do it? J Clin Oncol. 2008;26:2131–8. doi: 10.1200/JCO.2007.15.5481. [DOI] [PubMed] [Google Scholar]
- 9.Ackland SP, Garg MB, Dunstan RH. Simultaneous determination of dihydrofluorouracil and 5-fluorouracil in plasma by high-performance liquid chromatography. Anal Biochem. 1997;246:79–85. doi: 10.1006/abio.1996.9943. [DOI] [PubMed] [Google Scholar]
- 10.Di Paolo A, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, Allegrini G, Mini E, Bocci G, Conte PF, Del Tacca M. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol. 2001;12:1301–6. doi: 10.1023/a:1012294617392. [DOI] [PubMed] [Google Scholar]
- 11.Ciccolini J, Mercier C, Blachon MF, Favre R, Durand A, Lacarelle B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J Clin Pharm Ther. 2004;29:307–15. doi: 10.1111/j.1365-2710.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 12.Bocci G, Barbara C, Vannozzi F, Di Paolo A, Melosi A, Barsanti G, Allegrini G, Falcone A, Del Tacca M, Danesi R. A pharmacokinetic-based test to prevent severe 5-fluorouracil toxicity. Clin Pharmacol Ther. 2006;80:384–95. doi: 10.1016/j.clpt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Bocci G, Danesi R, Allegrini G, Innocenti F, Di Paolo A, Falcone A, Conte PF, Del Tacca M. Severe 5-fluorouracil toxicity associated with a marked alteration of pharmacokinetics of 5-fluorouracil and its catabolite 5-fluoro-5,6-dihydrouracil: a case report. Eur J Clin Pharmacol. 2002;58:593–5. doi: 10.1007/s00228-002-0534-6. [DOI] [PubMed] [Google Scholar]


