Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is the etiologic agent of adult T cell leukemia/lymphoma (ATLL), an aggressive CD4+ T lymphocyte malignancy. Activation of T lymphocytes is required for effective retroviral integration into the host cell genome and subsequent viral replication, but the molecular mechanisms involved in HTLV-1-mediated T cell activation remain unclear. HTLV-1 encodes various accessory proteins such as p12I, which has been demonstrated to be critical for HTLV-1 infectivity in vivo in rabbits and in vitro in quiescent primary human T lymphocytes. This hydrophobic protein localizes in the endoplasmic reticulum, increases intracellular calcium, and activates nuclear factor of activated T cell-mediated transcription. To further elucidate the role of p12I in regulation of cellular gene expression, we performed gene array analysis on stable p12I-expressing Jurkat T cells, using Affymetrix U133A arrays. Our data indicate that p12I altered the expression of genes associated with a network of interrelated pathways including T cell signaling, cell proliferation, and apoptosis. Expression of several calcium-regulated genes was found to be altered by p12I, consistent with known properties of the viral protein. Gene array findings were confirmed by semiquantitative RT-PCR in Jurkat T cells and primary CD4+ T lymphocytes. Furthermore, dose-dependent expression of p12I in Jurkat T cells resulted in significant increases in p300 and p300-dependent transcription. This is the first report of a viral protein influencing the transcription of p300, a rate-limiting coadapter critical in HTLV-1-mediated T cell activation. Collectively, our data strongly indicate that HTLV-1 p12I modulates cellular gene expression patterns to hasten the activation of T lymphocytes and thereby promote efficient viral infection.
INTRODUCTION
Human T-lymphotropic virus type 1 (HTLV-1), a deltaretrovirus, infects approximately 15 to 20 million people worldwide1 and is the etiologic agent of adult T cell leukemia/lymphoma (ATLL). ATLL is an aggressive malignancy of T lymphocytes characterized by prolonged latency, monoclonal proliferation of CD3+CD4+CD8−CD25+HLA-DR+ T lymphocytes, and viral persistence in infected individuals.2–4 HTLV-1 induces transformation of T lymphocytes in vitro, and activation of T lymphocytes appears to be necessary for efficient viral infection, as well as establishment of productive infection.3 However, details of the molecular mechanism of T cell activation and transformation by HTLV-1 remain to be completely understood.
HTLV-1 encodes regulatory proteins Tax and Rex as well as accessory proteins p12I, p27I, p13II, and p30II in the pX gene region of the viral genome.5 Studies have investigated the role of HTLV-1 accessory protein p12I in viral infection and T cell activation. Earlier studies reported that pX open reading frame (ORF) I is dispensable for HTLV-1 infection in vitro;6,7 however, findings from our laboratory demonstrated that selective ablation of ORF I from HTLV-1 proviral clone ACH dramatically reduced viral infectivity in vivo.8 ORF I deletion reduced viral infectivity of quiescent peripheral blood mononuclear cells (PBMCs) in the absence of mitogenic stimulation in vitro.9 Collectively, these findings established that p12I plays a critical role in T cell activation and efficient viral infection of quiescent T lymphocytes.
HTLV-1 p12I contains two putative transmembrane regions, four putative proline-rich SH3-binding domains,10 and a calcineurin-binding site,11 suggesting its possible involvement in T cell signaling pathways. p12I binds interleukin 2 (IL-2) receptor β and γ chains and enhances Stat5 DNA-binding activity.12,13 In addition, p12I localizes in the endoplasmic reticulum (ER) and cis-Golgi compartment and associates with calreticulin involved in calcium homeostasis.14,15 Importantly, expression of p12I elevates cytosolic calcium levels16 and selectively activates nuclear factor of activated T cells (NFAT)-mediated transcription in a calcium-dependent manner in Jurkat T cells.17 Taken together, these findings indicate a crucial role of HTLV-1 p12I in calcium-mediated cellular gene expression and T cell activation.
Gene array analysis of HTLV-1-immortalized T cells has provided insight into HTLV-1-associated alterations in genes that encode transcription factors, cell cycle-related genes, and genes involved in apoptosis.18,19 An extensive oligonucleotide array analysis of HTLV-1-immortalized and transformed cell lines described multiple genes potentially involved in cell proliferation or transformation.20 Selective expression of Tax appears to alter the expression of multiple genes involved in apoptosis, cell cycle, DNA repair, and cell adhesion in Jurkat T cells.21 T cells from patients who evolved from chronic to acute crisis ATLL were used to identify cancer progression-associated genes, using oligonucleotide array analysis.22 In addition, many genes including T cell differentiated antigen (MAL) and a lymphoid-specific member of the G protein-coupled receptor family (EBI-1/CCR7) were found to be upregulated in PBMCs from chronic ATLL patients.23
On the basis of these previous studies, we hypothesized that HTLV-1 p12I regulates the expression of cellular genes in a calcium-dependent manner. Herein, we used recombinant lentiviral vectors, gene array, and reverse transcription-polymerase chain reaction (RT-PCR) analysis to test the influence of p12I in gene expression in T lymphocytes. Our data indicated the viral protein expression resulted in alteration of genes in a predominant calcium-dependent manner in multiple pathways involving cell proliferation or signaling. Moreover, using RT-PCR, we have confirmed that p12I expression altered key signaling pathways in primary human CD4+ T lymphocytes. Intriguingly, p12I expression was associated with increased expression of p300, a rate-limiting, yet central transcriptional coadaptor, which plays a crucial role in HTLV-1 pathogenesis. We are the first to demonstrate a viral protein-mediated enhancement of p300 expression in T lymphocytes. Our data indicate that p12I appears to play a vital role in HTLV-1-mediated T cell activation by activating calcium-mediated transcription and augmenting the amounts of p300 within T lymphocytes. Collectively, our data indicate that this complex retrovirus, which is associated with lymphoproliferative diseases, relies on the p12I accessory protein to modify the cellular environment by enhancement of T cell activation and thereby facilitates early events of the viral infection.
MATERIALS AND METHODS
Cells
The 293T cell line (American Type Culture Collection, Manassas, VA), which stably expresses the simian virus 40 (SV40) T antigen, was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), streptomycin–penicillin (100 μg/mL), and 2 mM L-glutamine (complete DMEM, cDMEM). Human peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats of healthy donors from the Red Cross and maintained in RPMI 1640 medium (Invitrogen) supplemented with 15% FBS, streptomycin–penicillin (100 μg/mL) and 2 mM L-glutamine (complete RPMI, cRPMI). Jurkat T cells (clone E6-1; American Type Culture Collection) were maintained in cRPMI containing 10 mM HEPES (Invitrogen).
Lentiviral vectors and other plasmids
The internal ribosome entry site (IRES) sequence from pHR′CMV/Tax1/eGFP24 was cloned into the pWPT-GFP plasmid (D. Trono, University of Geneva, Geneva, Switzerland) to create pWPT-IRES-GFP (control plasmid). pWPT-p12IHA-IRES-GFP was generated by cloning p12IHA from pME-p12I 12 (G. Franchini, National Cancer Institutes, National Institutes of Health, Bethesda, MD) into pWPT-IRES-GFP (see Fig. 1A). Fidelity of plasmids was checked by Sanger sequencing and expression of GFP and p12I-HA was confirmed by flow cytometry (ELITE ESP flow cytometer; Beckman Coulter, Fullerton, CA) and Western blot (monoclonal anti-hemagglutinin antibody [diluted 1:1000]; Covance Research Products, Cumberland, VA), respectively, as previously described.25 Plasmid p5×GT-TATA-Luc (P. Quinn, Pennsylvania State University, Hershey, PA), contains five tandem Gal4 DNA-binding sequences upstream of a TATA box, derived from positions −264 to +11 of the phosphoenolpyruvate carboxykinase (PEPCK) gene in a luciferase reporter gene plasmid. pRSV-B-Gal and 12SE1A (T. Kouzarides, University of Cambridge, Cambridge, UK) have been described previously.26,27 pCMV-p300 expresses the full-length p300 protein from a cytomegalovirus immediate/early (CMV I/E) promoter (Upstate Cell Signaling Solutions, Lake Placid, NY).
FIG. 1.
(A) Schematic illustration of lentiviral vector expressing both p12I-HA and GFP (sample vector) as bicistronic messages and GFP alone (control vector) from elongation factor 1α promoter. Abbreviations: LTR, long terminal repeats; RRE, Rev response element; EFIα, elongation factor 1α promoter; IRES, internal ribosome entry site; WPRE, woodchuck hepatitis posttranscriptional regulatory element. (B) Flow cytometric analysis illustrating the expression of GFP in Jurkat T cells 7 days postinfection with lentiviral vectors. Both sample (expressing p12I-HA and GFP) and control (GFP alone) groups contain relatively high and similar levels of GFP. (C) RT-PCR demonstrating the expression of p12I-HA in Jurkat T cells 7 days postinfection with lentiviral vectors. Jurkat cells spin infected with sample vector express p12I whereas control vector spin-infected cells do not express p12I. RT-PCR was performed with triplicate samples and controls. GAPDH was used as a control for the integrity of the message.
Recombinant lentiviral vector production and stable p12I expression in Jurkat T lymphocytes
Recombinant lentiviral vectors, pseudotyped with vesicular stomatitis virus envelope glycoprotein (VSV-G), expressing p12I and GFP, were produced as described previously.25 Briefly, 5 μg of pHCMV-G, 25 μg of pCMVΔR8.2, and 25 μg of pWPT-p12IHA-IRES-GFP or pWPT-IRES-GFP were transfected into 293T cells, using the calcium phosphate method. Supernatant was collected 24, 48, and 72 hr posttransfection, cleared of cellular debris by centrifugation at 1000 rpm for 10 min at room temperature and then filtered through a 0.2-μm pore size filter. Recombinant vectors were then concentrated by centrifugation of the supernatant at 6500 × g for 16 hr at 4°C. The viral pellet was suspended in cDMEM overnight at 4°C and concentrated virus was aliquoted and stored at −80°C until use. Virus titer was determined by spin infecting 293T cells.25 Jurkat T lymphocytes were infected with recombinant lentiviral vectors at a multiplicity of infection (MOI) of 3 in the presence of Polybrene (8 μg/ml; Sigma, St. Louis, MO) at 2700 rpm for 1 hr at 28°C. At 7 days postinfection, cells were tested for green fluorescent protein (GFP) production by fluorescence-activated cell sorting (FACS) analysis and p12I expression was tested by RT-PCR.
Isolation of primary CD4+ T lymphocytes and expression of p12I
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat using Ficoll separation as described by the manufacturer (Amersham Biosciences, Piscataway, NJ). Primary CD4+ T cells were extracted with a CD4-positive isolation kit according to the manufacturer’s instructions (Dynal Biotech, Lake Success, NY). The purity of CD4+ T cell isolation was tested by staining the cells with phycoerythrin (PE)-conjugated anti-CD4 antibody and subsequent FACS analysis. Primary CD4+ T cells were stimulated with phytohemagglutinin (PHA) for 48 hr, transduced with recombinant virus at a multiplicity of infection of 25 in the presence of Polybrene (8 μg/mL; Sigma), and spin infected at 2700 rpm for 1 hr at 28°C. At 7 days postinfection, GFP expression of controls and samples was verified to be above 90% by FACS analysis and the presence of p12I mRNA expression in the samples (and absence in controls) was verified by RT-PCR. Expression of selected genes was also quantified by semiquantitative RT-PCR from these cells at 7 days postinfection.
RNA isolation and probe preparation
Total cellular RNA was isolated from Jurkat T lymphocytes, using RNAqueous according to the manufacturer’s instructions (Ambion, Austin, TX). Complementary DNA was synthesized with a GeneChip T7-Oligo(dT) Promoter Primer kit (Affymetrix, Santa Clara, CA) and a SuperScript double-stranded cDNA synthesis kit (Invitrogen) and in vitro transcription was done with an ENZO BioArray HighYield RNA transcript labeling kit (Affymetrix). Biotin-labeled cRNA was fragmented and hybridized to U133A (Affymetrix) arrays in a GeneChip hybridization oven. Arrays were washed and stained at a GeneChip Fluidics Station 400 and scanned with a Gene-Array scanner (Affymetrix). Quality controls included (1) comparisons of ratios of 3′ signal to 5′ signal of two housekeeping genes, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (between 0 and 3), (2) testing hybridization controls BioB, BioC, BioD, and Cre, which were all present and in a linear relationship of intensity, (3) testing scale factors between arrays, which did not vary by more than 3-fold, (4) determining the background intensity, which was not significantly higher than expected, and (5) ensuring that gene expression percentages were not less than the standard 30%. To determine the quantitative RNA level, the average difference representing the perfectly matched minus the mismatched for each gene-specific probe set was calculated. Differential gene expression and comparative analysis were done with the Data Mining Tool (Microarray Suite 5) to identify probes with at least a 1.5-fold difference in expression between control and p12I and verified for cluster formation by dChip software.28 Functional grouping of these probes was done with the Gene Ontology Mining Tool (Affymetrix).29
Semiquantitative RT-PCR in Jurkat and primary CD4+ T lymphocytes
Total cellular RNA was isolated from control and p12I spin-infected Jurkat T lymphocytes as well as primary CD4+ T lymphocytes, using RNAqueous as described by the manufacturer (Ambion). One microgram of RNA was converted to cDNA with the Reverse Transcription system (Promega, Madison, WI) as described by the manufacturer. cDNA from 100 ng of total RNA was then amplified by PCR with AmpliTaq DNA polymerase (PerkinElmer Life and Analytical Sciences, Boston, MA). The PCR primers for p12I were as follows: CCTCTTTCTCCCGCTCTTTT (forward) and GGCCAAGCTAGCGTAATCTG (reverse). The PCR primers for candidate genes selected for confirmation by semiquantitative RT-PCR were as follows:
TNFSF10: GGCCGCAAAATAAACTCCTG (forward), CCGAAAAAACTGGCTTCATG (reverse)
GADD45A: CTGAACGGTGATGGCATCTG (forward), CCAAAAATACCCAAACTATGGCT (reverse)
BAK1: AGAGCTGTCTGAACTCACGTGTC (forward), GGAGGATCCACCTCTGGGA (reverse)
IL6ST: TGTCCAGTATTCTACCGTGGTACAC (forward), GCATGCCTTCATCAGTCGC (reverse)
STK18: GCAGAATGAAACTTGAGTCACTTAC (forward), CCAGCAGGTTTTGTCCATG (reverse)
CDC2L1: CTGCTGACTCAGAAGCCTCTGT (forward), GCTTCACACGCTGCTGCT (reverse)
P300: GTAGCCTAAAAGACAATTTTCCTTG (forward), ATGTCAACCATCTGCACCAGTA (reverse)
PCR products were separated by agarose gel electrophoresis. Densitometric analysis was done by AlphaImager spot densitometry (Alpha Innotech, San Leandro, CA) as described previously.30 Statistical analysis was performed by Student t test, p < 0.05. DNA contamination was ruled out by performing a control with no reverse transcriptase.
Functional gene expression analysis
Jurkat T lymphocytes (2 × 106) were transfected with 500 ng of p5×67-TATA-LUC, 100 ng of pM-VP16, 500 ng of pRSV-β-gal, and increasing amounts of either pME-p12IHA, pME-18s, or pCMV-p300 in the presence or absence of 1 μg 12sE1A, using the SuperFect transfection protocol (Qiagen, Valencia, CA). At 72 hr posttransfection, cells were lysed with passive lysis buffer (Promega) at room temperature for 15 min. Twenty microliters of each lysate was used to test luciferase reporter gene activity, using an enhanced luciferase assay kit (Promega) according to the manufacturer’s protocol. Transfection efficiency was normalized by staining with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal; Sigma) and counting β-galactosidase (β-Gal)-expressing cells. Results were expressed as the mean of normalized luciferase activity in arbitrary light units (ALU) with standard error (SE) from a minimum of triplicate experiments. Statistical analysis was performed using the Student t test, p < 0.05.
RESULTS
Expression of HTLV-1 p12I in Jurkat T lymphocytes
To establish a stable expression system to test the effects of p12I in T lymphocytes, we constructed lentivirus vectors to express the viral protein in Jurkat T cells (Fig. 1A). The lentivirus vector system allowed us to stably express p12I as a bicistronic RNA concurrently with green fluorescent protein (GFP) marker gene. Jurkat T cells spin infected with recombinant lentivirus expressing GFP alone (controls) or p12I and GFP (samples) were analyzed 7 days posttransduction by FACS analysis for GFP expression (Fig. 1B). All the controls and samples expressed more than 95% GFP. Expression of HTLV-1 p12I mRNA, in control and sample Jurkat T lymphocytes, was tested 7 days postinfection by RT-PCR. All the samples were positive for p12I mRNA by RT-PCR, as indicated by the presence of an ~221-bp band (Fig. 1C) while the controls remained negative.
Calcium-dependent gene expression in Jurkat T lymphocytes expressing p12I
Critical to reproducible gene array data are quality control methods and analysis of standards. In this study, we used triplicate samples with vector-matched controls and multiple software for data analysis, as well as an Affymetrix chip method to minimize nonspecific hybridization and background signals.31 Quality controls included comparisons of ratios of 3′ signal to 5′ signal of two housekeeping genes, β-actin and GAPDH, and testing the linear relationship of hybridization controls. Additional controls included testing scale factors between arrays to ensure interassay quality and determining the background intensity. Established methods were used to test differential gene expression using commercial software to identify genes with at least a 1.5-fold difference in expression between p12I and empty vector-expressing cells.28
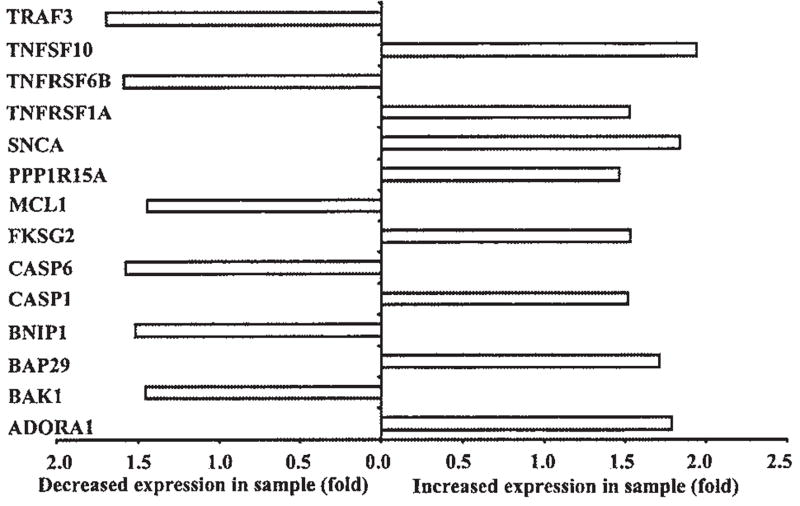
Overall, HTLV-1 p12I expression was associated with the enhanced expression of 288 genes and decreased expression of 407 genes with known biological functions. We classified these genes on the basis of their function in various cellular processes, such as apoptosis, cell proliferation, T cell activation or cell signaling, cell adhesion, and immune response (Figs. 2–6). Consistent with our previous findings of calcium-dependent T cell activation by p12I, our data indicated that Jurkat T cells expressing p12I exhibited a prominent pattern of calcium-related gene expression, including cell division cycle-2 like-1 (CDC2L1), interleukin 6 signal transducer (IL6ST), transcription factor E2F5, colony-stimulating factor 1 (CSF-1), tumor necrosis factor (TNF) superfamily members, adenosine receptor, TNF receptor-associated factor (TRAF), and serine threonine kinase 18 (STK18).32 Consistent with its known effects on intracellular calcium, p12I expression was associated with increased expression of adenosine A1 receptor (ADORA1) and lowered levels of protein phosphatase 1 inhibitor subunit 15A (PPP1R15A) and apoptosis inhibitor 5 (API5), all known to be calcium-dependent genes. Similarly, our data indicated that HTLV-1 p12I expression in Jurkat T cells was associated with downregulation of inositol polyphosphate phosphatase (SHIP2), responsible for increased biosynthesis of inositol triphosphate (IP3), a critical receptor for calcium flux in the ER (Fig. 4). This gene pattern would be consistent with compensatory changes related to enhancement calcium-mediated signaling.
FIG. 2.
Graph illustrating the differential expression of apoptosis-related genes in control and p12I-expressing Jurkat T cells from gene array. Critical to reproducible gene array data are quality control methods and analysis of standards. For all gene expression data represented in Figs. 2–6, triplicate samples with vector-matched controls were tested and data were analyzed with multiple software packages as detailed in Materials and Methods. An Affymetrix chip method was used to minimize nonspecific hybridization and background signals31 and quality controls included comparisons of ratios of 3′ signal to 5′ signal of two housekeeping genes, β-actin and GAPDH, and testing the linear relationship of hybridization controls. Additional controls included testing scale factors between arrays to ensure interassay quality and determining the background intensity. Established methods were used to test differential gene expression, using commercial software to identify genes with at least a 1.5-fold difference in expression between p12I and empty vector-expressing cells.28 Gene symbols are given on the left side of the graph. The complete list of genes modulated by p12I is given as a table elsewhere. (For a listing of genes modulated by HTLV-1 p12I, see www.vet.ohio-state.edu/docs/retrovirus/pubs.html.) The supplement material includes probe set ID of the gene used in Affymetrix HG-U133A gene chip, name of the gene, gene symbol, and chromosome map location of each gene.
FIG. 6.
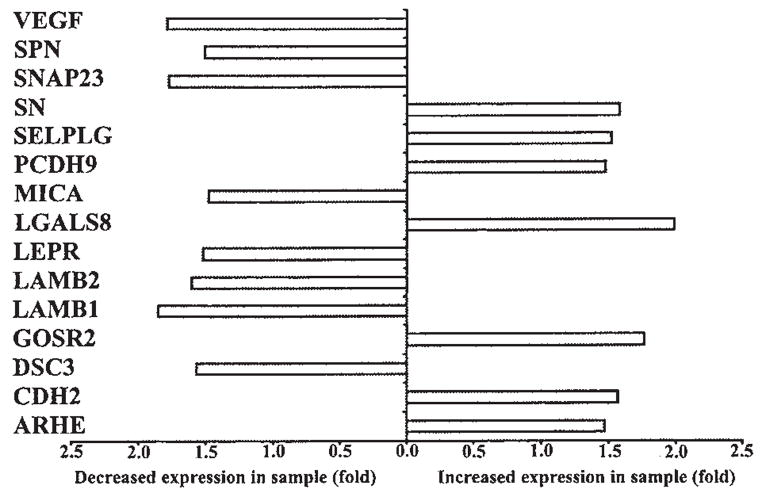
Graph illustrating the differential expression of genes involved in cell adhesion in control and p12I-expressing Jurkat T cells from gene array. A difference of at least 1.5-fold between control and sample was considered significant. Gene symbols are given on the left side of the graph. The complete list of genes modulated by p12I is given as a table elsewhere (www.vet.ohio-state.edu/docs/retrovirus.html). The supplement material includes probe set ID of the gene used in Affymetrix HG-U133A gene chip, name of the gene, gene symbol, and chromosome map location of each gene.
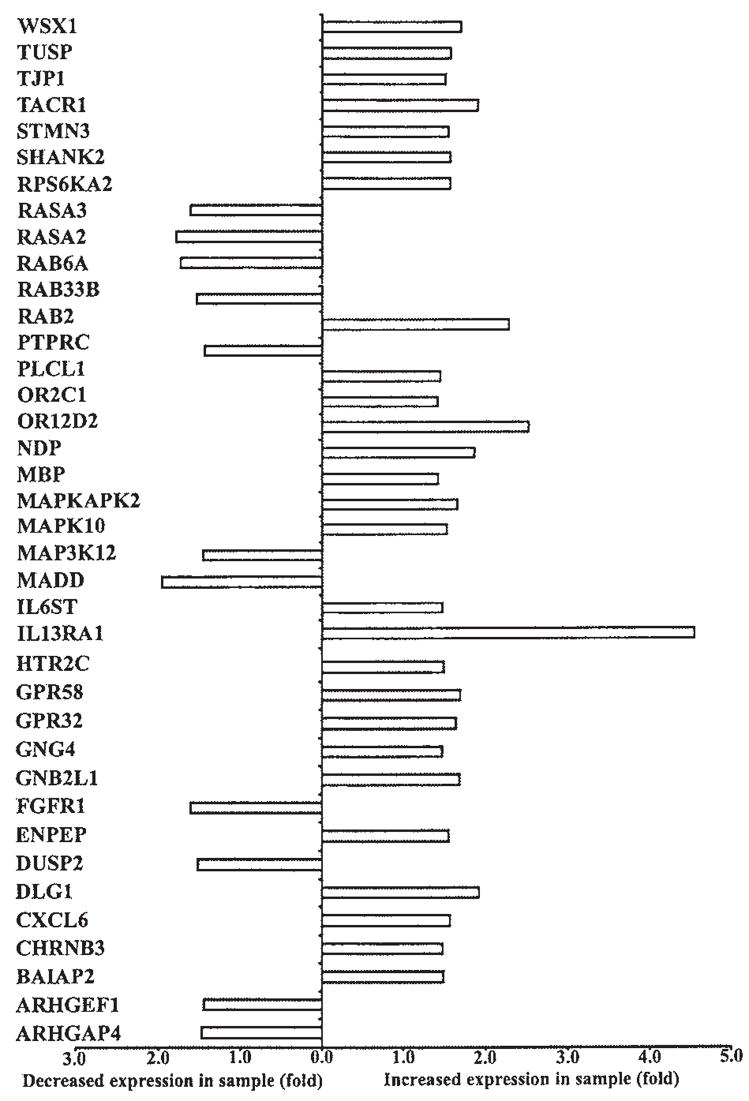
FIG. 4.
Graph illustrating the differential expression of genes associated with signal transduction in control and p12I-expressing Jurkat T cells from gene array. A difference of at least 1.5-fold between control and sample was considered significant. Gene symbols are given on the left side of the graph. The complete list of genes modulated by p12I is given as a table elsewhere (www.vet.ohio-state.edu/docs/retrovirus/pubs.html). The supplement material includes probe set ID of the gene used in Affymetrix HG-U133A gene chip, name of the gene, gene symbol, and chromosome map location of each gene.
Mobilization of endoplasmic reticulum calcium stores initiates the activation of cytoplasmic death pathways, which sensitize mitochondria to direct proapoptotic stimuli. Regulation of the mitochondrial checkpoint is obtained through a complex interplay between members of the Bcl-2 family and may include calcium release from the ER.33,34 Transport of Ca2+ between juxtaposed ER and mitochondrial membranes may sensitize mitochondria to the effects of proapoptotic Bcl-2 family members through Ca2+-induced opening of the mitochondrial permeability transition pore.34 Cells expressing p12I demonstrated altered expression of a variety of key apoptosis-related proteins, likely the result of altered calcium fluxes16 (Fig. 2). Expression of p12I also induced downregulation of Bcl-2-related/interacting genes such as proapoptotic Bcl-2-antagonist/killer 1 (BAK1), antiapoptotic protein, Bcl-2-associated athanogene 4 (BAG4), and myeloid cell leukemia sequence (MCL1). p12I expression also correlated with decreased expression of several genes associated with Fas-mediated apoptosis pathways such as TNF receptor-associated factor 3 (TRAF3), TRAF-interacting antiapoptotic protein BIRC4, and TNF receptor superfamily member-6b decoy (TNFRSF6B). Jurkat T cells expressing p12I also had upregulation of genes such as TNF superfamily members 10 and 1A, as well as caspase 1.
Gene expression patterns of cell activation and survival in p12I-expressing Jurkat T cells
Jurkat T cells expressing p12I also had altered patterns of gene expression of multiple cell cycle-dependent kinases, proteins involved in cytokine signaling, DNA replication, and G protein-coupled signaling (Fig. 3). These included increased expression of cell division cycle-2 like-1 and -5 (CDC2L1 and CDC2L5) and reduced expression of cyclin-dependent kinase 9 (CDK9) and CDC45 cell division cycle-45 like (CDC45L). Interestingly, CDK9 has been reported to associate with the HIV-1 preinitiation complex, where it interacts with HIV-1 Tat protein to cause increased transcriptional elongation.35 Jurkat T cells expressing p12I had decreased gene expression for cell cycle-regulatory proteins such as growth arrest and DNA damage-inducible α (GADD45A), which plays an important role in the G2–M checkpoint, FK506-binding protein 12–rapamycin-associated protein 1 (FRAP1), a phosphatidylinositol kinase-related kinase, critical for the progression through G1 phase of the cell cycle, mitogen-activated protein kinase kinase 6 (MAP2K6), a signaling protein known to induce G2 arrest, and cell cycle progression 2 protein (CPR2), which helps to overcome G1 arrest. Cells expressing p12I exhibited increased gene expression for certain cytokines such as interleukin 12A (IL12A) and CSF1. Interestingly, p12I-expressing cells had enhanced expression of TNF receptor superfamily member 1 (TNFRSF1), which when stimulated increases the synthesis of phosphatidylinositol diphosphate (PIP2), a key molecule in calcium-mediated T cell signaling. HTLV-1 p12I was associated with increased expression of origin recognition complex subunit 4-like (ORC4L) and E2F transcription factor 5 (E2F5), both essential for initiation of DNA replication. p12I expression was associated with decreased expression of several proteins associated with Ras-mediated signaling such as Ras p21 protein activator 2 and 3 (RASA2 and RASA3).
FIG. 3.
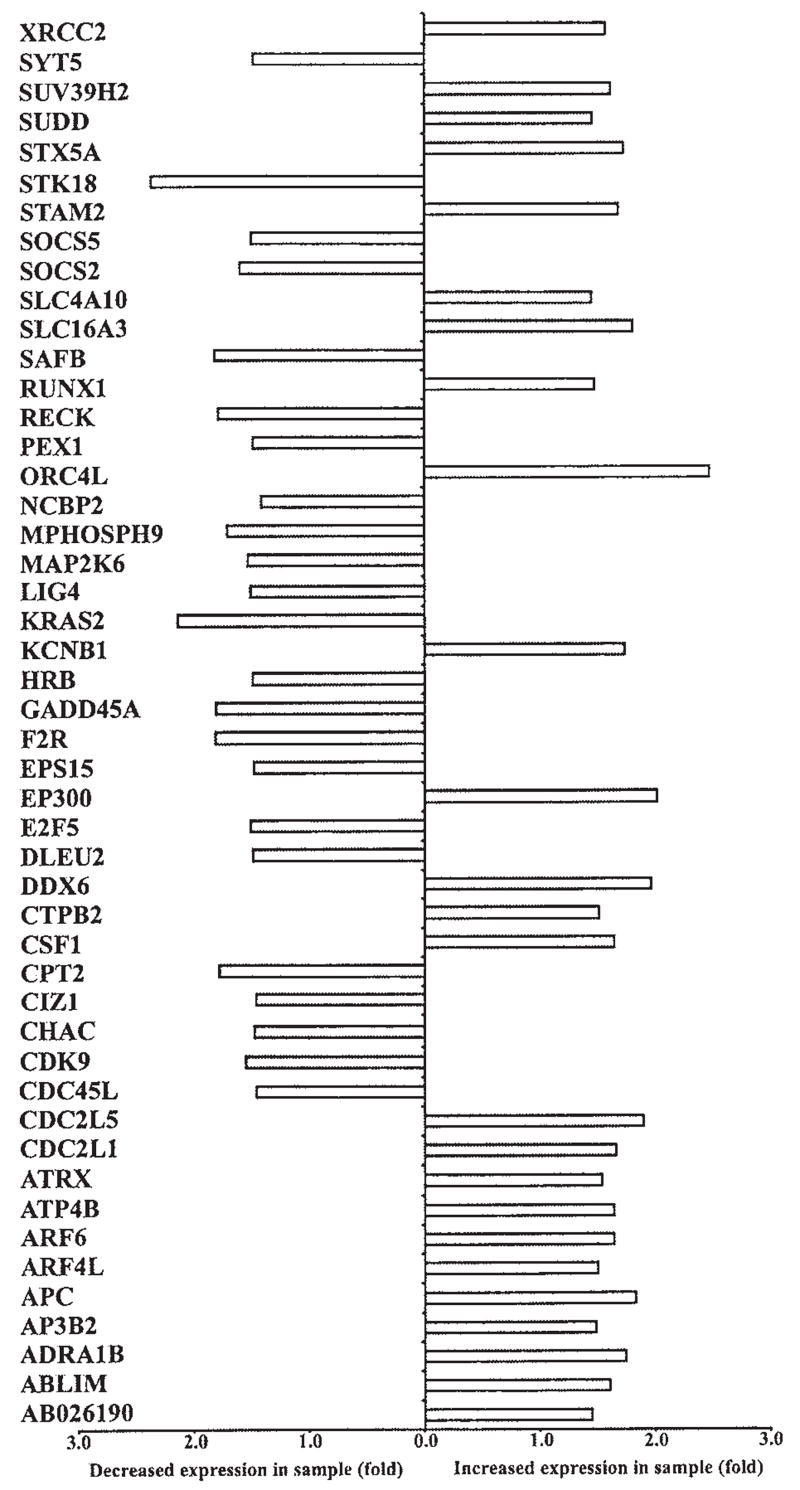
Graph illustrating the differential expression of cell proliferation-related genes in control and p12I-expressing Jurkat T cells from gene array. A difference of at least 1.5-fold between control and sample was considered significant. Gene symbols are given on the left side of the graph. The complete list of genes modulated by p12I is given as a table elsewhere (www.vet.ohio-state.edu/docs/retrovirus/pubs.html). The supplement material includes probe set ID of the gene used in Affymetrix HG-U133A gene chip, name of the gene, gene symbol, and chromosome map location of each gene.
Interestingly, Jurkat T cells expressing p12I had higher levels of p300, a critical transcriptional coadaptor. Transcription factors such as NFAT, c-Jun, Fos, p53, Stat1, Ets-1, NF-κB, cMyb, and CREB form active complexes when bound with p300 at their respective DNA-binding sites.36–38 p300 forms complexes with several of these transcription factors at the HTLV-1 promoter to regulate HTLV-1 transcription in infected T cells.39 In addition, two proteins of HTLV-1, Tax and p30II, bind p300 at the KIX domain and regulate viral gene transcription from the long terminal repeat (LTR).27,40 Thus, p12I expression may play a role in increasing p300, which is in limited concentrations in actively proliferating cells.41
HTLV-1 p12I expression correlated with a decrease in protein tyrosine phosphatase receptor type C (PTPRC, CD45), a signaling molecule involved in the regulation of a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. In particular, PTPRC regulates T cell antigen receptor signaling either by directly interacting with components of the antigen receptor complexes or by activating various Src family kinases required for antigen receptor signaling.42 Interestingly, IL-2-independent HTLV-1-transformed T cell lines also have lower expression of CD45RO, an isoform of PTPRC. More importantly, HTLV-1 Tax alone was not able to suppress the expression of CD45RO43 and therefore it is possible that p12I contributes to the suppression of CD45RO.
Patterns of gene expression in p12I-expressing cells related to cell adhesion and the immune response
The cellular immune response against HTLV-1 and the ability of the virus to influence cell-to-cell transmission are critical in establishing persistent HTLV-1 infection,44 in part, by modifying intracellular calcium concentration. Consistent with its ability to increase cytosolic calcium levels, we found that p12I modulated the expression of various proteins involved in cell adhesion or the immune response (Figs. 5 and 6). Cells expressing p12I had increased levels of two calcium-dependent cell adhesion molecules, namely, cadherin-2 type-1 (CDH2) and protocadherin 9 (PCDH9), two lectin family proteins that promote cell adhesion, namely, galectin 8 and sialoadhesin (CD169) and selectin P ligand (CD162). HTLV-1 p12I has been reported to downregulate MHC-1 and is believed to help infected cells evade immune recognition.45,46 Interestingly, in our study, p12I expression was associated with increased expression of calcium-dependent ADP-ribosylation factor 6 (ARF6) (Fig. 6), which functions to increase receptor-mediated endocytosis of MHC-1.47 Intriguingly, p12I expression was also associated with decreased expression of genes encoding proteins critical in peptide loading of MHC class II molecules such as MHC-II-DMβ(HLA-DMB), MHC-II-DRβ5 (HLA-DRB5), as well as MHC-II-DRβ1 (HLA-DRB1) and increased expression of interferon γ-inducible MHC-II-DPα1 (HLA-DPA1) and cathepsin-S (CTSS), a cysteine proteinase involved in MHC-II antigen presentation.
FIG. 5.
Graph illustrating the differential expression of immune response-related genes in control and p12I-expressing Jurkat T cells from gene array. A difference of at least 1.5-fold between control and sample was considered significant. Gene symbols are given on the left side of the graph. The complete list of genes modulated by p12I is given as a table elsewhere (www.vet.ohio-state.edu/docs/retrovirus.html). The supplement material includes probe set ID of the gene used in Affymetrix HG-U133A gene chip, name of the gene, gene symbol, and chromosome map location of each gene.
Confirmation of the role of p12I-mediated gene expression in Jurkat T cells and primary CD4+ T lymphocytes by RT-PCR
To confirm our gene array data, we selected several candidate genes on the basis of the crucial role of these gene products in various cellular processes such as transcriptional regulation (p300), apoptosis (BAK1 and TNFSF10), cell cycle regulation (CDC2L1 and GADD45), and signal transduction (STK18 and IL6ST). CDC2L1, IL6ST, and STK18 were of additional interest because their expression is calcium regulated.32 We confirmed the expression of these selected genes in response to p12I by performing semiquantitative RT-PCR and subsequent densitometric analyses. Our RT-PCR results were, in all cases, parallel to our gene array data. Jurkat T cells expressing p12I demonstrated increased expression of p300, IL6ST, CDC2L1, and TNFSF10 by 2.2-, 2.1-, 2.2-, and 1.8-fold, respectively, and decreased expression of BAK1, GADD45, and STK18 by 1.9-, 2.0-, and 2.4-fold, respectively (Fig. 7A and B). Because the target cells of HTLV-1 infection are CD4+ T lymphocytes, we similarly tested the ability of p12I to modulate gene expression in primary CD4+ T cells. In parallel with our gene array data and our findings in Jurkat T cells, primary CD4+ T lymphocytes expressing p12I exhibited increased expression of p300, IL6ST, CDC2L1, and TNFSF10 by 3.9-, 2.7-, 2.1-, and 1.7-fold, respectively, and decreased expression of BAK1, GADD45, and STK18 by 2.4-, 2.9-, and 1.8-fold, respectively (Fig. 8A and B).
FIG. 7.
(A) Semiquantitative RT-PCR demonstrating the differential expression of selected genes in Jurkat T cells expressing p12I. Total cellular RNA was extracted 7 days postinfection with recombinant lentiviral vectors. Semiquantitative RT-PCR was performed on cDNA from 100 ng of total cellular RNA. RT-PCR was performed with triplicate samples and controls. GAPDH was used as a control for the integrity of the message. (B) Graph demonstrating densitometric analysis of semiquantitative RT-PCR of selected genes in Jurkat T cells expressing p12I. Fold difference between control and sample is given at the top of the column for each gene. Results are expressed as means with standard error (SE) from a minimum of triplicate experiments. BAK1, GADD45, and STK18 were downregulated whereas p300, CDC2L1, TNFSF10, and IL6ST were upregulated by p12I. Statistical analysis was performed using Student t test. *p < 0.05.
FIG. 8.
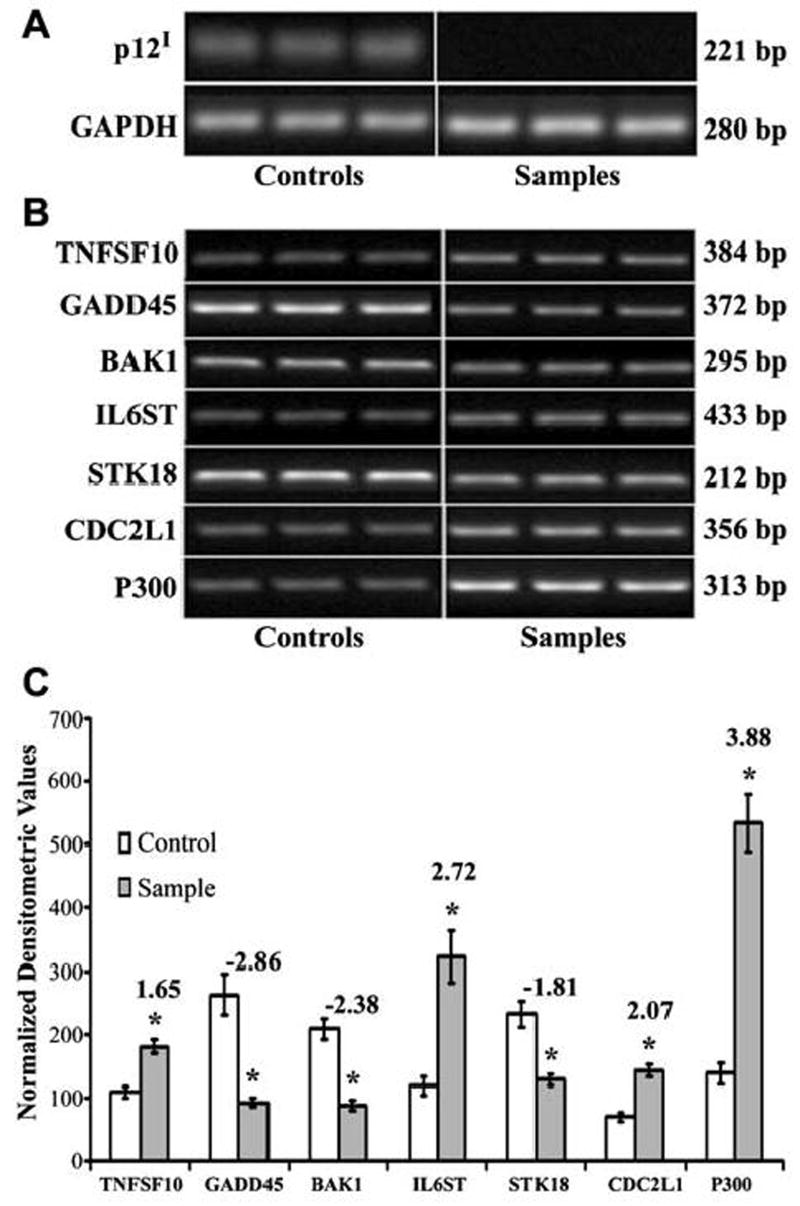
(A) RT-PCR demonstrating the expression of p12I-HA in primary CD4+ T cells 7 days postinfection with lentiviral vectors. Primary CD4+ T cells infected with sample vector express p12I whereas cells infected with control vector do not express p12I. RT-PCR for GAPDH was used as a control for the integrity of the message. (B) Semiquantitative RT-PCR demonstrating differential expression of selected genes in primary CD4+ T cells expressing p12I. Total cellular RNA was extracted 7 days postinfection with recombinant lentiviral vectors. Semiquantitative RT-PCR was performed on cDNA from 100 ng of total cellular RNA. RT-PCR was performed with triplicate samples and controls. GAPDH was used as a control for the integrity of the message. (C) Graph demonstrating densitometric analysis of semiquantitative RT-PCR of selected genes in primary CD4+ T cells expressing p12I. Fold difference between control and sample is given at the top of the column for each gene. Results are expressed as means with standard error (SE) from a minimum of triplicate experiments. BAK1, GADD45, and STK18 were downregulated whereas p300, CDC2L1, TNFSF10, and IL6ST were upregulated by p12I. Statistical analysis was performed using Student t test. *p < 0.05.
HTLV-1 p12I enhances p300 transcription in Jurkat T lymphocytes
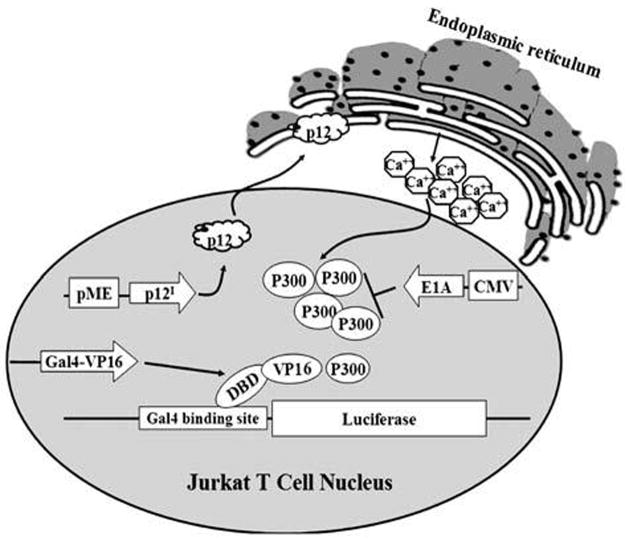
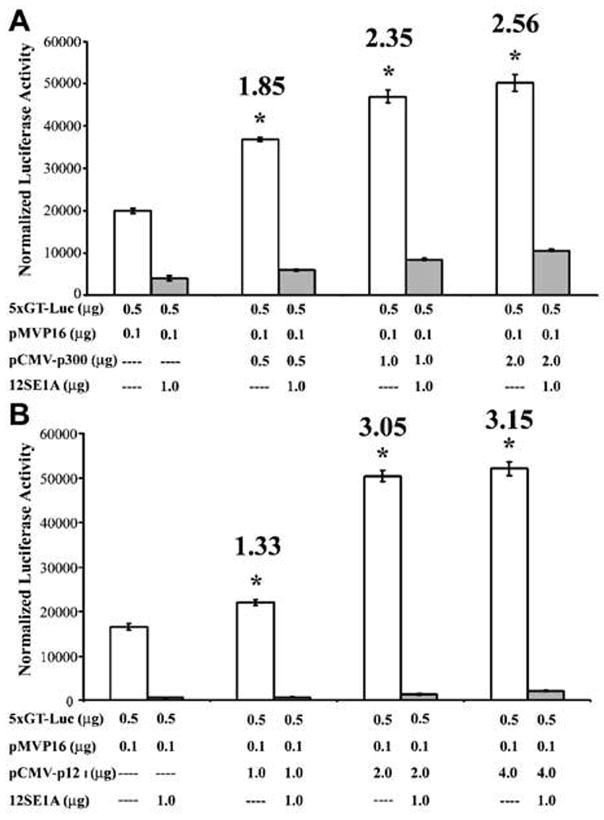
The cellular transcriptional coadaptor, p300, is known to interact with key transcriptional molecules involved in T cell activation such as NFAT, NF-κB, and AP-1.48–50 To test the influence of p12I expression on transcription mediated by this important cellular coadaptor, a p300-dependent reporter system was used (Fig. 9). This well-established method utilizes the acidic activation domain of herpes simplex virus protein VP16 to interact with p300 and VP16 fused to the Gal4 DNA-binding domain to activate transcription from a minimal promoter containing a Gal4-binding site.51 To validate the functional gene expression analysis system, we transfected a p300 expression plasmid (pCMV-p300) into Jurkat T cells along with a luciferase reporter plasmid driven by a Gal4 promoter and a Gal4 DBD-VP16 fusion plasmid (Fig. 10A). As expected, p300 enhanced VP16-mediated transcription in a dose-dependent manner and the trans-activation was blocked in the presence of adenovirus E1A protein (Fig. 10A). E1A protein is known to bind p300 and make it unavailable for transcription.52 We tested the ability of p12I to enhance p300 levels by transfecting Jurkat T cells with increasing concentrations of pME-p12IHA or pME-18s empty plasmid control in the presence of the Gal4 luciferase reporter gene construct and a Gal4 DBD-VP16 fusion plasmid. HTLV-1 p12I caused up to a 3.2-fold increase in VP16-mediated activation of transcription in a dose-dependent manner (Fig. 10B). To confirm that the increase in VP16-mediated transcription was due to p300, we transfected an adenovirus E1A expression plasmid into the Jurkat T cells along with the plasmids. We found that the enhancement of VP16-mediated trans-activation by p12I could be blocked in the presence of the competitive p300-binding E1A protein (Fig. 10B).
FIG. 9.
Schematic illustration of functional gene expression analysis. Jurkat T cells (12 × 106) were transfected with 500 ng Gal4-luciferase reporter plasmid, 100 ng of pM-VP16 expression plasmid, and increasing concentrations of p12I-HA expression plasmid. Luciferase activity was measured 72 hr posttransfection. To block p300-mediated transcription, 1.0 μg of E1A expression plasmid was transfected into these cells and luciferase activity was measured 72 hr posttransfection.
FIG. 10.
(A) Graph showing luciferase activity from Jurkat T cells transfected with p300 expression plasmid along with Gal4-luciferase reporter plasmid and pM-VP16 expression plasmid to confirm that VP16-mediated transcription is p300 dependent. Various plasmids used for transfection and the amounts are given on the x axis. There was a dose-dependent increase in luciferase activity with increasing amounts of p300 up to 2.6-fold. Fold differences are given above each column. Results are expressed as mean luciferase activity with standard error (SE) from a minimum of triplicate experiments. Statistical analysis was performed by Student t test. *p < 0.05. (B) Graph showing luciferase activity from Jurkat T cells transfected with p12I expression plasmid along with Gal4-luciferase reporter plasmid and pM-VP16 expression plasmid to confirm that p12I enhances p300 to biologically significant levels. There was a dose-dependent increase in luciferase activity with increasing amounts of p12I up to 3.3-fold. Fold differences are given above each column. Results are expressed as mean luciferase activity with standard error (SE) from a minimum of triplicate experiments. Statistical analysis was performed by Student t test. *p < 0.05.
DISCUSSION
Our study presents a comprehensive analysis of changes in gene expression patterns associated with HTLV-1 p12I expression, an accessory protein critical for virus replication in vivo. We included methods to strengthen the reliability of our data specifically by (1) using triplicate samples and appropriate controls, (2) using multiple software for data analysis, (3) minimizing nonspecific hybridization and background signals by using an Affymetrix chip,31 (4) using a well-characterized T lymphocyte system (Jurkat T cells), and (5) validating microarray data by RT-PCR and functional gene expression assays, all of which were consistent with our gene array data. Overall, this study confirms that p12I is a regulator of cellular genes, and identifies several potential new functional roles for p12I in T cell function.
We have previously reported that HTLV-1 p12I increases intracellular calcium concentration by releasing calcium from the ER and also by capacitative calcium entry.14 Calcium plays an essential role in lymphocyte activation and maturation. NFAT, a calcium/calcineurin-dependent transcriptional factor, is vital for proliferation of peripheral lymphocytes during HTLV-1 infection.53 Similarly, activation of NFAT is necessary for efficient infectivity in primary lymphocytes and early stages of HIV replication, especially at the completion of reverse transcription.54,55 HTLV-1 p12I specifically activates NFAT-mediated transcription in a calcium-dependent manner and thereby increases infectivity of the virus to quiescent T cells.17 Feske et al.32 identified calcium-dependent genes encoding proteins involved in T cell signaling and cell cycle regulators such as CDC2L1; cytokines and associated proteins such as IL6ST, TNF superfamily members, TRAF, and CSF-1; transcription factors such as E2F5; cell surface receptors including adenosine receptor; and protein kinases such as STK18. Interestingly, these gene expression profiles were similar to patterns displayed by Jurkat T cells expressing p12I. In addition, we found that HTLV-1 p12I modulated the expression of several genes that control calcium signaling pathways in Jurkat T cells, including IP4, PDPK1, and SHIP2. These proteins are responsible for increased biosynthesis of IP3 and DGKE, which is involved in the regeneration of phosphatidylinositol.
Using human cDNA array analysis of normal and HTLV-1-immortalized T cells, Harhaj et al.19 demonstrated deregulation of genes involved in apoptosis in HTLV-1-immortalized T cells. This same type of cDNA array was employed by De La Fuente et al.18 to study regulation of transcription factors in HTLV-1-infected cells. Gene expression profiles of peripheral blood mononuclear cells (PBMCs) from acute and chronic ATLL patients were used to identify genes associated with cancer progression.23 Tsukasaki et al.22 reported increased expression of genes encoding membrane receptors such as selectin and CD47, cell cycle regulatory proteins, and adhesion molecules such as galectins in acute ATLL. Using a National Institutes of Health-generated cDNA array representing 2304 cancer-related genes, Ng et al.,21 compared normal and Tax-expressing Jurkat T cells and identified Tax-associated changes in genes regulating a variety of cellular pathways including apoptosis, cell cycle, and DNA repair. Affymetrix microarrays representative of ~7000 genes were used to compare the expression profiles of normal activated peripheral blood lymphocytes with those of HTLV-1-immortalized and transformed cell lines.20
Our data are the first to demonstrate that a viral protein, HTLV-1 p12I, augments the expression of p300 and enhances p300-mediated transcription. p300 and cAMP response element-binding protein (CREB)-binding protein (CBP) are coactivators involved in the regulation of transcription and chromatin with histone acetyltransferase (HAT) properties. Histone acetyltransferases and histone deacetylases play a crucial role in transcriptional activation and repression of multiple genes.38 Interaction of p300 with components of the general transcriptional machinery, such as TFIID, TFIIB, and RNA polymerase II holoenzyme (RNAPII), is thought to be critical for its transcriptional function. Simultaneous interaction of multiple transcription factors with p300 contributes to transcriptional synergy, while competition to interact with limited amounts of p300 represses transcription.38 Interestingly, coactivators such as p300 are recruited in p53-dependent signaling pathways important in tumorigenesis.56,57 Furthermore, certain cases of acute myeloid leukemia have been linked to recurrent chromosomal translocations that result in frame fusions of CBP or p300 to the monocytic leukemia zinc finger protein58 and myeloid/lymphoid leukemia gene products.59,60 The adenoviral oncoprotein E1A inhibits host gene transcription by binding and presumably using the HAT activities of p300/CBP.57 Overall, p300-mediated transcriptional regulation have been extensively investigated; however, the transcriptional regulation of the p300 gene itself remains unclear. p300 interacts with transcription factors such as NFAT, AP-1, and NF-κB, which are essential for IL-2 production, T cell activation, and proliferation.48–50 These interactions are critical for viral gene expression and replication in retroviral infections such as HIV-1 and HTLV-1.52 Moreover, chromatin immunoprecipitation analysis of the integrated HTLV-1 provirus in infected T cells revealed the presence of Tax, a variety of ATF/CREB and AP-1 family members (CREB, CREB-2, ATF-1, ATF-2, c-Fos, and c-Jun) and p300 at the HTLV-1 promoter.39 We have demonstrated that the HTLV-1 accessory protein, p30II, competes with Tax for binding p300 at the KIX domain of p300.26,27 This competition between different proteins of the same virus is thought to play an important role in balancing viral gene expression during different phases of HTLV-1 replication. Our data, presented herein, suggest that HTLV-1 uses another accessory protein, p12I, to reprogram the cellular environment to favor the expression of p300, ultimately promoting lymphocyte survival and clonal expansion of the viral genome.
HTLV-1 persists in immunocompetent infected individuals, suggesting strategies for immune evasion. MHC molecules essential for presentation of foreign peptides are the targets of many viral proteins and HTLV-1 p12Ihas been reported to reduce MHC-1 cell surface expression.46 Interestingly, in our study p12I expression enhanced the expression of MHC-1-associated protein calcium dependent ADP-ribosylation factor 6, which is known to increase receptor-mediated endocytosis and thereby decrease cell surface expression of MHC-1.47 Importantly, in our p12I-expressing Jurkat T cells decreased expression of genes encoding MHC-II-associated proteins such as HLA-DMB, HLA-DRB5, and HLA-DRB1 had a similar pattern as observed in acute crisis ATLL patients.22 Because alteration in the expression of MHC proteins is a well-known mechanism of cellular defense against viral infection, the role of p12I in the modulation of MHC-1 and -II may correlate with the ability of HTLV-1 to maintain proviral loads in vivo. Other viral gene contributions to MHC-I or -II expression must be considered as HTLV-1 cell lines that are immortalized with full-length proviruses lacking pX ORF 1 expression do not show gross alterations of MHC-I or -II expression by flow cytometry.61
HTLV-1-mediated interference with normal T cell apoptosis is thought to be a mechanism of tumorigenesis,3 but specific mechanisms by which HTLV-1 may influence T cell survival are not fully understood.3 Similar to the effect of HTLV-1 Tax on apoptosis-related genes,19,21 we found that p12I was associated with the alteration of expression of multiple genes with both proapoptotic and antiapoptotic properties. Several members of the cell cycle machinery display alterations in gene expression in HTLV-1-infected cells20 and a number of studies examined the aberrations in cell cycle caused by HTLV-1 Tax.3 On the basis of data presented herein, p12I appears to modulate the cell cycle. Further studies that are beyond the scope of our current study will be required to test the role of accessory gene products of HTLV-1 in the context of other regulatory proteins such as Tax.
Overall, our current study extends our earlier reports8,9,14,16,17,25 and sheds light on the novel mechanisms by which p12I functions in HTLV-1 pathogenesis. It is possible that HTLV-1 accessory proteins act synergistically. We postulate that HTLV-1 differentially uses its regulatory and accessory gene products to subvert the cellular milieu in order to support optimal viral replication and maintain persistent infection. However, because information on the expression profile of HTLV-1 proteins during different stages of the infection is limited, additional studies will be required to explore these possibilities. These future studies will provide new directions in the development of therapeutic interventions against HTLV-1 lymphoproliferative disorders.
Acknowledgments
We thank M. Kotur and R. Meister for technical assistance in FACS, A. Bakaletz for technical support with data analysis, and Y. Liu-Stratton for technical help and valuable suggestions in design of the microarray experiment. We also thank W. Ding, S. J. Kim, P. Green, and L. Mathes for critical review of the manuscript, and G. Franchini and D. Trono for sharing valuable reagents. This work was supported by NIH grants CA100730 and RR14324 awarded to Dr. Michael Lairmore and CA-70529 from the NCI, awarded through the OSU Comprehensive Cancer Center.
Footnotes
This article has been cited by:
1. Christophe Nicot, Robert L Harrod, Vincenzo Ciminale, Genoveffa Franchini. 2005. Human T-cell leukemia/lymphoma virustype 1 nonstructural genes and their functions. Oncogene 24:39, 6026-6034. [CrossRef]
References
- 1.Bangham CR. HTLV-1 infections. J Clin Pathol. 2000;53:581–586. doi: 10.1136/jcp.53.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangham CR. The immune response to HTLV-I. Curr Opin Immunol. 2000;12:397–402. doi: 10.1016/s0952-7915(00)00107-2. [DOI] [PubMed] [Google Scholar]
- 3.Hollsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht B, Lairmore MD. Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis. Microbiol Mol Biol Rev. 2002;66:396–406. doi: 10.1128/MMBR.66.3.396-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 7.Robek MD, Wong FH, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 9.Albrecht B, Collins ND, Burniston MT, Nisbet JW, Ratner L, Green PL, Lairmore MD. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J Virol. 2000;74:9828–9835. doi: 10.1128/jvi.74.21.9828-9835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 11.Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J Biol Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- 12.Mulloy JC, Crownley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I proteins bind the interleukin-2 receptor β and γ chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, Trovato R, Fullen J, Leonard WJ, Franchini G. HTLV-1 p12I protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood. 2001;98:823–829. doi: 10.1182/blood.v98.3.823. [DOI] [PubMed] [Google Scholar]
- 14.Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12I: Association with calreticulin and calnexin. J Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim S, Altschuld RA, Lairmore MD. Human T lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht B, D’Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12I. J Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La Fuente C, Deng L, Santiago F, Arce L, Wang L, Kashanchi F. Gene expression array of HTLV type 1-infected T cells: Up-regulation of transcription factors and cell cycle genes. AIDS Res Hum Retroviruses. 2000;16:1695–1700. doi: 10.1089/08892220050193164. [DOI] [PubMed] [Google Scholar]
- 19.Harhaj EW, Good LF, Xiao GT, Sun SC. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18:1341–1349. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- 20.Pise-Masison CA, Radonovich M, Mahieux R, Chatterjee P, Whiteford C, Duvall J, Guillerm C, Gessain A, Brady JN. Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 2002;62:3562–3571. [PubMed] [Google Scholar]
- 21.Ng PW, Iha H, Iwanaga Y, Bittner M, Chen Y, Jiang Y, Gooden G, Trent JM, Meltzer P, Jeang KT, Zeichner SL. Genome-wide expression changes induced by HTLV-1 Tax: Evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-κB activation. Oncogene. 2001;20:4484–4496. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- 22.Tsukasaki K, Tanosaki S, DeVos S, Hofmann WK, Wachsman W, Gombart AF, Krebs J, Jauch A, Bartram CR, Nagai K, Tomonaga M, Said JW, Koeffler HP. Identifying progression-associated genes in adult T-cell leukemia/lymphoma by using oligonucleotide microarrays. Int J Cancer. 2004;109:875–881. doi: 10.1002/ijc.20028. [DOI] [PubMed] [Google Scholar]
- 23.Kohno T, Moriuchi R, Katamine S, Yamada Y, Tomonaga M, Matsuyama T. Identification of genes associated with the progression of adult T cell leukemia (ATL) Jpn J Cancer Res. 2000;91:1103–1110. doi: 10.1111/j.1349-7006.2000.tb00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripp A, Liu Y, Sieburg M, Montalbano J, Wrzesinski S, Feuer G. Human T-cell leukemia virus type 1 tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J Virol. 2003;77:12152–12164. doi: 10.1128/JVI.77.22.12152-12164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding W, Kim SJ, Nair AM, Michael B, Boris-Lawrie K, Tripp A, Feuer G, Lairmore MD. Human T-cell lymphotropic virus type 1 p12I enhances interleukin-2 production during T-cell activation. J Virol. 2003;77:11027–11039. doi: 10.1128/JVI.77.20.11027-11039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Nisbet JW, Bartoe JT, Ding W, Lairmore MD. Human T-lymphotropic virus type 1 p30II functions as a transcription factor and differentially modulates CREB-responsive promoters. J Virol. 2000;74:11270–11277. doi: 10.1128/jvi.74.23.11270-11277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30II regulates gene transcription by binding CREB binding protein/p300. J Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: Tool for the unification of biology. Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albrecht B, Collins ND, Newbound GC, Ratner L, Lairmore MD. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. doi: 10.1016/s0166-0934(98)00087-1. [DOI] [PubMed] [Google Scholar]
- 31.Murphy D. Gene expression studies using microarrays: Principles, problems, and prospects. Adv Physiol Educ. 2002;26:256–270. doi: 10.1152/advan.00043.2002. [DOI] [PubMed] [Google Scholar]
- 32.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 33.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Ling S, Yu XD, Venkatesh LK, Subramanian T, Chinnadurai G, Kuo TH. Modulation of mitochondrial Ca2+ homeostasis by Bcl-2. J Biol Chem. 1999;274:33267–33273. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- 35.Zhou M, Halanski MA, Radonovich MF, Kashanchi F, Peng J, Price DH, Brady JN. Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol Cell Biol. 2000;20:5077–5086. doi: 10.1128/mcb.20.14.5077-5086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–668. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- 37.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 38.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 39.Lemasson I, Polakowski NJ, Laybourn PJ, Nyborg JK. Transcription factor binding and histone modifications on the integrated proviral promoter in human T-cell leukemia virus-I-infected T-cells. J Biol Chem. 2002;277:49459–49465. doi: 10.1074/jbc.M209566200. [DOI] [PubMed] [Google Scholar]
- 40.Yan JP, Garrus JE, Giebler HA, Stargell LA, Nyborg JK. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J Mol Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- 41.Blobel GA. CBP and p300: Versatile coregulators with important roles in hematopoietic gene expression. J Leukoc Biol. 2002;71:545–556. [PubMed] [Google Scholar]
- 42.Tchilian EZ, Beverley PC. CD45 in memory and disease. Arch Immunol Ther Exp (Warsz) 2002;50:85–93. [PubMed] [Google Scholar]
- 43.Moro H, Iwai K, Mori N, Watanabe M, Fukushi M, Oie M, Arai M, Tanaka Y, Miyawaki T, Gejyo F, Arakawa M, Fujii M. Interleukin-2-dependent but not independent T-cell lines infected with human T-cell leukemia virus type 1 selectively express CD45RO, a marker for persistent infection in vivo. Virus Genes. 2001;23:263–271. doi: 10.1023/a:1012565105098. [DOI] [PubMed] [Google Scholar]
- 44.Bangham CR. Human T-lymphotropic virus type 1 (HTLV-1): Persistence and immune control. Int J Hematol. 2003;78:297–303. doi: 10.1007/BF02983553. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JM, Mulloy JC, Ciminale V, Fullen J, Nicot C, Franchini G. The MHC class I heavy chain is a common target of the small proteins encoded by the 3′ end of HTLV type 1 and HTLV type 2. AIDS Res Hum Retroviruses. 2000;16:1777–1781. doi: 10.1089/08892220050193308. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JM, Milloy JC, Ciminale V, et al. The MHC class I heavy chain is a common target of the small proteins encoded by 3′ end of HTLV type 1 and HTLV type 2. AIDS Res Hum Retroviruses. 2000;16:1777–1786. doi: 10.1089/08892220050193308. [DOI] [PubMed] [Google Scholar]
- 47.D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) J Exp Med. 1998;187:2031–2036. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 50.Subbaramaiah K, Lin DT, Hart JC, Dannenberg AJ. Peroxisome proliferator-activated receptor γ ligands suppress the transcriptional activation of cyclooxygenase-2: Evidence for involvement of activator protein-1 and CREB-binding protein/p300. J Biol Chem. 2001;276:12440–12448. doi: 10.1074/jbc.M007237200. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Grossman SR, Kieff E. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyl-transferases in activation of the LMP1 promoter. Proc Natl Acad Sci USA. 2000;97:430–435. doi: 10.1073/pnas.97.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hottiger MO, Nabel GJ. Viral replication and the coactivators p300 and CBP. Trends Microbiol. 2000;8:560–565. doi: 10.1016/s0966-842x(00)01874-6. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida H, Nishina H, Takimoto H, Marengere LE, Wakeham AC, Bouchard D, Kong YY, Ohteki T, Shahinian A, Bachmann M, Ohashi PS, Penninger JM, Crabtree GR, Mak TW. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 54.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 55.Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 56.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 57.Shikama N, Lyon J, Lathangue NB. The p300/CBP family: Integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 58.Borrow J, Stanton VP, Andresen JM, Becher R, Behm FG, Chaganti RSK, Civin CI, Disteche C, Dube I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;l6)(pll p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 59.Ida K, Kitabayashi I, Taki T, Taniwaki M, Noro K, Yamamoto M, Ohki M, Hayashi Y. Adenoviral E1A-associated protein p300 is involved in acute myeloid leukemia with t(11;22)(q23;q13) Blood. 1997;90:4699–4704. [PubMed] [Google Scholar]
- 60.Sobulo OM, Borrow J, Tomek R, Reshmi S, Harden A, Schlegel-berger B, Housman D, Doggett NA, Rowley JD, Zeleznik L. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3) Proc Natl Acad Sci USA. 1997;94:8732–8737. doi: 10.1073/pnas.94.16.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins ND, D’Souza C, Albrecht B, Robek MD, Ratner L, Ding W, Green PL, Lairmore MD. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]