Abstract
Mutations in ligand-gated ion channel genes associated with idiopathic generalized epilepsies have been reported in excitatory acetylcholine receptor α4 and β2 subunit genes linked to autosomal dominant nocturnal frontal lobe epilepsy and in inhibitory GABAA receptor α1, β3, γ2, and δ subunit genes associated with childhood absence epilepsy, juvenile myoclonic epilepsy, pure febrile seizures, generalized epilepsy with febrile seizures plus, and generalized epilepsy with tonic–clonic seizures. Recent studies suggest that these mutations alter receptor function or biogenesis, including impaired receptor subunit messenger RNA stability, receptor subunit protein folding and stability, receptor assembly, and receptor trafficking.
Idiopathic generalized epilepsies (IGEs) presumably are a group of genetic epilepsies that exhibit several clinical phenotypes, including childhood absence epilepsy, juvenile myoclonic epilepsy, generalized epilepsy with febrile seizures plus (GEFS+) and tonic–clonic seizures (1), and are among the most common neurological disorders, affecting about 1% of the population worldwide (2). Genetic advances and functional characterization of the pathophysiological alterations in receptor channel trafficking and function are enhancing the understanding of the cellular pathophysiology of IGEs. It has become clear that transmembrane ion channel mutations are the underlying cause of many IGEs (3). Mutations in voltage-gated ion channels (sodium, calcium, and potassium) and ligand-gated ion channels (nicotinic cholinergic receptor and GABAA receptor) have been reported to be associated with IGEs. This article will review the molecular pathology of IGEs caused by mutations in one of the ligand-gated ion channels, the GABAA receptor, which is the primary mediator of fast inhibitory synaptic transmission in the CNS.
Mutations in GABAA Receptor Subunit Genes
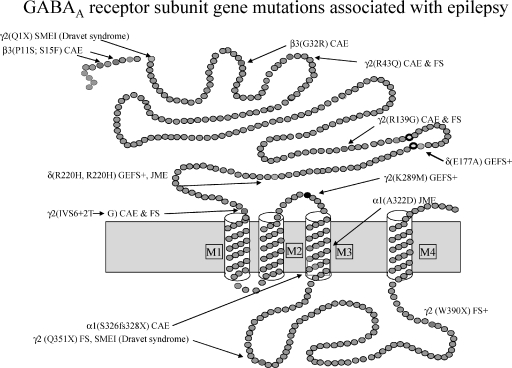
GABAA receptors are members of the cys-loop family of ligand-gated ion channels, which also includes nicotinic cholinergic, serotonin 5-HT3, and glycine receptors. GABAA receptors are formed by pentameric assemblies of different subunit subtypes (α1-α6, β1-β3, γ1-γ3, δ, ɛ, π, θ, and ρ1-ρ3), and form chloride ion selective channels (4). The majority of GABAA receptors contain two α subunits, two β subunits, and a γ or δ subunit (5). GABAA receptors mediate both phasic inhibitory synaptic transmission and tonic perisynaptic inhibition, and the mechanism of action of several antiepileptic drugs is the enhancement of GABAA receptor currents. GABAA receptor mutations or variants associated with IGEs have been reported in α1, β3, γ2, and δ subunits (see Figure 1) (6). Mutations in subunit genes can be divided broadly into four classes: 1) missense, 2) nonsense, and 3) frameshift mutations in coding sequences as well as 4) mutations in noncoding sequences (intronic, 3′ downstream, or 5′ upstream mutations).
FIGURE 1.
Schematic representation of the GABAA receptor subunit topology, showing the location of autosomal dominant epilepsy mutations associated with different idiopathic generalized epilepsies. SMEI, myoclonic epilepsy of infancy; CAE, childhood absence epilepsy; GEFS+, generalized epilepsy with febrile seizures plus; JME, juvenile myoclonic epilepsy; FS, febrile seizures.
Missense mutations alter the nucleotide sequence in a codon, resulting in the incorporation of a different amino acid in the mature peptide or the signal peptide and altering the triplet genetic code. The altered amino acid can result in a benign polymorphism, a variant that can confer susceptibility to a disease, or a mutation that is associated with a disease. In general, GABAA receptor subunit missense mutations impair surface expression of receptors and/or impair receptor channel kinetic properties (6). Reduced surface expression of receptors harboring missense mutations is due to multiple mechanisms, such as altered folding, impaired assembly, and endoplasmic reticulum retention, often leading to loss of the mutant proteins by endoplasmic reticulum-associated degradation. GABAA receptor missense mutations in mature subunits include: GABRA1(A322D), GABRB3(G32R), GABRG2(R43Q), GABRG2(R139G), and GABRG2(K289M); GABAA receptor subunit missense mutations in signal peptides include: GABRB3(P11S) and GABRB3(S15F); and GABAA receptor subunit missense mutations producing susceptibility variants include: GABRD(E177A) and GABRD(R220H).
Nonsense mutations produce alteration of the nucleotide sequence in codons that result in the introduction of a stop codon or premature translation–termination codon. Premature translation–termination codon-generating mutations in the last exon of a multi-exon gene or less than 50–55 nucleotides upstream of the last exon–exon junction produce a truncated protein, while premature translation–termination codon-generating mutations that are not in the last exon of a multi-exon gene or are more than 50–55 nucleotides upstream of the last exon–exon junction produce mRNA degradation through activation of nonsense-mediated decay (NMD)—a cellular mRNA quality-control system that eliminates production of the mutant protein (7). GABAA receptor nonsense mutations include: GABRG2(Q1X), GABRG2(Q351X), and GABRG2(W390X).
Frameshift mutations occur because the deletion or insertion of one or two nucleotides causes a change in downstream codons, with or without a change in the frameshifted codon. In addition to altering the downstream amino acid sequence, these frameshifts often result in production of a premature translation–termination codon. One GABAA receptor subunit frameshift mutation, GABRA1(975delC, S326fs328X), has been reported. Mutations can also occur in noncoding sequences, such as in introns, or either upstream or downstream of the GABAA receptor open reading frame. A GABAA receptor subunit mutation in a noncoding intron splice donor site GABRG2(IVS6 + 2T→G) has been described.
GABAA Receptor Subunit Missense Mutations
γ2 subunit missense mutation, R43Q
The GABAA receptor γ2 subunit mutation, R43Q, is located in the distal N-terminus of the γ2 subunit and is associated with childhood absence epilepsy and febrile seizures (see Figure 1) (8). The functional and cellular consequences of this mutation have been shown in vitro and in vivo due to reduction of surface γ2 subunit protein with heterozygous expression (9). Investigators have demonstrated that expression in HEK 293-T cells of heterozygous α1β2γ2/γ2(R43Q) or homozygous α1β2γ2(R43Q) receptors resulted in reduced peak current and surface receptors (9,10). In vivo, in mice engineered with the mutation γ2(R43Q), a small but significant reduction of GABAA receptors in cortical pyramidal neurons also was observed (11). Using fusion proteins, in which DNA-encoded enhanced yellow fluorescent protein was inserted between amino acids four and five of the mature γ2 subunit and fluorescence microscopy, Tan et al. demonstrated that the reduced surface expression of the mutant protein resulted from endoplasmic retention of the mutant protein. It has been shown that the mutation impairs subunit oligomerization (12). Thus, the reduced surface expression of heterozygous α1β2γ2S(R43Q) receptors was a consequence of receptor endoplasmic retention, secondary to impaired subunit folding or assembly and/or to impaired receptor trafficking.
γ2 subunit missense mutation, K289M
The GABAA receptor γ2 subunit mutation, K289M, is located in the short extracellular loop between transmembrane domains M2 and M3 (M2–M3 loop) (see Figure 1), a region implicated in the gating of ligand-gated ion channels and associated with an autosomal dominant generalized epilepsy, a condition similar to GEFS+ (13). The effects of this mutation were investigated using a rapid application concentration jump technique (open tip application rise time of 1 msec), applying GABA for brief (2–5 msec) durations, and the excised outside-out patch clamp recording technique (14); α1β3γ2L(K289M) receptors had more rapid current deactivation than wild-type receptors. Single channel currents from homozygous α1β3γ2(K289M) receptors had mean open times that were one-fourth as long as wild-type α1β3γ2L receptor currents, consistent with its more rapid whole cell current deactivation time (10). Brief, rapid GABA applications to excised macropatches evoked currents that were similar to IPSCs. Therefore, reduction of the duration of rapid GABA-evoked current by the γ2L(K289M) subunit mutation suggests that it would result in reduced IPSC duration, thus producing disinhibition that may lead to epilepsy.
γ2 subunit missense mutation, R139G
The GABAA receptor γ2 subunit mutation, R139G, is in the N-terminus of the γ2 subunit and is associated only with febrile seizures (15). The R139 residue is conserved among γ2 subunits across species, and basic residues are conserved among other γ subunits. Within the cys-loop family, polar and charged amino acid residues occur at this position. Residue R139 is located at a highly conserved and likely aqueous accessible part of the amino terminus in a hairpin loop between β sheets 5 and 6. It has been demonstrated that mutant α1β3γ2L(R139G) receptors had subtly altered current kinetics and reduced benzodiazepine sensitivity. Future study of the mutant protein maturation and receptor trafficking will further elucidate the molecular defect of this mutation in epilepsy.
α1 subunit missense mutation, A322D
The GABAA receptor α1 subunit mutation (A322D) introduces a negatively charged aspartate into the middle of the M3 transmembrane helix of the α1 subunit at residue A322 and is associated with autosomal dominant juvenile myoclonic epilepsy (16). Cossette and colleagues found that when co-expressed with wild-type β2 and γ2 subunits, the mutant α1(A322D) subunit reduced both total and surface α1 subunit levels and had an intermediate effect on heterozygous subunit expression (α1:α1(A322D – 1:1 cDNA ratio) (17). Peak GABA-evoked currents were significantly reduced in both heterozygous and homozygous conditions. Gallagher et al. demonstrated that this nonconserved mutation in a transmembrane domain destabilized M3 α helix formation and impaired α1 subunit folding and pentamer assembly (18). The loss of the misfolded mutant protein was due to an endoplasmic retention quality-control process, endoplasmic retention-associated degradation (19), and lysosomal degradation (20).
β3 subunit missense mutations, P11S, S15F, and G32R
Two mutations (P11S, S15F) in the GABAA receptor β3 subunit signal peptide in exon 1a and a mutation (G32R) in the mature β3 subunit of exon 2 have been associated with childhood absence epilepsy (21). The underlying molecular defect might be abnormal protein expression and/or impaired receptor trafficking, but the mechanism is still under study. Heterozygous and homozygous null mutant GABRB3 mice show absence-like features, including the EEG characteristics and pharmacological responses to antiepileptic drugs that are typical for absence seizures. GABRB3 deletion also has been associated with Angelman syndrome in which absence seizures are present. In addition, a GABRB3 promoter haplotype impairing transcriptional activity has been associated with childhood absence epilepsy. Thus, mutation of the GABRB3 subunit likely contributes to generation of childhood absence epilepsy, but this involvement needs to be clarified further.
δ Subunit Missense Variants, E177A, R220C, and R220H
Monogenic mutations only account for the pathogenesis of a small portion of IGE syndromes. Most of the idiopathic epilepsies are polygenic, requiring additive actions of a set of susceptibility genes. Dibbens et al. reported the first GABAA receptor susceptibility gene, GABRD, for IGEs (22). Two putative missense mutations in GABRD were identified: δ(E177A) was detected in a small GEFS+ family and δ(R220C) was detected in a second small GEFS+ family. Both mutations were heterozygously associated with epilepsy in these pedigrees. In addition, a polymorphic allele, δ(R220H), has been associated with juvenile myoclonic epilepsy patients but also is found in the general population.
The δ subunit variant, E177A, is adjacent to one of the invariant cysteines that form a disulfide bond—the signature feature of cys-loop receptors. The δ(R220) residue is localized about in the middle, between the δ(E177A) variant and the entrance to the first transmembrane domain (M1). The current amplitudes of heterozygous or homozygous receptors harboring either δ(E177A) or δ(R220H) subunits were significantly reduced compared to those of wild-type receptors (23). The current amplitudes of heterozygous or homozygous α1β2δ(R220C) receptors were not significantly different from those of wild-type receptors, but the finding requires further investigation. Combining both single channel recording and biochemistry data, the basis for the reduced peak current of these δ variant harboring receptors was determined to be primarily due to the reduced mean channel open time and a small reduction in the surface receptor expression (23).
GABAA Receptor Subunit Nonsense Mutations
γ2 subunit nonsense mutation, Q1X
A GABAA receptor γ2 subunit mutation that introduced a premature translation–termination codon, Q1X, between the signal peptide and mature peptide was identified in a family with severe myoclonic epilepsy of infancy (24). The γ2 subunit Q1X mutation likely triggers NMD, although activation of NMD has not been demonstrated. However, if the Q1X mutation resulted in NMD completely and if the mutant γ2S subunit does not interfere with transcription of the wild-type γ2S subunit at the mRNA level, no epilepsy would result (as observed in γ2 [+/–] gene deletion heterozygous mice). It is also unclear why, in the pedigree, both the twins carrying the mutation were diagnosed with myoclonic epilepsy of infancy, while the father with the de novo mutation was seizure-free. Future studies focusing on gene expression and the completeness of NMD may elucidate the underlying molecular pathology.
γ2 subunit nonsense mutation, Q351X
A GABAA receptor γ2 subunit mutation, Q351X, is associated with a family with GEFS+, including two family members with febrile seizures and a member with severe myoclonic epilepsy of infancy (25). The Q351X mutation is located in the γ2 subunit intracellular loop between transmembrane domains M3 and M4 (see Figure 1) and results in a premature translation–termination codon with loss of the downstream 78 amino acids. This mutation was studied by using green fluorescent protein-tagged γ2 subunits (25). The receptor containing this mutation was not expressed on the surface, but was retained in the endoplasmic reticulum. Consistent with this finding, no GABA-evoked current was recorded from oocytes expressing the mutant receptors. Genetic mutations producing premature translation–termination codons can result in C-terminally truncated proteins that can produce dominant negative inhibition of full-length proteins, thus potentially harming the cells. It is also intriguing that there is a great intrafamilial phenotype variation with this mutation; yet, the basis for this variation is unknown.
γ2 subunit nonsense mutation, W390X
A GABAA receptor γ2 subunit mutation, W390X, is associated with a family with GEFS+, with the majority of family members having febrile seizures plus and one member having febrile seizures (26). The W390X mutation is located in the γ2 subunit intracellular loop between transmembrane domains M3 and M4 (see Figure 1) and results in a premature translation–termination codon with loss of the downstream 39 amino acids. Identification of this mutation was published recently, and the underlying molecular mechanisms are still unknown.
GABAA Receptor Subunit Frameshift Mutations
α1 subunit mutation, 975delC, S326fs328X
The GABAA receptor α1subunit mutation, 975delC, S326fs328X, is an autosomal dominant mutation associated with childhood absence epilepsy (27), and thus, the patients are heterozygous. The mutation causes frameshift in GABRA1 that results in a premature translation–termination codon in an early exon, exon 8, and is 84 base pairs upstream of intron 8. Based on the 50–55 nucleotides rule, this frameshift mutation is likely to trigger NMD. Using an intron-inclusion minigene (MG) approach, which elicits NMD should it occur, it has been shown that heterozygous expression of α1MG/α1(975delC, S326fs328X)MGβ2γ2S subunits were found in HEK 293-T cells and resulted in functional haploinsufficiency. Using real-time polymerase chain reaction, mutant mRNA was demonstrated to be substantially reduced with concomitant loss of the mutant transcripts. Only a minimal amount of mutant α1(975delC, S326fs328X) subunit protein was detected, and the loss of the mutant protein likely was due primarily to the activation of NMD.
GABAA Receptor Subunit Splice Donor Site Mutations
γ2 subunit splice donor site mutation, IVS6 + 2T→G
The GABAA receptor γ2 subunit mutation, IVS6 + 2T→G, is the first mutation identified in a noncoding region of a GABAA receptor gene. The point mutation is in the splice-donor site in the GABRG2 intron 6 (IVS6 + 2T→G) and has been identified in a family with childhood absence epilepsy and febrile seizures (see Figure 1) (28). The effect of this mutation on GABAA receptor function is unknown but was predicted to lead to a nonfunctional protein through exon skipping, which would result in a new premature translation–termination codon at the 5th and 7th exon junction site. Thus, it is very likely that this premature translation–termination codon may also trigger NMD, thus eliminating expression of mutant protein at the mRNA level. Therefore, the underlying mechanism for this splice donor site mutation also may be due to haploinsufficiency. In fact, with the NMD-sensitive minigene approach, it was shown that the mutant γ2(IVS6 + 2T→G) subunit minigene only produced a minimal amount of the mutant protein compared with the wild-type γ2 minigene, suggesting that the mutation may activate NMD. Ongoing research at the transcription level will further elucidate the underlying molecular mechanisms.
Conclusions
Despite substantial advances in the understanding of the molecular pathogenesis of various GABAA receptor epilepsy mutations, there are still many unanswered questions about the pathology of IGEs. It has been difficult to develop an appropriate experimental model that adequately reproduces the functional alterations evidenced by the mutations in these patients. The majority of the studies of the pathophysiology of IGEs have been obtained using heterologous expression systems. Available hemizygous subunit gene deletion mice may reflect the function of some gene mutations, but gene knockout may be different from a loss-of-function mutation. In addition, most of the mutations are not fully penetrant, and there is a great intrafamilial phenotypic variation, suggesting that there are other modifying genes in the background that influence the development and persistence of the epilepsy, but this possibility needs to be clarified. Another unknown variable is the cellular response to the presence of mutant protein. Cells that are overloaded with misfolded proteins are under stress and activate the unfolded protein response, which may alter the biogenesis of wild-type receptors and, thus, produce further compromise of GABAergic inhibition and/or differential response upon cellular stress. The developmental aspects of the expression of a mutant subunit and the compensatory expression of other functionally overlapping subunits have not been studied. Different GABAA receptor subunit genes are active at different stages of brain development, and the impact of this developmentally regulated expression of subunits on neuronal excitability is likely to be substantial. It has been shown in a number of studies that neurons can compensate for loss of a subunit by regulating partnering subunits either up or down, which may compensate for or exacerbate the loss of inhibition stemming from the loss of a functional subunit. Ultimately, it is essential to determine the specific effect of a mutation on expression of GABAA receptors in a network context during development. This effect is a critical piece of the puzzle that will allow blending of the molecular pathogenesis of these mutations and the process of epileptogenesis.
Given the complexity of gene transcription and translation, as well as complications involved in receptor trafficking from the endoplasmic reticulum to cell surface and synapses in neurons, it would not be surprising if mutations occurring in other genes of associated proteins (e.g., chaperones, kinases involved in phosphorylation signaling pathways) are also associated with genetic epilepsies. For example, the nonion channel gene mutation in LGI1 has been identified in familial lateral temporal lobe epilepsy, suggesting an additional pathological mechanism for epileptogenesis. Future studies of the effects of GABAA receptor mutations on gene expression, mRNA stability, posttranslational protein modification, subunit folding and assembly, receptor trafficking, receptor synaptic targeting, and receptor endocytosis will eventually present a more accurate picture of the molecular pathogenesis of epileptogenesis—and hopefully lead to alternative therapeutic approaches.
References
- 1.Hirose S, Mitsudome A, Okada M, Kaneko S. Genetics of idiopathic epilepsies. Epilepsia. 2005;46(suppl 1):38–43. doi: 10.1111/j.0013-9580.2005.461011.x. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 3.Mulley JC, Scheffer IE, Petrou S, Berkovic SF. Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol. 2003;16:171–176. doi: 10.1097/01.wco.0000063767.15877.c7. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald RL, Olsen RW. Palo Alto, CA: Annual Reviews Inc; 1994. GABAA Receptor Channels, in Annu Rev of Neurosci, vol 17; pp. 569–602. [DOI] [PubMed] [Google Scholar]
- 5.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha 1beta 2gamma 2 GABAA Receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Kang J, Gallagher MJ, Feng H-J. GABAA receptor mutations epilepsy associated with generalized epilepsies. Adv Pharmacol. 2006;54:147–169. doi: 10.1016/s1054-3589(06)54007-4. [DOI] [PubMed] [Google Scholar]
- 7.Maquat LE. Nonsense-mediated mRNA decay: Splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 9.Kang JQ, Macdonald RL. The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. J Neurosci. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22:5321–5327. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan HO, Reid CA, Single FN, Davis PJ, Chiu C, Murphy S, Clarke AL, Dibbens L, Krestel H, Mulley JC, Jones MV, Seeburg PH, Sakmann B, Berkovic SF, Sprengel R, Petrou S. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci USA. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hales TG, Tang H, Bollan KA, Johnson SJ, King DP, McDonald NA, Cheng A, Connolly CN. The epilepsy mutation, gamma2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABAA receptors. Mol Cell Neurosci. 2005;29:120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: A mutation in the gamma2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi MT, Macdonald RL. Agonist trapping by GABAA receptor channels. J Neurosci. 2001;21:9083–9091. doi: 10.1523/JNEUROSCI.21-23-09083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL, De Jonghe P. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–690. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- 16.Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher MJ, Song L, Arain F, Macdonald RL. The juvenile myoclonic epilepsy GABA(A) receptor alpha1 subunit mutation A322D producesasymmetrical, subunit position-dependent reduction. J Neurosci. 2004;24:5570–5578. doi: 10.1523/JNEUROSCI.1301-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. The GABA-A receptor epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc Natl Acad Sci USA. 2007;104:12999–13004. doi: 10.1073/pnas.0700163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher MJ, Shen W, Song L, Macdonald RL. Endoplasmic reticulum retention and associated degradation of a GABAA receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha 1 subunit. J Biol Chem. 2005;280:37995–38004. doi: 10.1074/jbc.M508305200. [DOI] [PubMed] [Google Scholar]
- 20.Bradley CA, Taghibiglou C, Collingridge GL, Wang YT. Mechanisms involved in the reduction of GABAA receptor alpha1-subunit expression caused by the epilepsy mutation A322D in the trafficking-competent receptor. J Biol Chem. 2008;283:22043–22050. doi: 10.1074/jbc.M801708200. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Olsen RW, Medina MT, Schwartz E, Alonso ME, Duron RM, Castro-Ortega R, Martinez-Juarez IE, Pascual-Castroviejo E, Machado-Salas J, Silva R, Bailey JN, Bai D, Ochoa A, Jara-Prado A, Pineda G, Macdonald RL, Delgado-Escueta AV. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet. 2008;82:1249–1261. doi: 10.1016/j.ajhg.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- 23.Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirose S. A new paradigm of channelopathy in epilepsy syndromes: Intracellular trafficking abnormality of channel molecules. Epilepsy Res. 2006;70(suppl 1):S206–S217. doi: 10.1016/j.eplepsyres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MD, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Zhang Y, Liang J, Liu X, Ma X, Wu H, Xu K, Qin J, Qi Y, Wu X. SCN1A, SCN1B, and GABRG2 gene mutation analysis in Chinese families with generalized epilepsy with febrile seizures plus. J Hum Genet. 2008;53:769–774.. doi: 10.1007/s10038-008-0306-y. [DOI] [PubMed] [Google Scholar]
- 27.Maljevic, Krampfl K, Cobilanschi J, Tilgen N, Beyer S, Weber YG, Schlesinger F, Ursu D, Melzer W, Cossette P, Bufler J, Lerche H, Heils A. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann Neurol. 2006;59:983–987. doi: 10.1002/ana.20874. [DOI] [PubMed] [Google Scholar]
- 28.Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A, Steinlein OK. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]