Abstract
The mechanisms underlying neuronal ischemic preconditioning, a phenomenon in which brief episodes of ischemia protect against the lethal effects of subsequent periods of prolonged ischemia, are poorly understood. Ischemia can be modeled in vitro by oxygen-glucose deprivation (OGD). We report here that OGD preconditioning induces p21ras (Ras) activation in an N-methyl-d-aspartate receptor- and NO-dependent, but cGMP-independent, manner. We demonstrate that Ras activity is necessary and sufficient for OGD tolerance in neurons. Pharmacological inhibition of Ras, as well as a dominant negative mutant Ras, block OGD preconditioning whereas a constitutively active form of Ras promotes neuroprotection against lethal OGD insults. In contrast, the activity of phosphatidyl inositol 3-kinase is not required for OGD preconditioning because inhibition of phosphatidyl inositol 3-kinase with a chemical inhibitor or with a dominant negative mutant does not have any effect on the development of OGD tolerance. Furthermore, using recombinant adenoviruses and pharmacological inhibitors, we show that downstream of Ras the extracellular regulated kinase cascade is required for OGD preconditioning. Our observations indicate that activation of the Ras/extracellular regulated kinase cascade by NO is a critical mechanism for the development of OGD tolerance in cortical neurons, which may also play an important role in ischemic preconditioning in vivo.
Ischemic preconditioning or ischemic tolerance is a well known phenomenon in which brief episodes of sublethal ischemia induce a robust protection against the deleterious effects of subsequent, prolonged, lethal ischemia in a variety of organ systems, including brain, heart, liver, intestine, kidney, and lung (1–3). In the brain, ischemic tolerance is mediated largely by the activation of the N-methyl-d-aspartate (NMDA) glutamate receptors through increases in intracellular calcium (4–6). Although the importance of ischemic tolerance in providing profound protection against ischemic injury has been established (1–3, 7), the intracellular signaling mechanisms accounting for this phenomenon are still very poorly understood. Recent data suggest that activation of adenosine A1 receptors and KATP channels or generation of nitric oxide (NO) may be important for the induction of tolerance (8–14). In addition, ischemic preconditioning has been linked to changes in protein phosphorylation, including increased phosphorylation of extracellular signal-regulated protein kinases (Erks) (15–17). However, the functional consequences of these changes have not been demonstrated.
Because ischemic tolerance induces such dramatic protection, understanding the early intracellular events that underlie this process could lead to the development of novel protective strategies that could be used in the treatment of ischemic or traumatic injury in a variety of organs, as well as protective approaches against neurodegenerative diseases. Ischemia can be modeled in vitro by oxygen-glucose deprivation (OGD). To advance our understanding of the early intracellular events that mediate the induction of tolerance to ischemia, we have explored the role of two signaling cascades, the p21ras (Ras)-dependent and the phosphatidyl inositol 3-kinase (PI3K)-dependent pathways, in OGD preconditioning in cortical neurons. We demonstrate that Ras activation is required and sufficient for OGD tolerance, which also requires Raf/Erk signaling downstream of Ras, and we exclude the role of PI3K in OGD preconditioning.
Materials and Methods
Cell Culture.
Primary cortical cell cultures were prepared from gestational day 15 fetal rats as previously described in detail (18, 19). Experiments were performed at DIV (day in vitro) 14. Neurons represent 70–90% of the culture (18, 19).
Recombinant Adenovirus or Herpes Virus.
Cultures were exposed to 8 × 105 pfu/ml of recombinant adenovirus. All recombinant adenoviruses used are described elsewhere (20, 21). Cultures were exposed to 106 pfu/ml of recombinant herpes simplex virus. All herpes simplex recombinant viruses are described elsewhere (22, 23). We consistently achieved 90–100% infection efficiency of cortical neurons with minimal toxicity.
Oxygen-Glucose Deprivation.
Combined OGD was performed as described (24) by complete exchange of media with deoxygenated, glucose-free Earle's balanced salt solution, bubbled with 10% H2/85% N2/5% CO2. Cultures were kept in an anaerobic chamber for 5 or 60 min at 37°C. OGD was terminated by replacement of the Earle's balanced salt solution with oxygenated growth media. Experimental reagents were added to the exposure solution as indicated. For pharmacologic antagonist experiments, the compound was added to cultures 15 min before preconditioning and was maintained during 5 min of OGD.
Survival Assay.
Neuronal survival was assessed as described (25) 24 h after last treatment and is presented as percent of cell death. Viability was determined by computer-assisted cell counting after staining with 1 μg/ml Hoechst 33342 (Molecular Probes) and 7 μM propidium iodide (Sigma). Glial nuclei fluoresce at a lower intensity and were gated out (25). Data were analyzed by ANOVA with the Student's t test for independent means. At least two separate experiments using four separate wells were performed for a minimum of 16,000–30,000 neurons counted for each data point.
In Situ Ras Activation Assay.
Ras activity was assessed as described in detail (26). Primary cultures of cortical neurons were metabolically labeled for 4 h at 37°C with 1 mCi/ml H3[32P]PO4. After metabolic labeling, cells were exposed to OGD for 5 min. Cells were lysed 1 min later in the presence of 2 μg of anti-Ras monoclonal antibody Y13–259 (Oncogene Science). After immunoprecipitation of Ras, samples were spotted onto polyethyleneimine-cellulose thin layer chromatography plates (EM Science), and guanine nucleotides were eluted and separated in 1 M KH2PO4 (pH 3.4) for 3 h.
Western Blot Analysis of Erk Phosphorylation.
Cultures were exposed to 5 min of OGD in the absence or presence of inhibitors. Ten minutes later, total cellular protein was collected for protein analysis (26). Samples were separated by 12% SDS/PAGE and were electrotransferred to nitrocellulose membranes (Bio-Rad). Phosphorylated Erks were detected by using a rabbit phosphospecific Erk polyclonal antibody (New England Biolabs) and were visualized by using an Enhanced ChemiLuminiscence (ECL) detection kit (Kirkegaard & Perry Laboratories).
Results
OGD Preconditioning Is NMDA Receptor-, Ca2+-, and NO-Dependent.
To investigate the potential pathways of OGD preconditioning, a model of OGD tolerance in rat primary cortical cultures was established (Fig. 1A). Combined oxygen and glucose deprivation (OGD) for 60 min causes ≈60% neuronal death 24 h later. In contrast, a brief, nonlethal, 5-min exposure of the cultures to OGD 24 h before the 60-min OGD exposure has a robust neuroprotective effect and leads to neuronal survival levels that are not significantly different from buffer-treated control cells. Five minutes of OGD exposure does not affect Ca2+ influx induced by sixty minutes of OGD 24 h later (data not shown). Thus, perturbations of Ca2+ influx are unlikely to account for OGD tolerance. OGD preconditioning in this model system is predominantly NMDA receptor-dependent (Fig. 1A), as indicated by the complete blockade of OGD tolerance when the NMDA receptor antagonists, MK801 (10 μM) and AP5 (25 μM), are applied during the preconditioning episode. Non-NMDA receptor activation plays a less prominent role because the AMPA and kainate receptor blocker DNQX (25 μM) only partially prevents OGD tolerance (Fig. 1A). The voltage-dependent Ca2+ channel blockers, nifedipine (100 μM) and nimodipine (100 μM), also partially block OGD preconditioning (Fig. 1A). The Ca2+ chelator EGTA (10 mM) significantly blocks neuroprotection by OGD preconditioning, indicating that increases in intracellular Ca2+ levels [Ca2+]i mediate the development of tolerance (Fig. 1A).
Figure 1.
Tolerance to OGD is NMDA receptor-, Ca2+-, and NO- dependent. (A) Sixty minutes of oxygen-glucose deprivation (60′ OGD) causes ≈60% neuronal death. Brief, sublethal 5-min OGD 24 h before the lethal 60-min OGD (5′/60′ OGD) results in survival of primary cortical neurons. OGD tolerance is blocked by the NMDA receptor antagonists MK801 (10 μM) and AP5 (25 μM) when added 15 min before and during the 5 min of OGD. The non-NMDA receptor antagonist DNQX (25 μM) has a modest effect. The Ca2+ chelator EGTA (10 mM) blocks OGD tolerance. The voltage-dependent Ca2+channel blockers, nifedipine (100 μM) and nimodipine (100 μM), partially block OGD preconditioning. Significance was determined by a balanced two-way ANOVA with a Student's t test: *, P = 0.001 when comparing 60′ OGD to 5′/60′ OGD, 5′ + DNQX/60′ OGD, 5′ + nifedipine/60′ OGD, or 5′ + nimodipine/60′ OGD; †, P = 0.001 when comparing 5′/60′ OGD to 5′ + MK801/60′ OGD, 5′ + AP5/60′ OGD, or 5′ + EGTA/60′ OGD. (B) The NOS inhibitor N-Arg (100 μM) blocks OGD tolerance (5′ + N-Arg/60′OGD). Coadministration of excess NOS substrate, l-Arg (1 mM), restores OGD tolerance (5′ + N-Arg + l-Arg/60′ OGD). *, P = 0.001 when comparing 60′ OGD to 5′/60′ OGD, or 5′ + N-Arg + l-Arg/60′ OGD. †, P = 0.001 when comparing 5′/60′ OGD to 5′ + N-Arg/60′ OGD. (C) OGD tolerance can be partially induced by a 5-min exposure to 10 μM of the NO donors sodium nitroprusside (SNP), diethylenetriamine nitric oxide adduct (DETA/NO), or ethyl-2-hydroxyimino-5-nitro-3-hexeneamide (NOR3). *, P = 0.001 when comparing 60′ OGD to 5′ SNP/60′ OGD, 5′ DETA/NO/60′ OGD, or 5′ NOR3/60′ OGD. Each data point is the mean ± SEM (n = 8) of at least two separate experiments. Each data point reflects a minimum of 16,000–30,000 neurons counted.
Because NMDA receptor activation and elevation of [Ca2+]i leads to increased generation of NO, we examined the effect of inhibition of NO production on OGD tolerance by applying the NOS inhibitor nitro-l-arginine (N-Arg) (100 μM) during the preconditioning episode (Fig. 1B). N-Arg blocks the protective actions of preconditioning by ≈70%, and coadministration of an excess of the NOS substrate, l-arginine (l-Arg) (1 mM), reverses the blockade. These results implicate NO as a key mediator in processes leading to tolerance to lethal OGD. Because a majority of NO's physiologic actions are thought to be mediated through increases in cGMP after activation of soluble guanylyl cyclase (GC), we evaluated the effects of the potent and selective inhibitor of GC, 1H-[1,2,4]oxidiazolo[4,3-α]quinoxalin-1-one (27) on OGD preconditioning. 1H-[1,2,4]oxidiazolo[4,3-α]quinoxalin-1-one (10 μM), at a concentration that completely inhibits GC, does not block OGD preconditioning (data not shown), which excludes GC and implicates other NO targets in this phenomenon.
To confirm the role of NO in the induction of OGD tolerance, we tested NO donors for their ability to protect neurons from death induced by 60 min of OGD. Application of three different and independent NO donors to neuronal cultures at low doses (10 μM) for 5 min 24 h before exposure of the cells to 60 min of OGD leads to significant neuroprotection (Fig. 1C), which further supports the role of NO in the development of tolerance to OGD. NO donors depleted of NO have no effect on tolerance (data not shown). To exclude alterations in Ca2+ influx induced by NO donors as a cause for the observed neuroprotection, we monitored Ca2+ influx 24 h after the 5-min preconditioning exposure to NO donors and compared it to the Ca2+ influx induced by 60 min of OGD in control cells that were not preconditioned. We did not detect any effect of the NO donors on Ca2+ influx after a lethal OGD episode 24 h later (data not shown), which indicates that the preconditioning stimuli are not influencing Ca2+ dynamics.
Glutamate receptor activation can elicit the production and release of the neurotrophin brain-derived neurotrophic factor (BDNF), which could mediate subsequent neuronal survival (28). BDNF has also been implicated in tolerance to ischemia induced by spreading depression (29). We tested the potential role of BDNF in OGD preconditioning. A 5-min application of BDNF (100 ng/ml) as a preconditioning stimulus did not induce OGD tolerance. Addition of specific anti-BDNF antibodies (25 μg/ml) or TrkB receptor bodies (35 μg/ml) to the cultures during and after the OGD preconditioning episode did not block tolerance to OGD (data not shown). Thus, our observations exclude BDNF as a potential mediator of OGD preconditioning in our model system.
Ras Is Activated by 5 Min of OGD.
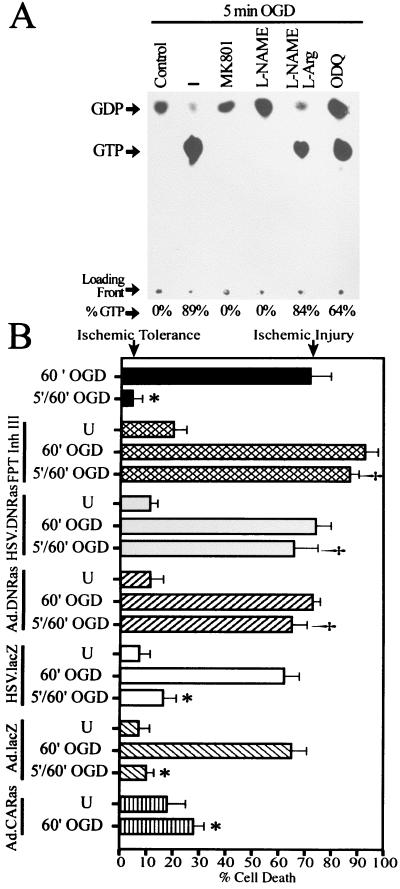
To gain insight into the early intracellular signaling pathways that potentially mediate the development of tolerance to ischemia, we explored the role of the Ras cell survival signaling pathway in OGD preconditioning. Five minutes of OGD induces a dramatic increase in the proportion of GTP bound to Ras (Fig. 2A), reflecting Ras activation (26, 30). This activation is completely blocked by the NMDA receptor antagonist MK801 (10 μM) and by the competitive NOS inhibitor l-nitroarginine methyl ester (l-NAME) (500 μM). Coadministration of an excess of NOS substrate, l-Arg (5 mM), reverses the l-NAME inhibition of Ras activation (Fig. 2A). These results indicate that 5 min of OGD activate Ras in cortical neurons through NMDA receptor stimulation and production of NO, and are consistent with the potential role of NMDA receptor activation and NO generation in the development of OGD tolerance. The GC inhibitor 1H-[1,2,4]oxidiazolo[4,3-α]quinoxalin-1-one (10 μM) does not inhibit 5 min of OGD-induced activation of Ras, which rules out a possible activation of Ras by NO through GC.
Figure 2.
Ras activity is necessary and sufficient for OGD preconditioning. (A) Activation of Ras is indicated by the ratio between GDP and GTP detected by thin layer chromatography. Ras is activated by 5 min of OGD in a NMDA receptor- and NO-dependent manner. Five minutes of OGD (–) induces robust activation of Ras. The activation of Ras by 5 min of OGD is blocked by the NMDA antagonist MK801 (10 μM) and the NOS inhibitor l-NAME (500 μM). Excess l-Arg (5 mM) reverses the l-NAME inhibition of Ras activation. The GC inhibitor 1H-[1,2,4]oxidiazolo[4,3-α]quinoxalin-1-one (ODQ) (10 μM) has no significant effect on Ras activation. Experiments were replicated three times with similar results. (B) Activation of Ras mediates OGD tolerance. OGD tolerance is blocked by the Ras inhibitor FPT Inh III (250 μM) and by expression of a dominant negative mutant Ras (DNRas) via adenovirus or herpes simplex virus vectors. DNRas does not have an effect on the neurotoxicity induced by 60 min of OGD. Viruses containing the lacZ gene were used to control for nonspecific effects of the recombinant viral systems. A constitutively active form of Ras (CARas) expressed by an adenoviral vector induces neuroprotection against 60 min of OGD. U, cultures infected with recombinant virus, but not exposed to OGD. Each data point is the mean ± SEM (n = 8) of at least two separate experiments. Each data point reflects a minimum of 16,000–30,000 neurons counted. Significance was determined by a balanced two-way ANOVA with a Student's t test: *, P = 0.001 comparing 60′ OGD to 5′/60′ OGD, lacZ-5′/60′ OGD, or CARas-60′ OGD; †, P = 0.001 when comparing 5′/60′ OGD to FPT Inh III-5′/60′ OGD, or DNRas-5′/60′ OGD.
Ras Is Required and Sufficient for OGD Preconditioning in Neurons.
Ras-dependent pathways have been implicated in transducing survival and differentiation signals in neurons (30, 31). To elucidate the role of Ras in neuronal OGD preconditioning, we first tested the effect of inhibition of Ras activity on the development of tolerance (Fig. 2B). To perturb Ras signaling, we used the widely used chemical inhibitor of farnesyl protein transferase (FPT), FPT Inhibitor III (FPT Inh III) (32), a recombinant adenovirus system (21) and a recombinant herpes simplex virus system (22) expressing a dominant negative (DN) mutant form of Ras (N17) (23). FPT Inh III is a potent and selective inhibitor of farnesyl transferase and, therefore, inhibits Ras farnesylation and processing. Addition of FPT Inh III (250 μM) to the cultures 15 min before and during the preconditioning episode blocks OGD tolerance, and both DN Ras viral constructs prevent the development of tolerance (Fig. 2B). To control against effects induced by the recombinant viral systems, an adenovirus and a herpes simplex virus expressing the β-galactosidase (lacZ) gene were used. Neurons infected with the lacZ-containing viral vectors are susceptible to lethal exposure to 60 min of OGD and develop OGD tolerance in a manner identical to noninfected control cells (Fig. 2B). Thus, the recombinant viral systems that we use do not affect neuronal susceptibility to OGD or the development of tolerance to OGD damage. Taken together, these results indicate that Ras activity is required for OGD preconditioning in cortical neurons.
Because Ras activation appears to be critical for OGD preconditioning, we tested whether Ras activity is sufficient to induce tolerance. Cortical neurons were infected with an adenovirus expressing a constitutively active (CA) form of Ras (V12) (20, 33), and 48 h after infection cultures were exposed to 60 min of OGD (Fig. 2B). CA Ras is capable of inducing a three-fold neuroprotection against lethal OGD. Therefore, Ras activity is not only necessary but is sufficient for OGD tolerance.
PI3K Activity Is Not Required for OGD Preconditioning in Neurons.
Ras signaling mediates cell survival through downstream effector cascades including the PI3K/Akt and Raf/Erk pathways (30, 31, 34). Because PI3K has been implicated in anti-apoptotic signaling in various cell types, including neurons (35), we tested PI3K activity for a role in neuronal OGD preconditioning (Fig. 3). Perturbation of PI3K signaling with the chemical inhibitor LY294002 (36), or via a recombinant herpes simplex virus expressing a DN mutant of the p85 subunit of PI3K (37), does not block OGD tolerance (Fig. 3). These results indicate that PI3K activity is not required for OGD preconditioning in neurons.
Figure 3.
PI3K does not mediate OGD tolerance. Inhibition of PI3K by the selective inhibitor LY294002 (15 μM) or by a dominant negative PI3K mutant (DN PI3K) has no effect on OGD tolerance induced by 5 min OGD. U, cultures infected with recombinant virus but not exposed to OGD. A herpes simplex virus containing the lacZ gene was used to control for nonspecific effects of the recombinant viral system. Each data point is the mean ± SEM (n = 8) of at least two separate experiments. Each data point reflects a minimum of 16,000–30,000 neurons counted. Significance was determined by a balanced two-way ANOVA with a Student's t test: *, P = 0.001 when comparing 60′ OGD to 5′/60′ OGD, to LY2940002–5′/60′ OGD, to DN PI3K-5′/60′ OGD, or to lacZ-5′/60′ OGD.
Role of the Erk Cascade in Neuronal OGD Preconditioning.
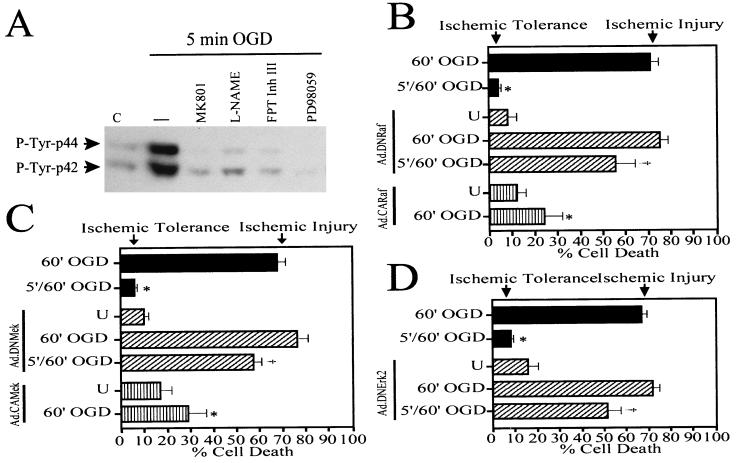
Because Ras is required and sufficient for OGD preconditioning in neurons, but PI3K is not, we tested another prominent downstream Ras effector cascade, the Raf/Erk pathway, for a role in the development of tolerance to OGD. We examined the effect of 5 min of OGD on Erk activity by measuring the level of Erk phosphorylation in cortical neurons (Fig. 4). The preconditioning stimulus induces a robust increase in the phosphorylation levels of both isoforms of Erk (42- and 44-kDa) within 10 min after the 5 min of OGD exposure. The NMDA receptor antagonist, MK801 (10 μM), and the NOS inhibitor, l-NAME (500 μM), completely block Erk phosphorylation induced by 5 min of OGD (Fig. 4A), which strengthens our previous observations implicating NMDA receptors and NO as key mediators in the signaling pathways activated by OGD preconditioning. To determine whether Erk is an effector of Ras signaling during OGD preconditioning, we added the Ras inhibitor, FPT Inh III (250 μM), to the neuronal cultures 10 min before and during the 5 min of OGD and examined its effect on Erk phosphorylation (Fig. 4A). FPT Inh III (250 μM) completely abolishes Erk phosphorylation induced by the preconditioning episode, indicating that Erk is a downstream effector of Ras activation during OGD preconditioning. To test further the involvement of Erk in Ras signaling during OGD preconditioning, we tested the effect of an inhibitor selective for Mek signaling, PD98059 (38), on preconditioning-induced Erk phosphorylation (Fig. 4A). PD98059 (50 μM) completely blocks Erk phosphorylation caused by 5 min of OGD, implicating Mek, the upstream activator of Erk, in OGD preconditioning-initiated intracellular signaling.
Figure 4.
The Raf/Mek/Erk cascade is required for OGD preconditioning. (A) Western blot analysis of tyrosine-phosphorylated Erk (42 and 44 kDa). OGD induces Erk phosphorylation (–) over saline control (C). OGD-induced Erk phosphorylation is blocked by the NMDA receptor antagonist MK801 (10 μM), the NOS inhibitor l-NAME (500 μM), the Ras inhibitor FPT Inh III (250 μM), and the Mek inhibitor PD98059 (50 μM). These experiments have been replicated three times with similar results. (B–D) Functional analysis of the role of Raf/Mek/Erk signaling in OGD preconditioning in neurons. (B) Expression of DNRaf in primary cortical neurons blocks the development of OGD tolerance, and CARaf is capable of conferring neuroprotection against lethal OGD. Significance was determined by a balanced two-way ANOVA with a Student's t test: *, P = 0.001 when comparing 60′ OGD to 5′/60′ OGD, or to Ad.CARaf/60′ OGD; †, P = 0.001 when comparing 5′/60′ OGD to Ad.DNRaf-5′/60′ OGD. (C) Expression of DNMek prevents OGD tolerance, and CAMek induces protection against 60 min of OGD. *, P = 0.001 when comparing 60′ OGD to 5′/60′ OGD, or to Ad.CAMek/60′ OGD; ‡, P = 0.001 when comparing 5′/60′ OGD to Ad.DNMek-5′/60′ OGD. (D) Expression of DNErk2 blocks OGD tolerance in a similar manner as its upstream signaling mediators. †, P = 0.001 when comparing 5′/60′ OGD to Ad.DNErk-5′/60′ OGD. Each data point is the mean ± SEM (n = 8) of at least two separate experiments. Each data point reflects a minimum of 16,000–30,000 neurons counted.
Because Erk is activated by 5 min of OGD in an NMDA receptor-, NO-, Ras-, and Mek-dependent manner, we next explored the functional role of the Erk cascade in the development of OGD tolerance (Fig. 4B). We tested recombinant adenoviruses (21) expressing DN mutants of the Raf/Erk cascade (i.e., DN of Raf, Mek, and Erk2) and CA forms of Raf and Mek for their ability to affect OGD preconditioning. The DN mutants of Raf, Mek, and Erk2 are all capable of blocking the development of OGD tolerance (Fig. 4 B–D). These results are consistent with our previous observations that DN Ras blocks OGD tolerance, although such a blockade by DN Ras is more dramatic, although not significantly different, than the blockade induced by the DN Raf, DN Mek, and DN Erk2 (Figs. 2B and 4B). All DN mutants inhibited Erk phosphorylation to similar levels (data not shown). These observations indicate that not only Ras, but also Raf, Mek, and Erk are required for OGD preconditioning.
The CA forms of Raf and Mek (Fig. 4 B and C) induced neuroprotection against 60 min of OGD applied 48 h after adenoviral infection of the cultures. All CA mutants were capable of causing similar levels of Erk phosphorylation (data not shown). These results strongly suggest that the Ras effector Raf/Erk cascade is important in the mechanisms leading to OGD tolerance.
Discussion
The studies presented here identify Ras as an essential early intracellular mediator of OGD preconditioning in cortical neurons. We show that neuronal preconditioning by 5 min of OGD induces activation of Ras. The activity of Ras is not only necessary for OGD preconditioning, but is also capable of inducing tolerance to OGD in the absence of an OGD preconditioning stimulus. By using recombinant adenoviruses and aided by chemical inhibitors, we were able to systematically dissect the role of each intermediate of the Ras/Erk cascade in OGD tolerance in neurons. Our results indicate that all of these signaling mediators, Ras, Raf, Mek, and Erk, are required for the development of tolerance to OGD. In contrast, by using recombinant herpes virus and chemical inhibitors, we demonstrate that PI3K activity is not essential for OGD preconditioning in neurons. The signaling pathway from PI3K to the serine/threonine protein kinase Akt/protein kinase B is involved in some cellular responses induced by growth factors such as insulin, platelet-derived growth factor, epidermal growth factor, and insulin-like growth factor I (34, 39–42). Although this pathway has been proposed to protect cells from apoptosis induced by various stimuli (41), it does not appear to play a role in the development of neuronal OGD tolerance.
Confirming previous studies (4–6, 10, 12, 17), we show that OGD preconditioning is blocked by NMDA receptor antagonists (MK801) and NOS inhibitors (l-NAME). We demonstrate that both MK801 and l-NAME block the activation of Ras and Erk induced by 5 min of OGD. These results are consistent with previous observations implicating NO as a mediator of Ras activation (43) by NMDA receptor stimulation (26) and support a key role for NO in the induction of tolerance. Our data indicate that during OGD preconditioning in neurons a signaling cascade is initiated by activation of the NMDA receptors, Ca2+ influx, and production of NO, which in a Ras/Erk-dependent manner leads to the development of neuronal tolerance to OGD.
Previous studies have reported Erk activation in the hippocampus after sublethal ischemia (16) and in myocardium after ischemia-reperfusion (44, 45). Erks have been proposed as the downstream mediators of protein kinase C signaling during ischemic preconditioning in the heart (45). Although the results of those studies are suggestive, they do not demonstrate a functional requirement for Erk activity in ischemic tolerance. We have examined directly the functional role not only of Erk, but also of each intermediate of the Erk cascade, including Ras, Raf, Mek, and Erk2 in the induction of neuronal OGD tolerance. Ischemic preconditioning may require activation of diverse intracellular signaling pathways, including Ras-dependent pathways as demonstrated by our studies, or protein kinase C-dependent pathways as reported previously (17). NO may play a role in both of these and in other pathways because our data indicate that NO mediates activation of Ras in OGD preconditioning in neurons, and Ping et al. (45) have suggested that NO activates protein kinase C in ischemic preconditioning in the heart. The impressive ability of the CA form of Ras to promote neuroprotection to OGD in the absence of an OGD preconditioning stimulus indicates that this signaling cascade may be a predominant effector of OGD preconditioning in neurons.
How the Ras/Erk pathway leads to neuronal survival in general and to resistance to neuronal OGD damage in particular is not yet known but may be linked to an Erk-dependent inhibition of pro-apoptotic proteins. Consistent with this notion, in Drosophila, Ras has been shown to cause cell survival through Erk-induced phosphorylation and inactivation of the pro-apoptotic product of the head involution defective (hid) gene (46). Another plausible hypothesis is that Ras activation is linked to Erk-dependent modulation of gene expression and new protein synthesis. Tolerance develops over many hours after the preconditioning episode (4, 47, 48), and protein synthesis inhibitors block OGD preconditioning (data not shown; ref. 49). Consistent with this hypothesis, changes in transcription have been associated with the development of ischemic tolerance, including up-regulation of protective genes such as HSP70, HSP72, and bcl-2 (50, 51). Also, in Drosophila, Ras has been shown to promote cell survival by down-regulating hid transcription (52).
The finding that the Ras/Erk pathway is a critical mediator of OGD tolerance is likely to have considerable biological significance and may possibly explain how ischemic preconditioning can effect changes in neuronal vulnerability by inducing gene expression within the nucleus. For instance, one of the characteristic features of Erks is their ability to translocate to the nucleus, where they phosphorylate and activate transcription factors, thereby regulating gene expression (53). Expression of immediate early genes such as c-Jun and c-Fos as well as activity of the transcription factors Elk1 and CREB are induced by Erks (53). Identification of genes that mediate Ras-dependent OGD preconditioning in neurons is warranted.
The data presented here contribute considerably to the understanding of the mechanistic basis of ischemic tolerance in neurons. We have confirmed the importance of NMDA receptors and NO production in OGD preconditioning and, most importantly, we have identified the Ras/Erk pathway as the downstream signaling effector of NMDA receptor/NOS activation that is necessary and sufficient for OGD preconditioning. These endogenous protective mechanisms may ultimately be harnessed as novel protective strategies against ischemic and traumatic injury as well as neurodegenerative disorders.
Acknowledgments
We thank Regeneron Pharmaceuticals, Inc., for providing BDNF and TrkB-IgG. This research was supported by the following: M.G.-Z. by the Boehringer Ingelheim Fonds (Stuttgart, Germany); R.G.K. by U.S. Public Health Service Grants NS29837 and NS33467; J.F.D. by U.S. Public Health Service Grant NS10635-01 and the National Stroke Association; L.F.P. by U.S. Public Health Service Grant NS34296; V.L.D. by U.S. Public Health Service Grant NS33142, the American Heart Association, and the Muscular Dystrophy Association; and T.M.D. by the American Heart Association, U.S. Public Health Service Grant NS37090, and the Paul Beeson Faculty Scholar Award in Aging Research.
Abbreviations
- Erk
extracellular regulated kinase
- PI3K
phosphatidyl inositol 3-kinase
- Ras
p21ras
- OGD
oxygen-glucose deprivation
- NMDA
N-methyl-d-aspartate
- GC
guanylyl cyclase
- BDNF
brain-derived neurotrophic factor
- l-NAME
l-nitroarginine methyl ester
- FPT Inh III
farnesyl protein transferase inhibitor III
- DN
dominant negative
- CA
constitutively active
- N-Arg
nitro-l-arginine
- l-Arg
l-arginine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tomai F, Crea F, Chiariello L, Gioffre P A. Circulation. 1999;100:559–563. doi: 10.1161/01.cir.100.5.559. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari R, Ceconi C, Curello S, Percoco G, Toselli T, Antonioli G. Am Heart J. 1999;138:61–68. doi: 10.1016/s0002-8703(99)70322-4. [DOI] [PubMed] [Google Scholar]
- 3.Ishida T, Yarimizu K, Gute D C, Korthuis R J. Shock. 1997;8:86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Grabb M C, Choi D W. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato H, Liu Y, Araki T, Kogure K. Neurosci Lett. 1992;139:118–121. doi: 10.1016/0304-3940(92)90871-4. [DOI] [PubMed] [Google Scholar]
- 6.Kasischke K, Ludolph A C, Riepe M W. Neurosci Lett. 1996;214:175–178. doi: 10.1016/0304-3940(96)12915-3. [DOI] [PubMed] [Google Scholar]
- 7.Murry C E, Jennings R B, Reimer K A. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Jones W K, Xuan Y T, Tang X L, Bao W, Wu W J, Han H, Laubach V E, Ping P, Yang Z, et al. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura T, Tsuchida A. Clin Exp Pharmacol Physiol. 1999;26:92–99. doi: 10.1046/j.1440-1681.1999.03003.x. [DOI] [PubMed] [Google Scholar]
- 10.Gidday J M, Shah A R, Maceren R G, Wang Q, Pelligrino D A, Holtzman D M, Park T S. J Cereb Blood Flow Metab. 1999;19:331–340. doi: 10.1097/00004647-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imagawa J, Yellon D M, Baxter G F. Br J Pharmacol. 1999;126:701–708. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukreja R C. Ann NY Acad Sci. 1999;874:211–221. doi: 10.1111/j.1749-6632.1999.tb09237.x. [DOI] [PubMed] [Google Scholar]
- 14.Baker J E, Holman P, Gross G J. Circulation. 1999;99:1249–1254. doi: 10.1161/01.cir.99.9.1249. [DOI] [PubMed] [Google Scholar]
- 15.Hu B R, Wieloch T. J Neurochem. 1994;62:1357–1367. doi: 10.1046/j.1471-4159.1994.62041357.x. [DOI] [PubMed] [Google Scholar]
- 16.Shamloo M, Rytter A, Wieloch T. Neuroscience. 1999;93:81–88. doi: 10.1016/s0306-4522(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 17.Ping P, Takano H, Zhang J, Tang X L, Qiu Y, Li R C, Banerjee S, Dawn B, Balafonova Z, Bolli R. Circ Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 18.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson V L, Dawson T M, Bartley D A, Uhl G R, Snyder S H. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klesse L J, Parada L F. J Neurosci. 1998;18:10420–10428. doi: 10.1523/JNEUROSCI.18-24-10420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klesse L J, Meyers K A, Marshall C J, Parada L F. Oncogene. 1999;18:2055–2068. doi: 10.1038/sj.onc.1202524. [DOI] [PubMed] [Google Scholar]
- 22.Neve R L, Howe J R, Hong S, Kalb R G. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 23.Feig L A, Cooper G M. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monyer H, Giffard R G, Hartley D M, Dugan L L, Goldberg M P, Choi D W. Neuron. 1992;8:967–973. doi: 10.1016/0896-6273(92)90211-u. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Zulueta M, Ensz L M, Mukhina G, Lebovitz R M, Zwacka R M, Engelhardt J F, Oberley L W, Dawson V L, Dawson T M. J Neurosci. 1998;18:2040–2055. doi: 10.1523/JNEUROSCI.18-06-02040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun H Y, Gonzalez-Zulueta M, Dawson V L, Dawson T M. Proc Natl Acad Sci USA. 1998;95:5773–5778. doi: 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southam E, Charles S L, Garthwaite J. Br J Pharmacol. 1996;119:527–532. doi: 10.1111/j.1476-5381.1996.tb15703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marini A M, Rabin S J, Lipsky R H, Mocchetti I. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara N, Croll S D, Wiegand S J, Klatzo I. Neurosci Res. 1997;29:37–47. doi: 10.1016/s0168-0102(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 30.Rosen L B, Ginty D D, Weber M J, Greenberg M E. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 31.Bading H, Greenberg M E. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- 32.Patel D V, Schmidt R J, Biller S A, Gordon E M, Robinson S S, Manne V. J Med Chem. 1995;38:2906–2921. doi: 10.1021/jm00015a013. [DOI] [PubMed] [Google Scholar]
- 33.Joneson T, White M A, Wigler M H, Bar-Sagi D. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 34.Downward J. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 35.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 36.Vlahos C J, Matter W F, Hui K Y, Brown R F. J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 37.Hara K, Yonezawa K, Sakaue H, Kotani K, Kotani K, Kojima A, Waterfield M D, Kasuga M. Biochem Biophys Res Commun. 1995;208:735–741. doi: 10.1006/bbrc.1995.1399. [DOI] [PubMed] [Google Scholar]
- 38.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan D R, Miller F D. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 40.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 41.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 42.Klesse L J, Parada L F. Microsc Res Tech. 1999;45:210–216. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<210::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Lander H M, Ogiste J S, Pearce S F, Levi R, Novogrodsky A. J Biol Chem. 1995;270:7017–7020. doi: 10.1074/jbc.270.13.7017. [DOI] [PubMed] [Google Scholar]
- 44.Maulik N, Watanabe M, Zu Y L, Huang C K, Cordis G A, Schley J A, Das D K. FEBS Lett. 1996;396:233–237. doi: 10.1016/0014-5793(96)01109-x. [DOI] [PubMed] [Google Scholar]
- 45.Ping P, Zhang J, Cao X, Li R C, Kong D, Tang X L, Qiu Y, Manchikalapudi S, Auchampach J A, Black R G, Bolli R. Am J Physiol. 1999;276:H1468–H1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann A, Agapite J, McCall K, Steller H. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- 48.Kato H, Liu Y, Araki T, Kogure K. Brain Res. 1991;553:238–242. doi: 10.1016/0006-8993(91)90831-f. [DOI] [PubMed] [Google Scholar]
- 49.Barone F C, White R F, Spera P A, Ellison J, Currie R W, Wang X, Feuerstein G Z. Stroke. 1998;29:1937–1951. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 50.Aoki M, Abe K, Kawagoe J, Sato S, Nakamura S, Kogure K. Brain Res. 1993;601:185–192. doi: 10.1016/0006-8993(93)91709-2. [DOI] [PubMed] [Google Scholar]
- 51.Shimazaki K, Ishida A, Kawai N. Neurosci Res. 1994;20:95–99. doi: 10.1016/0168-0102(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 52.Kurada P, White K. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 53.Gotoh Y, Nishida E. Mol Reprod Dev. 1995;42:486–492. doi: 10.1002/mrd.1080420417. [DOI] [PubMed] [Google Scholar]