Abstract
Experiencing repeatedly presented auditory stimuli during magnetoencephalographic (MEG) recording may affect how the sound is processed in the listener’s brain and may modify auditory evoked responses over the time course of the experiment. Amplitudes of N1 and P2 responses have been proposed as indicators for the outcome of training and learning studies. In this context the effect of merely sound experience on N1 and P2 responses was studied during two experimental sessions on different days with young, middle-aged, and older participants passively listening to speech stimuli and a noise sound. N1 and P2 were characterized as functionally distinct responses with P2 sources located more anterior than N1 in auditory cortices. N1 amplitudes decreased continuously during each recording session, but completely recovered between sessions. In contrast, P2 amplitudes were fairly constant within a session but increased from the first to the second day of MEG recording. Whereas N1 decrease was independent of age, the amount of P2 amplitude increase diminished with age. Temporal dynamics of N1 and P2 amplitudes were interpreted as reflecting neuroplastic changes along different time scales. The long lasting increase in P2 amplitude indicates that the auditory P2 response is potentially an important physiological correlate of perceptual learning, memory, and training.
Keywords: Hearing, Auditory plasticity, Perceptual learning, Aging, MEG
Introduction
In neuroimaging studies of the auditory system, acoustic stimuli are repeatedly presented, which in turn may alter the way these sounds are processed in the listener’s brain. Changes of brain function and recorded brain responses during the experiment may limit the observability of brain function or the observed change may be addressed misleadingly to an experimental condition. Components of sensory evoked responses, like the N1 and P2 waves of the auditory evoked potential (AEP) or auditory evoked magnetic field (AEF) occurring around 100 and 200 ms after sound onset, respectively, have been described as physiological markers of experience-induced plastic reorganization of brain function. Here the term “plasticity” refers to the capacity for physiological change (Lomber and Eggermont, 2006). Within this framework, amplitude differences among musicians and non-musicians have served as a model for studying long-term training induced plastic reorganization (Lopez et al., 2003; Pantev et al., 2001a). More specifically, enhanced N1 amplitudes have been found in musicians using magnetoencephalography (MEG) (Kuriki et al., 2006; Pantev et al., 1998; Pantev et al., 2003) and electroencephalography (EEG) (Shahin et al., 2003) with response enhancements being most evident for the timbre of the musician’s instrument than for pure tones (Pantev et al., 2001b). Amplitude differences between musicians and non-musicians have also been reported for the P2 wave of the AEP/AEF and have been attributed to long-term musical experience and training (Kuriki et al., 2006; Shahin et al., 2003; Shahin et al., 2005; Trainor et al., 2003). Moreover, the P2 wave is also responsive to short-term laboratory based training. For example, P2 amplitudes increased in individuals who underwent multiple days of speech-sound training (Tremblay et al., 2001), as well as in subjects who partook in four sessions of vowel segregation training that took place within one week (Reinke et al., 2003). In a subsequent study, these investigators reported rapidly occurring physiological changes during one hour of vowel segregation training (Alain et al., 2007). Despite the converging findings that changes in the components of AEP/AEF coincide with perceptual learning, the neural basis of the enhanced P2 is poorly understood.
Several features of neuroelectric and neuromagnetic responses have to be considered when studying the effects of training and learning. For instance, in addition to response facilitation during training, physiological responses may decrease because of habituation, especially when stimuli are repeatedly presented to listeners who are not engaged in a specific task (Naatanen and Picton, 1987). The effect of habituation on the N1 has been studied by comparing N1 amplitude changes between subsets of averaged responses within an experimental block. N1 responses to pure tone stimuli at the begin of a recording block are typically larger in amplitude than at the end of a block, and this decrease diminishes after some 10 s (Ritter et al., 1968).
Further complicating the interpretation of training studies is that the time course of changes in performance and physiological changes appear to be different. For example, Atienza et al. (2002) reported that the P2 amplitude did not increase between pre- and post-training ERP recordings in an auditory discrimination study, even though participants improved their ability to perceive the stimuli and performed almost perfectly during the post-training session in a task, which they initially could not do at all. However, 24 hrs later, after the subjects slept over night, an increase in P2 amplitude was observed. In addition, the mismatch negativity (MMN), which was absent before training, began to emerge immediately after training and continued to increase in amplitude over the following 36 hour time period. Different time-courses were also reported during speech-sound training (Tremblay et al., 1998), where improved behavioural performance lagged behind increasing physiological responses for about half of the subjects. The interpretation of these findings was that the MMN represents a pre-attentive process related to fast learning and a period of consolidation was necessary for some subjects before they could improve their performance on the perceptual task.
Exposure to repeatedly presented stimuli during EEG or MEG might also contribute to the observed learning effects and changes in physiological activity. Sheehan et al. (2005) reported that enhanced P2 responses could result solely from stimulus exposure and might not require explicit training. They found an increase in P2 amplitude in a trained group, and in a control group that did not partake in training speech sounds. Consequently, the authors questioned whether explicit training is necessary to induce an enhancement of the P2 wave of the AEP.
To learn more about the significance of P2 changes in relation to perceptual learning, and to determine if these changes reflect a person’s physiological capacity for change, the goals of this study were to investigate the time course of amplitude changes for N1 and P2 peaks of the AEF within and across test sessions. The hypotheses were that N1 and P2 responses are generated by different cortical sources and that the time courses of N1 and P2 changes would be distinctly different from each other. Whereas the N1 would show a decrease in amplitude within a session, but would recover between sessions; P2 amplitude would increase, between test sessions, recorded on different days. A secondary purpose was to examine these P2 effects across lifespan, because training programs are often aimed at clinical populations of diverse ages. The hypothesis was that the capacity of neuroplastic changes would be reduced with advanced age. To test this hypothesis, subjects from a wide range of ages were included and then equally distributed into three groups of young, middle-aged, and older participants. AEFs to two speech tokens as well as a noise sound were recorded in two sessions, collected on separate days prior, to a training program in which participants learned identification of small differences in the voice-onset time of syllables. The analysis of AEF recorded during the two pre-training sessions will be reported here, whereas results of the training part of the study will be reported elsewhere.
Methods
Participants
Forty-three normal-hearing, mono-lingual English speaking, right-handed adults in three age groups participated in this study, 15 participants in the young group (mean age 25.2 yrs, range 19–33 yrs, 8 female), 13 in the middle aged (mean age 46.8 yrs, range 37–55 yrs, 6 female), and 15 in the group of older adults (mean age 63.5 yrs, range 55–74, 10 female). Pure tone audiometric thresholds were tested before the first MEG recording session. Only participants with normal hearing bilaterally, defined as audiometric thresholds better than 25 dB nHL in the frequency range of 250–4000 Hz, were included in the study. This means the stimuli used in this experiment were clearly audible to all subjects because the acoustic content of the signals fell within this frequency range. Nonetheless, systematic threshold differences were found between the age groups (F(2,40)=16.3, p<0.0001). All participants had a flat audiogram between 250 and 4000 Hz and the mean threshold across these frequencies was lowest in the young (3.6 dB nHL), followed by the middle aged (9.2 dB nHL), and highest in the older group (13.6 dB nHL). The threshold increase between young and middle-aged group was significant (t(25)=3.43, p<0.0025) as well as the difference between middle-aged and older group (t(26)=2.31, p<0.03). Participants provided their written consent after receiving information about the nature of the study, which had been approved by the Research Ethics Board at Baycrest Centre well as the University of Washington.
Auditory stimulation
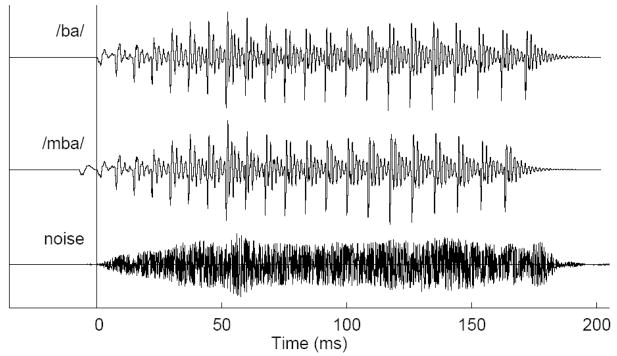
Auditory stimuli were two versions of a synthesized syllable /ba/ with one sound having a voice-onset time (VOT) of −20 ms and the other −10 ms (Figure 1). These same stimuli have been used in previous training studies (Tremblay et al.1997; Tremblay et al., 1998; Tremblay et al., 2001; Tremblay and Kraus, 2002). Without training, English speaking subjects identify these two pre-voiced stimuli as /ba/, but after training they can learn to detect differences in VOT and label the −20 ms stimulus as /mba/ and the −10 ms VOT stimulus as /ba/ (McClaskey et al., 1983). The third stimulus was a noise sound, which was generated by multiplying white Gaussian noise with the smoothed envelope of the /ba/ sound. Such noise stimulus is sometimes used as control stimulus in related training experiments. The sounds were presented with constant stimulus onset asynchrony (SOA) of 2175 ms, which was a quasi-optimal choice to evoke N1 and P2 responses of comparable size within shortest investigation time. Two hundred stimuli of the same type were presented in a block of 435 s in duration (7.25 min). Each block was repeated such that each subject listened to 400 presentations of the same stimuli, within one hour, on the same day. A set of blocks with /ba/, /mba/, and noise stimuli was always performed in the first half of the session and repeated in the second half. The order of blocks within the set of the three stimulus types was randomly permuted for each subject. Thus, the time interval between two blocks of same stimulus type varied between two blocks being consecutive and being separated by four blocks of different sound. The procedure was repeated on a second day. For some participants, this second session took place on two consecutive days, or at a later date no later than 20 days following the first sessions. Each stimulus was presented binaurally, at 85 dB SPL, using Etymotic ER 3A transducers transducers connected to the partcipants ears with 1.5 m of plastic tubing.
Figure 1.
Time series of auditory stimuli, the syllable /ba/ (top trace), a modification of the /ba/ syllable with 10 ms longer pre voicing time, termed the /mba/ sound (middle trace), and a noise stimulus created by modulating white noise with the envelope of the /ba/ sound.
MEG data acquisition
Prior to MEG recording the geometrical shape of the subject’s head was measured by recording the spatial coordinates of 100 to 200 representative points on the surface of the scalp using a three-dimensional digitizer (FastTrack, Polhemus, Colchester, VT). The head shape data were used to obtain a head model for MEG source analysis and for co-registration of dipole source locations with an anatomical MRI atlas brain. The head shape coordinates were referenced to three fiducials defined by the nasion and the left and right pre-auricular points. The fiducials established a head based Cartesian coordinate system with the x-axis pointing from the midpoint between the pre-auricular points to the nasion, the y-axis running from right to left in the plane formed by the three fiducials and the z-axis pointing in superior direction.
MEG recordings were performed in a quiet, magnetically shielded room using a 151-channel whole-head neuromagnetometer (VSM-Medtech, Port Coquitlam, BC, Canada) at the Rotman Research Institute. The detection coils of this MEG device are almost equally spaced on the helmet shaped surface and are configured as axial first order gradiometers (Vrba and Robinson, 2001). After low-pass filtering at 200 Hz, the magnetic field data were sampled at the rate of 625 Hz and stored continuously.
MEG data were collected during passive listening, meaning, that the subjects did not need to attend to the stimuli or execute a task but were asked to remain alert. In order to control for confounding changes in vigilance, the subjects watched a closed captioned movie of their choice, while the auditory stimuli were being presented. Compliance was verified using video monitoring. The subjects were in upright sitting position with the head resting inside the helmet shaped MEG sensor. The head position was registered at begin and end of each recording block of 7.25 min duration using the three detection coils attached to the subject’s nasion and the pre-auricular points. Subjects were able not to move during a recording block and no data had to be rejected because of head movements larger than 8 mm, which was defined as exclusion criterion.
MEG data analysis
Each block of continuously recorded MEG data was subdivided into 200 stimulus related epochs of 1500 ms duration including a 500 ms pre-stimulus interval. For artifact rejection, principal component analysis was performed on each epoch of magnetic field data. Principal components, which exceeded the threshold of 2 pT in at least one channel, were subtracted from the data. This approach is effective for removing artifacts with amplitudes larger than the brain signals of interest (Lagerlund et al., 1997) such as artifacts related to dental metal and eye-blinks (Bardouille et al., 2006). After artifact removal, the magnetic field data were averaged and magnetic source analysis was applied to the ±20 ms time intervals around the maximum of the N1 and P2 waves around 100 and 200 ms after stimulus onset. Separate source analyses for the N1 and P2 waves were based on the model of spatio-temporal equivalent current dipoles (ECD) in a spherical volume conductor.
MEG responses were modeled by single dipoles in left and right auditory cortices. Previous analyses, using a beamformer approach (Vrba and Robinson, 2001), which does not need a-priori assumptions about the source configuration, demonstrated that single dipoles are sufficient to describe the activation of the auditory cortex (Herdman et al., 2003). Single dipoles in both hemispheres were fit simultaneously to the 151-channel magnetic field distribution. First, we modeled the data with a mirror symmetric pair of dipoles. The resulting source coordinates were then used as starting points to fit the dipole in one hemisphere while the coordinates in the other hemisphere remained fixed. We then switched between hemispheres and repeated the last step until the source coordinates showed no further change. Dipole fits were accepted if their calculated fields explained at least 85% of the variance of the measured magnetic field. Up to 12 estimates of the N1m and P2m source locations were obtained for each subject from data sets corresponding to three stimulus types, two blocks per session, and two sessions. The median of spatial coordinates and orientations were used as individual models to measure the source waveforms for the auditory evoked responses.
Dipole moment waveforms were analyzed representing the source activity in the auditory cortices. The method of source space projection (Ross et al., 2000; Tesche et al., 1995), was applied to combine the 151 waveforms of magnetic field strength into a single waveform of a magnetic dipole moment measured in nanoAmpere-meter (nAm). The dipole moment measure is most sensitive for the localized area in the brain and less sensitive to electro-magnetic sources at other locations. Furthermore, source space projection results in waveforms with higher signal-to-noise ratio than the magnetic field waveforms (Ross et al., 2000). A further advantage of analysis in source domain is that the dipole moment is independent of the sensor position and the waveforms of cortical source activity can be combined across repeated sessions for a subject and across the group of subjects.
To analyze the temporal dynamics of N1 and P2 amplitudes, the 200 trials of MEG data in each experimental block were subdivided into quartiles with 50 trials in each, corresponding to 108 s of stimulation. The subsets of trials were averaged and corresponding source waveforms were calculated as described. Peak latencies were defined by the time point of zero crossing of the first derivative of the source waveform. The N1 and P2 peak amplitudes were measured as the amplitudes in the source waveforms at the latencies of the N1 and P2 peak in the grand average across all recordings in each individual subject. Repeated measures ANOVA with between groups factor ‘age’ and five within group factors ‘stimulus type’ (/ba/, /mba/, and noise), ‘quartiles’ (four levels), ‘block’ (two levels), ‘session’ (two levels), and ‘hemisphere’ (left, right) was applied to the N1 and P2 amplitudes and latencies. Scheffe’s procedure for pair wise comparison was used in the post-hoc tests. Greenhouse-Geiser adjustments were made if necessary, however the nominal degrees of freedom are reported in the results. Effects were accepted as significant on the α = 0.05 level. All calculations were performed using the SPSS Vers.11.
Behavioural tests
A stimulus identification task was used for behavioural tests immediately after each MEG recording session with the subject still sitting in the MEG scanner and using same stimulation equipment. Participants were instructed to listen to the single stimulus presentation and selecting what stimulus they heard by choosing one of two labels: /mba/ or /ba/. Each label was presented as text on a computer screen in front of the participant. The task was self-paced and no feedback was provided. Identifying the pre-voiced stimulus as /mba/, and the other as /ba/, was scored as a correct response using percent correct scores as well d-prime, which takes into consideration hits and false alarms (Macmillan and Creelman, 2005).
Results
Behavioural tests
Correct identification of the /ba/ and /mba/ sounds based on 10 ms difference in VOT is difficult for English speaking subjects and requires intense training. This was reflected in the rate of correct responses of 56.0% averaged across age groups at end of the first session and d′=0.384, which is above the chance level of 50% (t(42)=3.89, p<0.0004), and of 56.8% correct responses and d′=0.394 in the second session. The improvement in performance between first and second session did not reach significance according to percent correct scores (t(42)=0.39), or d′-values (t(41) = 0.15).
N1, P2 source analysis
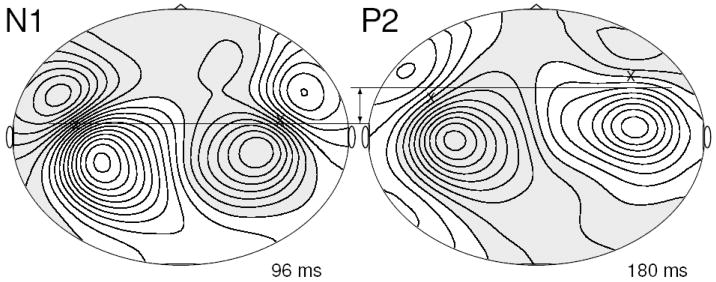
Typical maps of magnetic field distribution of the averaged evoked response above the head are shown for an individual subject listening to the /ba/ sound in flattened projections of the MEG sensors in Figure 2 at latencies of N1 and P2 amplitude peaks. The field maps for both components show characteristic dipolar patterns of opposite polarities above left and right temporal cortices, which justified modeling N1 and P2 waves with pairs of single equivalent current dipoles. Sources can be estimated close to locations below the mid points of lines connecting corresponding field maxima. Those points are indicated with x symbols in Figure 2 and suggest more anterior located P2 sources or a change towards anterior direction in dipole orientation between N1 and P2 sources.
Figure 2.
Top view of flattened projection of magnetic field above the head for an individual subject listening to the /ba/ stimulus at 96 ms after stimulus onset, the latency of the N1 peak, and at 180 ms, the latency of the P2 peak. Gray shaded areas indicate ingoing and white areas outgoing directions of the magnetic flux, respectively. Iso-field lines are drawn equally spaced every 20 fT. Dipolar magnetic field pattern have above the left and right temporal cortex have opposite polarity for both responses. A first approximation for the location of underlying sources is the midpoint between a pair of field maxima, indicated by ‘x’ symbols. The P2 sources are potentially more frontally located than the N1 sources.
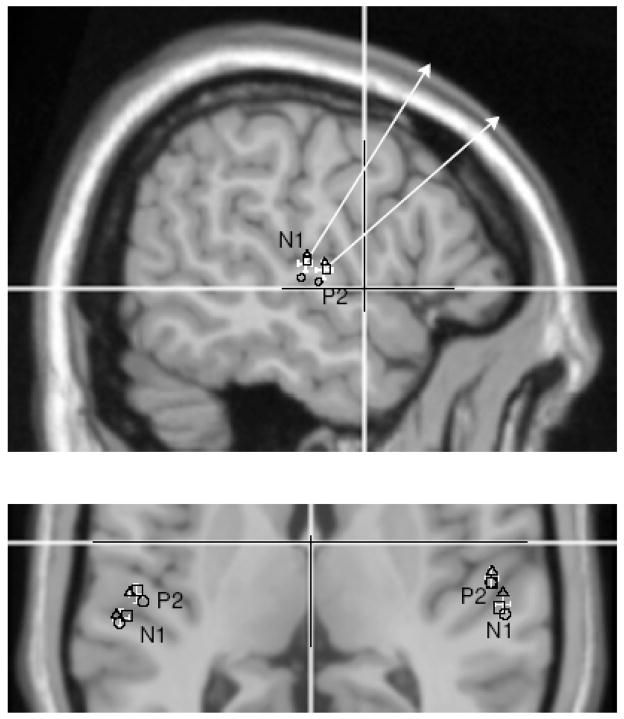
Dipole modeling for N1 and P2 components in left and right hemisphere was successful in 13 out of 15 young subjects, in 11 out of 13 middle-aged, and in all 15 older subjects. Overlaying group mean source locations onto an atlas brain allowed conversion from the original head based Cartesian coordinate system into Talairach coordinates. N1 sources were located at −27.8, −54.3, 7.8 mm (x,y,z) in left and −23.4, 54.9, 8.9 mm in right hemisphere, whereas P2 source coordinates were −20.8, −49.9, 6.1 mm left and −16.1, 51.7, 7.2 mm right. The 95% confidence intervals for the group mean coordinates were less than 6 mm in x, less than 5 mm in y and less than 6 mm in z direction. No significant differences between the three age groups were found. Figure 3 shows the locations of N1 and P2 sources in a section of a coronal MRI slice at z=8 mm. The error bars indicating the 95% confidence intervals for the grand mean do not overlap between N1 and P2 sources however separately calculated mean locations for each age group lay in close vicinity within the range indicated by the error bars. From the coronal MRI slice in Figure 3 it becomes obvious that N1 and P2 sources are located in different anatomical and likely different functional structures. The N1 sources lay in the posterior part of auditory cortex, the planum temporale, whereas the center of activity for P2 lay in anterior auditory cortex, the lateral part of Heschl’s gyrus.
Figure 3.
Overlay of group averaged dipole locations onto a MRI atlas brain. A section of a coronal slice at Talairach z-coordinate ztal=8. The white cursor lines correspond to xtal=0 and ztal=0. The white error bars indicate the 95% confidence limits for the group mean location in x and y direction for N1 and P2 sources in the left and right hemisphere. The open symbols indicate the group mean dipole locations for the young (circle), middle aged (square) and older (triangle) groups. P2 and N1 sources are separated with more medial and anterior locations of the P2 sources. Also the hemispherical asymmetry is obvious for both the anatomical structure and dipole locations. The upper figure shows a view from right onto a sagittal slice at xtal=−54. The white arrows represent a projection of mean dipole orientation onto the y–z plane. The orientation of the P2 dipole is rotated in anterior direction compared to the N1 orientation.
Pair wise comparisons between N1 and P2 source coordinates revealed statistical significance for more anterior located P2 sources in left (6.7 mm, t(38)=4.8, p<0.001) and right hemisphere (7.5 mm, t(38)=4.4, p<0.001) and more medial location of P2 sources in both hemispheres (left: 4.4 mm, t(38)=3.6, p<0.001, right: 3.2 mm, t(38)=2.7, p<0.012) however no differences in z direction were found. Also the characteristic anatomical asymmetry between right and left auditory cortices was reflected in significantly more anterior source locations in the right than left hemisphere (N1: 4.0 mm, t(38)=4.2, p<0.001, P2: 4.7 mm, t(38)=4.4, p<0.001).
The dipole orientation is characterized in a sagittal MRI slice in Figure 4. N1 and P2 dipoles were oriented almost parallel to this plane with the N1 orientation about 45° tilted from superior inferior direction toward the front of the head and the P2 oriented even more toward anterior direction. The differences in N1 and P2 dipole orientations were significant in both hemispheres (left: ϕ =13°, t(38)=4.3, p<0.001, right: ϕ =23°, t(38)=5.0, p<0.001). In summary the dipole modeling revealed distinct sources for the N1 and P2 wave of the AEF with the center of N1 sources locations in left and right planum temporale and the P2 sources in the lateral part of left and right Heschl’s gyri.
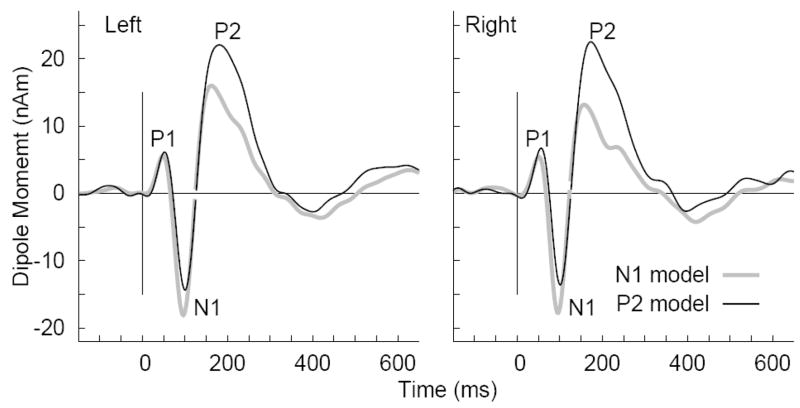
Figure 4.
Grand averaged source waveforms obtained from young subjects listening to the /ba/ stimulus in the second session. Two versions of source waveforms were calculated based on the N1 and P2 source model, respectively. The waveforms demonstrate that not using the appropriate source model may under estimate the N1 and P2 amplitudes.
Source waveforms
Two versions of source waveforms were calculated based on source models for the N1 and P2 response, respectively, which were kept constant across stimulus types and repeated measurements for each subject. Examples of source waveforms obtained with the N1 and P2 models, respectively, from left and right hemisphere in young subjects listening to the /ba/ stimulus are shown in Figure 4. Although the waveforms obtained with both models showed pronounced P1-N1-P2 waves, the N1 peak was larger when using the more appropriate N1 source model and the P2 peak was larger when using the P2 source model. The P1 response, which was not in the focus of this study and for which a source model was not feasible in all subjects, was almost equally well represented in the source waveforms using either the N1 or the P2 source model. For further analysis the N1 peak amplitudes were measured from source waveforms based on the N1 source model and vice versa for the P2 peak amplitudes.
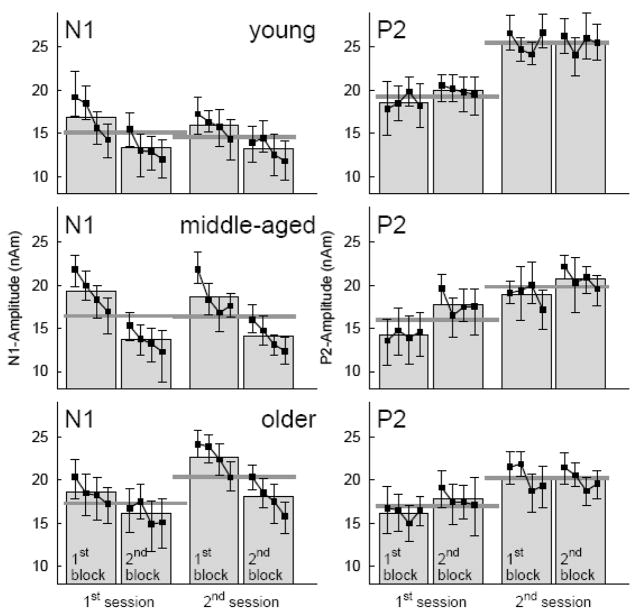
N1 and P2 peak amplitudes measured over the time course of the experiment are summarized for the three age groups in Figure 5. Generally, N1 peak amplitudes decreased within each experimental block and between blocks in all age groups with similar effect size in both sessions. After N1 decline during the first session the N1 amplitudes had been recovered at the beginning of the second session. There was no evidence of N1 decline between sessions. Even an increase between first and second session can be seen in the older group. P2 peak amplitudes did not change within a block but increased between blocks with larger effect size in the first than second session. As most pronounced effect P2 amplitudes in the second session were larger than in the first session.
Figure 5.
N1 and P2 amplitudes as measured on various time scales during the experiment. Square symbols and error bars denote mean and 95% confidence intervals for each quarter of trials within a block. Gray boxes represent the mean amplitudes within each of the two repeated blocks within a session and dark gray lines the mean amplitudes within first and second session, respectively. N1 and P2 amplitudes were averaged across the three stimulus types but shown separately for the three age groups.
Peak latencies
The subject’s age had a main effect on the N1 latency (F(2,35)=3.67, p=0.036) with shortest latency of 96 ms in the young, 104 ms in the middle-aged, and 105 ms in the older group (p=0.05). Mean P2 latencies increased with age from 182 to 185 and 197 ms, however, this effect did not reach significance (F(2,35)=1.36). P1 did not change with age, with mean latencies being 54, 54, and 55 ms in the young, middle-aged and older group, respectively. The stimulus type had main effects on the latencies of P1 (F(2,34)=78.4, p<0.0001), N1 (F(2,34)=53.5, p<0.0001), and P2 (F(2,34)=28.5, p<0.0001) with longer latencies for the /ba/ sound (58, 107, 191 ms) compared to /mba/ (53, 101, 187 ms) and shortest latencies for the noise sound (52, 98, 185 ms). No within and between session effects on the peak latencies and no interaction between factors were significant.
ANOVA of N1 peak amplitudes
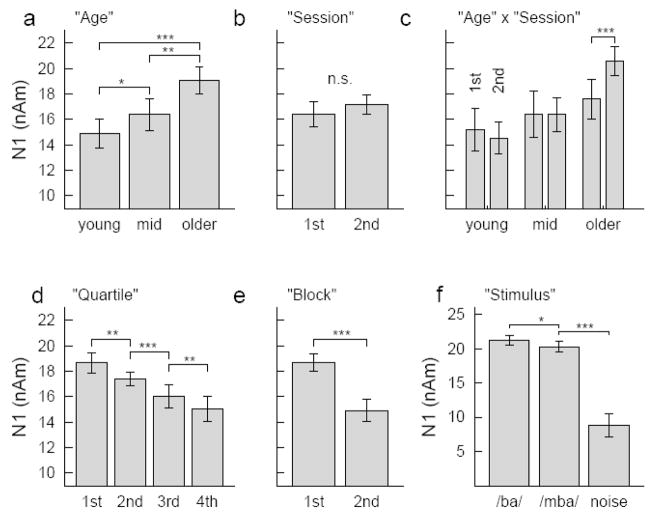
Analysis of variance was performed to study the effects of measuring response amplitudes over various time scales (two sessions on different days, two blocks within each session, and four quartiles within each block), the effect of ‘type of stimulus’ and ‘hemisphere’, where the response was measured, and the between groups factor ‘age’. The ANOVA revealed a main effect of ‘age’ on the N1 amplitude (F(2,36)=15.7, p<0.001). Pair wise comparisons confirmed significance for an increase in N1 amplitude between middle-aged and older group (p<0.003) and a tendency for N1 increase between the young and middle-aged group (p=0.073) (Figure 6a). No differences in the mean N1 amplitudes were observed in MEG recordings at the first and second session (F(1,36)=2.1, n.s.) (Figure 6b). However, an interaction between the factors ‘age’ and ‘sessions’ (F(2,36)=4.8, p<0.015) was caused by different N1 amplitude pattern in the first than second session in the older group only (Figure 6c). Pair wise comparison showed significant N1 decrease between first and second block in the second session (t(14)=4.54, p<0.001) but not in the first session (t(14)=1.38) and N1 increase between the first block in the first session and the first block in the second session (t(14)=3.37, p<0.005) but not for the second block between sessions (t(14)=1.88). Thus, the between session effect in the older group was caused by small N1 amplitudes during the very first block (see also Figure 5). Changes in the N1 amplitude during the MEG recording session became evident with main effects of the factors ‘quartile’ (F(3,36)=18.7, P<0.001) and ‘block’ (F(1,36)=79.5, p<0.001). The N1 amplitude decreased continuously between the measurements observed from quartiles within a block of 3 min recording time (Figure 6d). Also N1 amplitude decreased between repeated blocks within a session (Figure 6e). The type of stimulus was another factor affecting the N1 amplitude (F(2,36)=99.7, p<0.001) because the N1 elicited by the noise stimulus was noticeable smaller than the N1 response to the speech stimuli and the response to /ba/ was slightly larger than to the /mba/ sound (Figure 6f). No interactions between ‘age’, ‘stimulus’, ‘quartiles’, and ‘blocks’ were significant. This means especially, that the effects of decreasing N1 within the recording session was not different for the speech and noise stimuli and effects were preserved with increasing age.
Figure 6.
Summary of the Anova results for the N1 amplitudes. a: Main effect of ‘Age’ expressed by increasing N1 amplitudes with increasing age. b: No N1 difference between sessions was found, c: however and interaction ‘Age x Session’ because of N1 increase between first and second session in the older group only. d: N1 amplitudes were attenuated for each quarter of trials within a block, and e: between the two repeated blocks. f: ‘Stimulus type’ had a main effect on the N1 amplitude, which was noticeably larger for the speech than the noise sound and slightly larger for /ba/ than /mba/.
ANOVA of P2 peak amplitudes
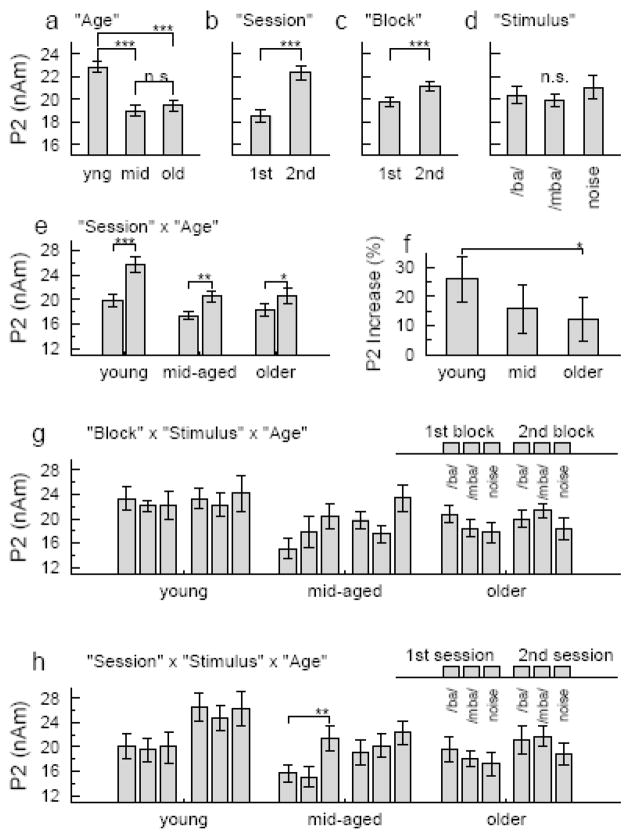
The repeated measures ANOVA for the P2 peak amplitudes revealed a main effect of the between groups factor ‘age’ (F(2,35)=90.2, p<0.001) expressed as larger P2 amplitude in the young group compared to the others (Figure 7a). Main effects of the within groups factors ‘session’ (F(1,35)=58.1, p<0.001) and ‘block’ (F(1,35)=18.0, p<0.001) were found with increasing P2 between first and second session (Figure 7b) and a smaller P2 increase between first and second block within a session (Figure 7c). The type of stimulus had no main effect on the P2 amplitude (F(2,35)=1.6, n.s.) (Figure 7d). Interactions were significant between ‘session’ and ‘age’ (F(2,35)=5.1, p<0.012), between ‘stimulus’ and ‘age’ (F(4,35)=5.5, p<0.004), and between ‘block’, ‘stimulus’, and ‘age’ (F(4,35)=6.0, p<0.002). The P2 increase between sessions was significant in all three age groups (young: F(1,12)=38.4, p<0.001, middle-aged: F(1,1)=22.5, p<0.002, and older: F(1,13)=7.2, p<0.020) (Figure 7e); however, the effect size decreased with increasing age (F(2,35)=3.6, p<0.038). The post hoc comparison was significant for larger between sessions increase in the young than older group (p<0.044) (Figure 7f). The stimulus type did not affect the P2 amplitude in the young group (F(2,12)=0.5, n.s.); however, it was a significant factor in the middle-aged group (F(2,10)=10.5, p<0.002) and the older group (F(2,13)=3.5, p<0.046). More complicated, the ANOVA revealed ‘stimulus’ x ‘block’ interaction in both groups (middle-aged: F(2,10)=4.5, p<0.027, older: F(2,13)=7.3, p<0.006). The noise stimulus elicited a larger P2 than the speech stimuli in the middle-aged group and this was stronger expressed for the /ba/-sound in the first block and the /mba/-sound in the second block. However, in the group of older subjects, the noise stimulus elicited a smaller P2 than the speech stimuli did and again this was expressed for the /ba/-sound in the first block and the /mba/-sound in the second block (Figure 7g). A ‘stimulus’ x ‘session’ interaction was significant in the middle-aged group only (F(2,10)=4.7, p<0.023) because of a larger P2 response to the noise compared to the speech stimuli in the first session and no further increase in the second session. (Figure 7h). N1 and P2 amplitudes above the right or left hemisphere were not different for each stimulus type, nor were there significant interactions between ‘hemisphere’ and other factors.
Figure 7.
Summary of the Anova results for the P2 amplitudes. Main effects were found for the factors a: ‘age’, b: ‘session’, and c: ‘block’. d: In contrast to the N1 response, in mean the P2 was not affected by the ‘stimulus type’. e: An interaction between ‘age’ and ‘session’ was found because f: the relative P2 increase from first to second session was larger in young than older participants. g: Interactions between ‘stimulus’, ‘block’, and ‘age’, h: Interactions between ‘stimulus’, ‘session’, and ‘age’
Changes in response waveforms over the time course of the experiment
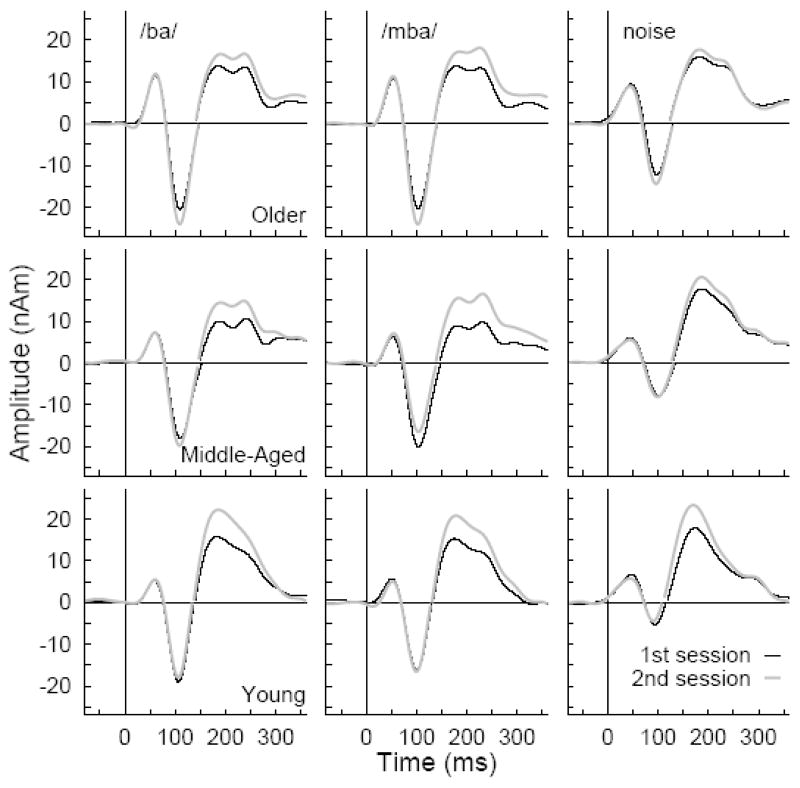
How the observed waveforms changed between first and second session is shown in Figure 8 for the different stimulus types and age groups. In each age group, responses to the /ba/ and /mba/ stimuli were almost identical except the latency of the /mba/ response was about 5 ms shorter than /ba/ because of the earlier onset in the more pre-voiced /mba/ sound. N1 responses were noticeable larger for the speech stimuli than for the noise stimulus, however, P2 amplitudes were of almost equal size for all stimuli P2 responses to speech stimuli expressed a distinct second peak, which was not as evident in the noise response. The second peak in the P2 wave was found in 8 of 15 young participants, 12 of 13 middle-aged and 13 of 15 older participants.
Figure 8.
Changes in the response waveforms between first and second session. The waveforms were averaged across hemispheres and shown separately for age groups and stimulus types. The first part of the waveforms until the zero crossing between N1 and P2 wave was calculated based on the N1 source model and the later part based on the P2 source model.
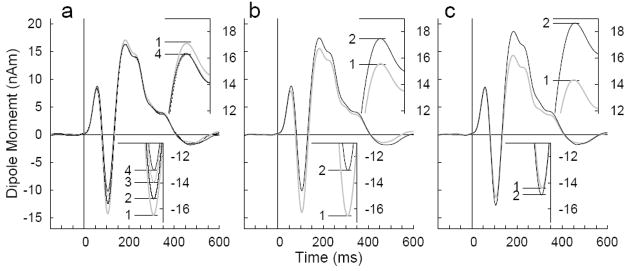
An overview about changes in the source waveforms over various time scales is given in Figure 9. Response waveforms were averaged across stimulus types and age groups and are based on the P2 model; however, figure inserts showing the N1 response on enlarged scale are based on the more appropriate N1 source model. Figure 9a shows a systematic decrease in N1 amplitude during a block of MEG recording from largest value during the first quartile of trials in each block to the smallest amplitude during the fourth quartile at the end of each block. The total effect size is on the order of 20%. In contrast, the P2 amplitude decreased during the first quartile of trials but did not decrease any further. The overall decrease in P2 amplitude during an experimental block of 7 min was on the order of 5%. No differences in the response waveforms outside narrow time intervals around the N1 and P2 peak are visible. Differences in response waveforms between the first and second repeated block within a session are visualized in Figure 9b. Because the order of stimulus types was randomized across subjects the actual time difference between repeated blocks varied. However, the first block was always in the first half of a recording session and the second block always in the second half. Between the first and the second block the N1 amplitude decreased by about 25% almost the same amount as the N1 decrement within a block. However, at the same time the P2 response showed the opposite effect with larger amplitude in the repeated block compared to the first block. The amount of P2 increase was on the order of 12%. The mean responses obtained in the first and second session are shown in Figure 8c. The N1 peak slightly increases by about 4% between the first and second session. More dominant is the effect of about 25% larger P2 amplitudes in the second session.
Figure 9.
Within and between session effects on the N1 and P2 amplitudes, a: Grand averaged waveforms across all stimulus conditions and sessions separately for quartiles within a block. The N1 amplitude decreases within a block of 7 min whereas the effect on the P2 is smaller. b: Grand averages separately for the first and second repeated run within a session. The N1 amplitude decreases whereas the P2 amplitude increases. c: Grand averages for both sessions on different days. The P2 increase from first to second session is dominant.
P1 amplitude
Figure 9 demonstrates also that in contrast to the observed changes over time in the N1 and P2 amplitudes the P1 amplitude changes were small.. P1 amplitudes were measured from the waveforms based on the N1 source model because modeling the P1 source was not feasible in all subjects, The stimulus type had a main effect on the P1 amplitude (F(2,34)=8.08, p=0.001) with larger amplitude for the speech sounds /ba/ (9.3 nAm) and /mba/ (9.0 nAm) compared to the noise stimulus (5.8 nAm). P1 amplitude increased with age (F(2,35)=3.44, p=0.044) with 5.9 nAm in the young, 7.2 nAm in the middle-aged and 10.9 nAm in the older group, which was a significant increase in P1 between the young and older group (p=0.05). No P1 change during a block was observed, however, P1 increased slightly between blocks within a session from 7.9 to 8.7 nAm (F(1,35)=16.5, p<0.001), which is visible in the waveforms shown in Figure 9b. No P1 change between sessions and no interactions between factors were significant.
P2 increase vs. time interval between sessions
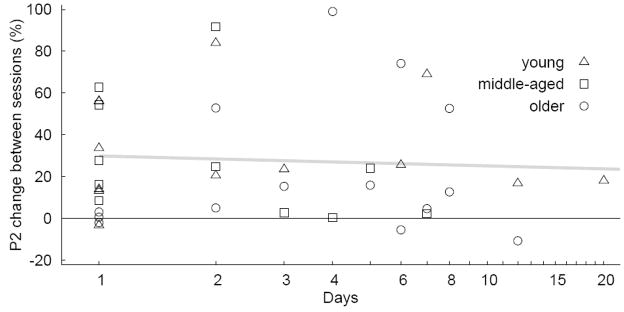
The number of days between first and second MEG session was not strictly controlled, which could have affected the measure of P2 difference between sessions. Figure 10 shows the relative P2 change versus the number of days between sessions. The between subject variability for the relative P2 change is quite large and the number of subjects with long time interval between sessions was small. Thus, an indication whether the size of P2 increase declined with progressing time is not conclusive from the current data. The linear regression did not show a significant slope.
Figure 10.
Relative change in P2 amplitude between first and second session as function of the number of days between sessions. The gray line indicates a linear regression to the data.
Discussion
Multiple dissociations between N1 and P2 waves of the averaged auditory evoked response were found in this study. Locations of underlying sources were different, and peak amplitudes of both components were modified differently while the subjects passively listened to the sound stimuli. Considerably diverse time courses of changes in N1 and P2 amplitudes imply specific functional meanings of the responses. The wide age range of participants allows of discussing effects of age on these observations.
Source localization
The finding of more anterior located P2 source compared to the N1 is consistent with previous MEG studies (Hari et al., 1987; Pantev et al., 1996a; Pantev et al., 1996b; Rif et al., 1991; Sams et al., 1985). Despite those converging reports of successfully explaining the P2 response with a pair of single dipoles, multiple P2 sources had been suggested in planum temporale and in Brodmann area 22, and auditory association cortices (Crowley and Colrain, 2004). In the current study, P2 source coordinates were anterior to the sulcus posterior to the first transverse gyrus of Heschl and the N1 posterior to this sulcus according to the anatomical characterization of human auditory cortex given by Leonard et al. (1998). N1 sources in the planum temporale and P2 sources in lateral Heschl’s gyrus have been reported from repeated MEG recordings in a single subject (Lutkenhoner and Steinstrater, 1998). However, those results should be interpreted with caution because single dipole solutions represent centers of activity and reasonable explanations for the underlying neural activation may consist of dipoles moving in space over time (Rogers et al., 1990) or multiple dipoles at fixed location in close vicinity which are activated with different time courses (Zouridakis et al., 1998). The latter model separated locations for N1 and P2 and demonstrated different time-courses of activation. Likely the N1 is generated in Heschl’s gyrus and planum temporale as it has been found in intra-cerebral recordings and MEG in same subjects (Godey et al., 2001). The finding that any type of acoustical onset or change elicits an N1 response (Hyde, 1997; Naatanen and Picton, 1987) and causes strong metabolic changes in planum temporale as seen in PET studies (Binder et al., 1996; Johnsrude et al., 1997) had been taken as evidences that the N1 mainly origins from the planum temporale (Engelien et al., 2000; Lutkenhoner and Steinstrater, 1998). Although distances between N1 and P2 sources were small, the difference in orientation may account for the observation of larger P2 responses in EEG at more frontal midline electrodes (Fz) than Cz, where the largest N1 amplitude has been observed (Sheehan et al., 2005).
Despite different locations and orientations of N1 and P2 sources it is common practice to display and discuss the waveforms obtained with a single source model. Implicitly this is done also when observing the waveform from a single electrode in EEG or a single sensor in MEG, which both are just other examples of spatial filters to the data. The current results showed that the N1 source amplitudes were underestimated when using the P2 model and vice versa. Thus, a practical implication is recommending the use of the correct model accordingly when comparing different AEF components.
Suppression of N1 response
Refractoriness, the reduced excitability of neural resources immediately after a response occurred, accounts for amplitude reduction of responses to repeatedly presented stimuli and this effect is typically settled within less than 10 to 20 s. Response habituation is the progressive decline in response strength with repetitions of same stimuli. Habituation of the N1 may occur with same time constants as refractoriness, which makes it difficult to differentiate between both. Dissociation between the mechanisms had been proposed because habituation should involve dishabituation, an enhanced response to previously habituated stimulus after presenting a deviant stimulus. However, this had not been shown convincingly for the N1 response (Budd et al., 1998) and it seems that sometimes the phenomenon of fast response decrement has been termed short-term habituation, even though the underlying neural mechanism is more likely refractoriness. Larger effects of short-term habituation has been reported for speech sounds compared to pure tones by Woods and Elmasian (1986), who also reported effects of a long-term amplitude decrement as N1 amplitude reduction continuing over the time course of 5 min of stimulation. The exact nature of short-term stimulus specific response reduction is not fully understood, however, fast plastic reorganizations of lateral inhibitory connections are thought to be a primary neurophysiological mechanism (Jaaskelainen et al., 2007). Long-term habituation has been described as continuing decline of the response over the time course of the experiment (Picton et al., 1976) and likely explains the decrement in N1 over several minutes (Naatanen and Picton, 1987). Consistently with previous reports (Picton et al., 1976), the N1 response reported in this study did not show dishabituation after long-term habituation and continued to decrease between blocks within a session. One explanation may be that the /ba/ and /mba/ sounds might have been not different enough to dishabituate the N1 response between blocks. In contrast, blocks of noise stimuli were always preceded by blocks of speech stimuli, which potentially could have had induced dishabituation. However, the ANOVA did not reveal an interaction between blocks and stimulus type, and no indication was given, that N1 decrement did not continue between the blocks of noise stimuli. Thus, consistently across stimulus types and age groups, the N1 amplitude decreased progressively over the time course of an experimental session. One neurophysiological explanation for a response decrement over the time course of several minutes had been suggested by Picton et al. (1976) as depletion of presynaptic neurotransmitters. Presumably different stores of neurotransmitters with different rates of depletion exist, which could explain fast and slow time courses of response decrement. Between sessions, the N1 amplitudes recovered from the decrement and were almost identical to the begining of both sessions. The only exception from this pattern was an initially reduced N1 response in the group of older participants. Because the smallest time difference between MEG recording sessions was one day, we did not gain information whether the N1 recovery required sleep over night or just hours of rest from repeated stimulation.
Within and between session effects on the P2 response
Despite N1 decrement, P2 amplitudes remained constant within an experimental block of 7.25 min in duration. Although it has been suggested that the P2 is not affected by habituation (Kenemans et al., 1989), little is known about the effect of response decrement on the P2 wave mainly because early ERP studies often treated the N1-P2 wave as a single entity and measure the difference amplitude between N1 and P2 peaks (i.e., peak-to-peak amplitude). One distinction between N1 and P2 came from sleep studies, which showed strong N1 decreases but P2 increases during early sleep stages (Crowley and Colrain, 2004; Naatanen and Picton, 1987). Although, N1 and P2 amplitude changes in those studies were related to sleep, the finding of different dynamics of N1 and P2 amplitudes is consistent. Thus, our current findings support the hypothesis that N1 and P2 are functionally different responses. Different effects of sleep and vigilance on N1 and P2 could suggest that changes in vigilance during the recording time could have been a factor. However, watching a movie of their own choice and verbal interaction with the experimenter after every recording block ensured that the subjects did not fall asleep. The possibility of multiple superimposed effects with opposite direction resulting in the net effect of constant P2 amplitude over the duration of a block cannot be excluded. Nevertheless, P2 increase was dominant and in contrast to not changing during a block, P2 amplitude increased between the first and the second block within a session (Figure 7c). Although the increase between blocks was larger in the first than second session (Figure 5) the session x block interaction was not significant. A similar tendency for P2 increase between blocks of the first session is indicated in Figure 2 of a paper by Sheehan et al. (2005), who did not report significance for this effect.
In contrast to the N1 changes observed within a session, which were reversible between sessions, two changes in the P2 responses continued for several days following the first session: P2 amplitudes increased between sessions and a smaller increase was observed during the second compared to the first session. Because these changes continued, the P2 dynamics can be described as plastic and likely represents neural reorganization. However, despite the current information, the functional meaning the P2 responses is still poorly understood. Whereas the N1 response seems to reflect multiple processes of signaling an unspecific change in the auditory environment, P2 has been said to relate to analyzing acoustical features and forming an auditory memory (Naatanen and Picton, 1987). Some speculation has been made about the positive AEP/AEF wave in the 200–250 ms latency range as being related to stimulus identification (Crowley and Colrain, 2004), that means at least partially the P2 response would reflect the outcome of comparison between the incoming stimuli with a memory. The observed P2 increase could result from a stronger memory of the stimulus. Naatanen et al. (2001) interpreted the gradually over time developing MMN response, which paralleled the subject’s improved performance in a pitch discrimination task, as indication that the MMN represents a rather permanent memory trace, Such permanent auditory memory, for example, provides the template for fast speaker recognition. The observed P2 effect in our study likely reflects the same type of auditory memory, which builds up over the time course of the experiment and likely consolidates over night.
The interpretation of a sound experience induced permanent auditory memory raises the possibility that the intervening behavioral task following session one might have contributed to the enhanced P2 seen during session two. However, the behavioral task involved the identification of the speech stimuli only. Thus, this alternative explanation would have been supported if the P2 enhancement had been specific for the speech stimuli. However, an interaction between ‘session’ and ‘stimulus type’ was not evident.
Multiple dissociations between N1 and P2 response
Distinct cortical sources of P2 responses anterior to N1 sources as observed in this study were the first indication for functional independence of both components. In a combined fMRI and MEG study Ahveninen et al. (2006) differentiated the functional meaning of anterior and posterior non-primary auditory cortices and suggested that analysis of the stimulus type especially for speech takes place in anterior-lateral Heschl’s gyrus, anterior the anterior superior temporal gyrus, and the planum polare in contrast to analysis of sound location in posterior auditory areas. Although, their study did not investigate differences in temporal dynamics of anterior and posterior non-primary auditory cortices, our finding of more anterior P2 sources support the hypothesis that the P2 response reflects evaluation of stimulus features.
Time courses of P2 and behavioural changes
Several studies have shown that the time course of change in physiological responses and behavioural performance are not always in line with each other, which complicates inferences about the relation between both measures. Between first and second session of MEG recording the subjects had at least one night of sleep and it could be speculated whether sleep was necessary to establish the P2 increase. The data provided by Atienza et al. (2002) support this speculation. They recorded pre-training responses at 9 am before performing pitch discrimination training. ERP recordings did not show P2 enhancement after the training as well as about 12 hours after the training. However, recordings at 9 am on the following day showed pronounced P2 increase, which continued at least for the next day. Although the underlying mechanism are not fully understand, consolidation of learning and memory over night assumes that a new memory is in fragile state until the first sleep occurred after exposure to the stimulus (Maquet, 2001). However, consolidation of performance in an auditory identification task had been shown during daytime without the requirement of sleep (Roth et al., 2005). Further research is necessary to clarify whether sleep is required for the P2 enhancement shown in this study.
Effects of age
N1 amplitudes increased significantly between young and middle-aged and between middle-aged and older participants. In contrast, the P2 was largest in the young and not different between middle-aged and older groups. These different characteristics are another indicator for the independence of N1 and P2 components. Although reports about N1 and P2 changes with advancing age are controversial likely because of large inter-subject variability and differences in experimental procedures between studies (Crowley and Colrain, 2004), there is evidence that N1 amplitude increases with aging. For example, N1 amplitude increase with age has been found in a study with amplitude modulated tonal stimuli (Ross et al., 2007), as well as using speech stimuli along a /ba/-/pa/ VOT continuum (Tremblay and Kraus, 2002; Tremblay et al., 2003b). Latency increases with increasing age of 10 ms for the N1 and 15 ms for the P2 were of similar size than found in a previous study (Ross et al., 2007), although the P2 latency effect did not reach statistical significance in this study.
One remarkable effect of age was that the P2 increase between sessions was largest in the young group, declined noticeably with increasing age, and this change commenced in mid life. The pattern of larger P2 increase during the first compared to second session did not change with increasing age, giving a hint that the amount of P2 increase but not the time course of plasticity changes with increasing age. This observation may indicate reduced amount of plasticity in the aging brain however the functional relevance of this finding needs further investigation.
The interactions between stimulus type and temporal effects on N1 and P2 in the group of older adults could possibly be attributed to normal but still elevated hearing thresholds at higher frequencies, which affects the stimuli differently because low frequency formants are dominant in the speech stimuli, whereas high frequency components contributed stronger to the noise stimulus.
Comparison with previous studies
In the present experiment, participants were tested and then retested. In this respect, the design is similar to that used to assess test re-test reliability of these same cortical responses. Numerous studies have shown good test-retest reliability for P1, N1 and P2 responses regardless of whether test re-test sessions take place within a day, week, or year (Escera and Grau, 1996; Kileny and Kripal, 1987; Kinoshita et al., 1996; Sandman and Patterson, 2000; Tremblay et al., 2003a; Uwer and von Suchodoletz, 2000; Williams et al., 2005). One could therefore argue that exposure to stimuli during one test session does not automatically affect the physiological representation of sound during a second test session. However, the majority of these studies did not analyze activity from the entire scalp, and did not use source analysis. Therefore, there might have been experience-related physiological changes that were not observed or reported.
There are also differences in the experimental procedure and data analysis that might explain why the reported P2 increases here were not seen in ERP data from test re-test studies. Many investigators used peak-to-peak amplitude, and not absolute amplitude, to define P2 amplitude. Also, the SOA in the current study was almost twice as long as others reported in the literature and different SOAs might have strongly effect the configuration of the evoked response. Then again, Sheehan and colleagues (2005) used an SOA that was almost half the length of the one used in the present study.
Implications for learning and training studies
Response decrement, as observed for the N1 response, and plastic neural reorganization, as suggested for the P2, are fundamental biological mechanism of learning and memory. Within this context, response suppression is an effective mechanism for avoiding unnecessary responses to repeated stimuli without new informational content. The different sound environment between sessions in our study likely diminished the response decrement completely as it was observed in the study. Because the P2 effect persisted between the sessions, we interpret our results as evidence that the P2 wave, more likely than N1, reflects physiological processes associated with learning and memory on a time scale similar to that of laboratory training. This interpretation is inline with reports of increased P2 amplitude during perceptual learning and training (Reinke et al., 2003; Trainor et al., 2003; Tremblay et al., 2001). The P2 enhancement in the current study was observed in participants who listened passively to the stimuli during AEF recordings, and were not required to complete a task. Their perceptual ability to identify the stimuli with different voice-onset time did not increase significantly. However, the absence of a behavioural effect should not be interpreted as absence of learning. With the lack of interaction between ‘session’ and ‘stimulus type’ effects, at last in the young group, we have no indication for a stimulus specific effect on the P2. More likely the subjects experienced the sound stimuli during MEG recording learned something general about the stimuli. Familiarization with the stimulus could be one explanation, and consolidation of long-term memory following the first MEG session, could account for the finding that P2 increases were observable at the beginning of the second session.
Learning through sensory experience is a valuable mechanism and, especially during development, has been demonstrated in the plasticity of cortical maps in animal experiments (Dahmen and King, 2007). Neurophysiological basis for sensory experience related plasticity in the adult cortex has been demonstrated for example by Trachtenberg et al. (2002), who showed in vivo expression and extinction of dendritic spines associated with synaptogenesis, as well as the elimination of synapses over various time courses including days to weeks. Certainly, the adult brain should show stability, which was expressed in their study as no change in the structure of dendritic branches over weeks. Further work is necessary to bridge the large gap between studies of neural plasticity at the single neuron level, and studying far-field brain activity using EEG or MEG in order to explain the neurobiological underpinning of experience related changes in the AEF. Nevertheless, it is a fascinating result of this study that gross measures like the N1 and P2 components of the auditory evoked response reflect neuroplastic changes and that they can be used to study physiological correlates of learning in humans over the lifetime.
Conclusions
Merely experiencing the sound stimuli during MEG recording resulted in a continuous decline in N1 amplitude within experimental sessions followed by a recovery between sessions. In contrast, P2 amplitude was relatively constant within a session but increased between first and second sessions taking place on different days. Both observations could be described as reflecting neural plasticity on different time scales. Sound experience induced increase of P2 continued to last for several days, which emphasizes the importance of the P2 wave of the neuromagnetic auditory response for studying effects of experience, memory, and learning. Neuroplastic modifications of P2 were observed in all age groups, demonstrating that brain functions are malleable throughout the lifespan. However, the effect size decreased with increasing age, indicating a reduced capacity for plastic reorganization in later life.
Acknowledgments
We are grateful for support by grants from the Canadian Institutes for Health Research (grant Nr. 81135) to BR and the National Institutes of Health (NIH NIDCD R01 DC007705) awarded to KT as well as the Virginia Merrill Bloedel Hearing Research Center traveling scholar program for KT. We like to thank Drs. Claude Alain and Terry Picton for helpful comments on a previous version of the manuscript.
Abbreviations
- AEF
auditory evoked field
- AEP
auditory evoked potential
- EEG
electroencephalography
- ERP
event related potential
- MEG
magnetoencephalography
- MMN
mismatch negativity
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SOA
stimulus onset asynchrony
- VOT
voice onset time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, Levanen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103:14608–13. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Snyder JS, He Y, Reinke KS. Changes in auditory cortex parallel rapid perceptual learning. Cereb Cortex. 2007;17:1074–84. doi: 10.1093/cercor/bhl018. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL, Dominguez-Marin E. The time course of neural changes underlying auditory perceptual learning. Learn Mem. 2002;9:138–50. doi: 10.1101/lm.46502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardouille T, Picton TW, Ross B. Correlates of eye blinking as determined by synthetic aperture magnetometry. Clin Neurophysiol. 2006;117:952–8. doi: 10.1016/j.clinph.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119 (Pt 4):1239–47. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Budd TW, Barry RJ, Gordon E, Rennie C, Michie PT. Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int J Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115:732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol. 2007;17:456–64. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Engelien A, Schulz M, Ross B, Arolt V, Pantev C. A combined functional in vivo measure for primary and secondary auditory cortices. Hear Res. 2000;148:153–60. doi: 10.1016/s0378-5955(00)00148-9. [DOI] [PubMed] [Google Scholar]
- Escera C, Grau C. Short-term replicability of the mismatch negativity. Electroencephalogr Clin Neurophysiol. 1996;100:549–54. doi: 10.1016/s0168-5597(96)95633-6. [DOI] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112:1850–9. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Hari R, Pelizzone M, Makela JP, Hallstrom J, Leinonen L, Lounasmaa OV. Neuromagnetic responses of the human auditory cortex to on- and offsets of noise bursts. Audiology. 1987;26:31–43. doi: 10.3109/00206098709078405. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Wollbrink A, Chau W, Ishii R, Ross B, Pantev C. Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. Neuroimage. 2003;20:995–1005. doi: 10.1016/S1053-8119(03)00403-8. [DOI] [PubMed] [Google Scholar]
- Hyde M. The N1 response and its applications. Audiol Neurootol. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen IP, Ahveninen J, Belliveau JW, Raij T, Sams M. Short-term plasticity in auditory cognition. Trends Neurosci. 2007;30:653–61. doi: 10.1016/j.tins.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Zatorre RJ, Milner BA, Evans AC. Left-hemisphere specialization for the processing of acoustic transients. Neuroreport. 1997;8:1761–5. doi: 10.1097/00001756-199705060-00038. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Verbaten MN, Roelofs JW, Slangen JL. “Initial-” and “change-orienting reactions”: an analysis based on visual single-trial event-related potentials”. Biol Psychol. 1989;28:199–226. doi: 10.1016/0301-0511(89)90001-x. [DOI] [PubMed] [Google Scholar]
- Kileny PR, Kripal JP. Test-retest variability of auditory event-related potentials. Ear Hear. 1987;8:110–4. doi: 10.1097/00003446-198704000-00008. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Inoue M, Maeda H, Nakamura J, Morita K. Long-term patterns of change in ERPs across repeated measurements. Physiol Behav. 1996;60:1087–92. doi: 10.1016/0031-9384(96)00130-8. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Kanda S, Hirata Y. Effects of musical experience on different components of MEG responses elicited by sequential piano-tones and chords. J Neurosci. 2006;26:4046–53. doi: 10.1523/JNEUROSCI.3907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerlund TD, Sharbrough FW, Busacker NE. Spatial filtering of multichannel electroencephalographic recordings through principal component analysis by singular value decomposition. J Clin Neurophysiol. 1997;14:73–82. doi: 10.1097/00004691-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb Cortex. 1998;8:397–406. doi: 10.1093/cercor/8.5.397. [DOI] [PubMed] [Google Scholar]
- Lomber S, Eggermont JJ, editors. Reprogramming the Cerebral Cortex: Plasticity Following Central and Peripheral Lesions. Oxford University Press; Oxford, UK: 2006. [Google Scholar]
- Lopez L, Jurgens R, Diekmann V, Becker W, Ried S, Grozinger B, Erne SN. Musicians versus nonmusicians. A neurophysiological approach. Ann N Y Acad Sci. 2003;999:124–30. doi: 10.1196/annals.1284.013. [DOI] [PubMed] [Google Scholar]
- Lutkenhoner B, Steinstrater O. High-precision neuromagnetic study of the functional organization of the human auditory cortex. Audiol Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide (2nd ed.) 2. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- McClaskey CL, Pisoni DB, Carrell TD. Transfer of training of a new linguistic contrast in voicing. Percept Psychophys. 1983;34:323–30. doi: 10.3758/bf03203044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends Neurosci. 2001;24:283–8. doi: 10.1016/s0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- Pantev C, Eulitz C, Hampson S, Ross B, Roberts LE. The auditory evoked “off” response: sources and comparison with the “on” and the “sustained” responses. Ear Hear. 1996a;17:255–65. doi: 10.1097/00003446-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses. Hear Res. 1996b;101:62–74. doi: 10.1016/s0378-5955(96)00133-5. [DOI] [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392:811–4. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- Pantev C, Engelien A, Candia V, Elbert T. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001a;930:300–14. [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Schulz M, Engelien A, Ross B. Timbre-specific enhancement of auditory cortical representations in musicians. Neuroreport. 2001b;12:169–74. doi: 10.1097/00001756-200101220-00041. [DOI] [PubMed] [Google Scholar]
- Pantev C, Ross B, Fujioka T, Trainor LJ, Schulte M, Schulz M. Music and learning-induced cortical plasticity. Ann N Y Acad Sci. 2003;999:438–50. doi: 10.1196/annals.1284.054. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA, Galambos R. Habituation and attention in the auditory system. In: Keidel WD, Neff WD, editors. Handbook of sensory physiology. Vol. 3. Springer; Berlin: 1976. pp. 343–389. [Google Scholar]
- Reinke KS, He Y, Wang C, Alain C. Perceptual learning modulates sensory evoked response during vowel segregation. Brain Res Cogn Brain Res. 2003;17:781–91. doi: 10.1016/s0926-6410(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Rif J, Hari R, Hamalainen MS, Sams M. Auditory attention affects two different areas in the human supratemporal cortex. Electroencephalogr Clin Neurophysiol. 1991;79:464–72. doi: 10.1016/0013-4694(91)90166-2. [DOI] [PubMed] [Google Scholar]
- Ritter W, Vaughan HG, Jr, Costa LD. Orienting and habituation to auditory stimuli: a study of short term changes in average evoked responses. Electroencephalogr Clin Neurophysiol. 1968;25:550–6. doi: 10.1016/0013-4694(68)90234-4. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Papanicolaou AC, Baumann SB, Saydjari C, Eisenberg HM. Neuromagnetic evidence of a dynamic excitation pattern generating the N100 auditory response. Electroencephalogr Clin Neurophysiol. 1990;77:237–40. doi: 10.1016/0168-5597(90)90043-d. [DOI] [PubMed] [Google Scholar]
- Ross B, Borgmann C, Draganova R, Roberts LE, Pantev C. A high-precision magnetoencephalographic study of human auditory steady- state responses to amplitude-modulated tones. J Acoust Soc Am. 2000;108:679–91. doi: 10.1121/1.429600. [DOI] [PubMed] [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27:11172–8. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DA, Kishon-Rabin L, Hildesheimer M, Karni A. A latent consolidation phase in auditory identification learning: time in the awake state is sufficient. Learn Mem. 2005;12:159–64. doi: 10.1101/87505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams M, Hamalainen M, Antervo A, Kaukoranta E, Reinikainen K, Hari R. Cerebral neuromagnetic responses evoked by short auditory stimuli. Electroencephalogr Clin Neurophysiol. 1985;61:254–66. doi: 10.1016/0013-4694(85)91092-2. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Patterson JV. The auditory event-related potential is a stable and reliable measure in elderly subjects over a 3 year period. Clin Neurophysiol. 2000;111:1427–37. doi: 10.1016/s1388-2457(00)00320-5. [DOI] [PubMed] [Google Scholar]
- Shahin A, Bosnyak DJ, Trainor LJ, Roberts LE. Enhancement of neuroplastic P2 and N1c auditory evoked potentials in musicians. J Neurosci. 2003;23:5545–52. doi: 10.1523/JNEUROSCI.23-13-05545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin A, Roberts LE, Pantev C, Trainor LJ, Ross B. Modulation of P2 auditory-evoked responses by the spectral complexity of musical sounds. Neuroreport. 2005;16:1781–5. doi: 10.1097/01.wnr.0000185017.29316.63. [DOI] [PubMed] [Google Scholar]
- Sheehan KA, McArthur GM, Bishop DV. Is discrimination training necessary to cause changes in the P2 auditory event-related brain potential to speech sounds? Brain Res Cogn Brain Res. 2005;25:547–53. doi: 10.1016/j.cogbrainres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O. Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol. 1995;95:189–200. doi: 10.1016/0013-4694(95)00064-6. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–94. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Shahin A, Roberts LE. Effects of musical training on the auditory cortex in children. Ann N Y Acad Sci. 2003;999:506–13. doi: 10.1196/annals.1284.061. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, Carrell TD, McGee T. Central auditory system plasticity: generalization to novel stimuli following listening training. J Acoust Soc Am. 1997;102:3762–73. doi: 10.1121/1.420139. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, McGee T. The time course of auditory perceptual learning: neurophysiological changes during speech-sound training. Neuroreport. 1998;9:3557–60. doi: 10.1097/00001756-199811160-00003. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, McGee T, Ponton C, Otis B. Central auditory plasticity: changes in the N1-P2 complex after speech-sound training. Ear Hear. 2001;22:79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N. Auditory training induces asymmetrical changes in cortical neural activity. J Speech Lang Hear Res. 2002;45:564–72. doi: 10.1044/1092-4388(2002/045). [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Friesen L, Martin BA, Wright R. Test-retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear Hear. 2003a;24:225–32. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003b;114:1332–43. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Uwer R, von Suchodoletz W. Stability of mismatch negativities in children. Clin Neurophysiol. 2000;111:45–52. doi: 10.1016/s1388-2457(99)00204-7. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–71. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115:1605–30. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- Woods DL, Elmasian R. The habituation of event-related potentials to speech sounds and tones. Electroencephalogr Clin Neurophysiol. 1986;65:447–59. doi: 10.1016/0168-5597(86)90024-9. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Simos PG, Papanicolaou AC. Multiple bilaterally asymmetric cortical sources account for the auditory N1m component. Brain Topogr. 1998;10:183–9. doi: 10.1023/a:1022246825461. [DOI] [PubMed] [Google Scholar]