Abstract
Background
Recent studies have demonstrated an association between mutations in CACNA1c or CACNB2b and Brugada syndrome (BrS). Previously described mutations all caused a loss of function secondary to a reduction of peak calcium current (ICa). We describe a novel CACNB2b mutation associated with BrS in which loss of function is caused by accelerated inactivation of ICa.
Methods and Results
The proband, a 32 yo male, displayed a Type I ST segment elevation in two right precordial ECG leads following a procainamide challenge. EP study was positive with induction of polymorphic VT/VF. Interrogation of implanted ICD revealed brief episodes of very rapid ventricular tachycardia. He was also diagnosed with vasovagal syncope. Genomic DNA was isolated from lymphocytes. All exons and intron borders of 15 ion channel genes were amplified and sequenced. The only mutation uncovered was a missense mutation (T11I) in CACNB2b. We expressed WT or T11I CACNB2b in TSA201 cells co-transfected with WT CACNA1c and CACNA2d. Patch clamp analysis showed no significant difference between WT and T11I in peak ICa density, steady-state inactivation or recovery from inactivation. However, both fast and slow decay of ICa were significantly faster in mutant channels between 0 and +20 mV. Action potential voltage clamp experiments showed that total charge was reduced by almost half compared to WT.
Conclusions
We report the first BrS mutation in CaCNB2b resulting in accelerated inactivation of L-type calcium channel current. Our results suggest that the faster current decay results in a loss-of-function responsible for the Brugada phenotype.
Keywords: genetics, ion channels, arrhythmia, calcium
INTRODUCTION
Brugada Syndrome (BrS) is characterized by an ST-segment elevation in the right precordial leads unrelated to ischemic or structural heart disease [1]. It is well established that mutations in SCN5A, the a-subunit of the cardiac Na+ channel, reduce the magnitude of the cardiac Na+ current by a variety of mechanisms [2] and are linked to the development BrS [3]. A second chromosome has been linked to the BrS in a large family in which the syndrome was also associated with progressive conduction disease, and a relatively good prognosis[4]. The gene was recently identified as the glycerol-3-phosphate dehydrogenase 1-like gene (GPD1L). The mutation in GPD1L has been shown to result in a reduction of INa [4,5]. Mutations in SCN1B (Navβ1)[6] have also recently been associated with BrS and shown to cause a loss of function of INa.
A recent study reported a mutation in ancillary subunit KCNE3 in patients diagnosed with BrS[7]. Co-transfection of the KCNE3 mutation with KCND3 (Kv4.3, the pore forming a-subunit) resulted in a significant increase in the magnitude of the Ca2+-independent transient outward current (Ito) compared to WT KCNE3+KCND3. Results of this study demonstrated a functional role of KCNE3 (MiRP2) in the modulation of Ito in the human heart and suggested that mutations in KCNE3 can underlie the development of BrS.
Blockade of the L-type Ca2+ channel (ICa) has been shown to lead to a BrS phenotype in isolated canine right ventricular wedge preparations [8]. Consistent with these findings, we identified a new clinical entity exhibiting ECG and arrhythmic manifestations of both BrS and short QT syndrome (SQTS) associated with loss of function mutations in the α1 (CACNA1C) and β (CACNB2b) subunits of the L-type cardiac calcium channel [9].
The balance of inward (typically INa and ICa) and outward (principally Ito) currents active during the early phase of the epicardial AP determine the magnitude of the AP notch and an outward shift in the balance of current can amplify the AP notch and predispose to loss of the AP dome, leading to the electrocardiographic and arrhythmic manifestations of BrS (for review see [10]). The outward shift can occur as a result of a reduction in the density or total charge of INa and ICa secondary to an accelerated inactivation of these currents. One of the first loss of function mutations in SCN5A associated with BrS was found to be due to an accelerated inactivation of the sodium channel [11]. A loss of function mutation associated with BrS due to accelerated inactivation of the L-type Ca2+ channel has as yet not been uncovered.
The cardiac L-type Ca2+ channel is believed to be comprised of a pore forming α1C-subunit as well as ancillary α2 and subunits [12]. The 2 subunit has been shown to enhance trafficking of the pore forming α1C-subunit to the sarcolemmal membrane and to alter the biophysical properties of the channel [12,13]. Here we report the first Brugada syndrome mutation in the 2b subunit of the L-type calcium channel (CaCNB2b) resulting in accelerated inactivation of ICa but not affecting trafficking. Our results suggest that the faster current decay results in a reduced total charge carried by ICa during the plateau of the action potential, thus predisposing to the BrS phenotype. Preliminary results have been presented as an abstract [14].
METHODS
ECG Measurement
The ECG was digitally scanned, magnified 4 to 8 times, and measured with digital calipers. The end of the T wave was defined as the intersection of a tangent, drawn to the descending portion of the T wave, with the isoelectric line.
Mutation Analysis
Genomic DNA was prepared from peripheral blood lymphocytes of patient (MMRL284) and available family members. All known exons of the principal BrS and candidate genes were amplified with intronic primers and sequenced in both directions to probe for mutations. The following genes were screened: SCN5A, SCN1B, SCN3B, KCNH2, KCNQ1, KCNJ2, KCNE1, KCNE2, KCNE3, KCND3 (Kv4.3), KCNIP2 (KCHiP2), KCNJ11, CACNA1C, CACNB2b, and CACNA2D1. No mutations were detected except in CACNB2b. All individuals studied in the control groups for the mutation, matched by race and ethnic background, were healthy and had no family history of cardiac arrhythmias based on written clinical history.
Cell Transfection/Mutagenesis
Site-directed mutagenesis was performed using QuikChange (Stratagene, LaJolla, CA) on full-length human wild type (WT) CACNA1C containing Exon 8A cDNA (accession number AJ224873) cloned in pcDNA3 [15,16]. CACNA1C, CACNA2D1 and CACNB2b cloned in pcDNA3 were kind gifts from Dr. Nikolai Soldatov and Dr. Igor Splawski. TSA201 cells were grown in DMEM with Glutamax supplemented with 10% FBS in 35mm culture dishes and placed in a 5% CO2 incubator at 37°C. To assess how T11I mutant channels altered the biophysical characteristics of ICa, TSA201 cells were co-transfected with a combination of mutant or WT CACNB2b. The cells were co-transfected using FuGene6 (Roche Diagnostics, Indianapolis, Ind) with a 1:1:1 molar ratio of WT human CACNA1C, WT or T11I mutant CACNB2b, and WT CACNA2D1. In addition, 0.40 μg of enhanced green fluorescent protein cDNA was added to the transfection mixture. Cells displaying fluorescence 48–72 h after transfection were used for electrophysiological study.
Measurement of Action Potentials
Single left ventricular myocytes were isolated from canine hearts using techniques previously described [17,18]. Action potentials of single cells were recorded using whole cell patch pipettes coupled to a MultiClamp 700A amplifier. The ventricular cells were superfused with a HEPES buffer of the following composition (mM): NaCl 126, KCl 4.0, MgCl2 1.0, CaCl2 2.0, HEPES 10, and glucose 11. pH adjusted to 7.4 with NaOH. The patch pipette solution had the following composition (mM): K-aspartate 90, KCl 30, glucose 5.5, MgCl2 1.0, EGTA 5, MgATP 5, HEPES 5, NaCl 10. pH = 7.2 with KOH. The resistance of the electrodes was 2–4 MΩ when filled with the pipette solution (described above). Action potentials were elicited using a 3 ms-current pulse at 120% threshold amplitude. Both Epi and Endo cells were paced at a cycle length of 0.5 Hz. The pre-recorded action potentials served as the waveforms for the AP clamp experiments. All myocyte recordings were made at 36±1° C.
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US NIH.
Electrophysiology
Voltage clamp recordings of ICa from transfected TSA201 cells were performed as previously described [15,9]. Briefly, patch pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, PA). The pipettes were pulled using a gravity puller (Narishige Corp., Tokyo, Japan) and filled with pipette solution of the following composition (mmol/L): 120 CsCl2, 2.0 MgCl2, 10 HEPES, 5 CaCl2, 2 MgATP and 10 EGTA, pH 7.25 (CsOH). The pipette resistance ranged from 1–4 MΩ when filled with the internal solution. The perfusion solution contained (mmol/L): 130 NMDG, 5 KCl, 15 CaCl2, 1 MgCl2, 5 mM TEA-Cl, 10 HEPES, pH 7.35 with HCl. Current signals were recorded using a MultiClamp 700A amplifier (Axon Instruments Inc., Foster City, CA) and series resistance errors were reduced by about 60–70% with electronic compensation. All recordings from TSA201 were made at room temperature.
Data Acquisition and Analysis
All signals were acquired at 20–50 kHz (Digidata 1322, Axon Instruments) with a microcomputer running Clampex 9 software (Axon Instruments, Foster City, CA). Voltage dependence of activation was measured from the current-voltage relation based on the equation: I = Gmax·(V−Vrev)/(1+exp(−(V V1/2)/k), where I is the peak current amplitude, Gmax the maximum conductance, V test potential, Vrev the reversal potential, V1/2 the midpoint of activation, and k the slope factor. Steady-state was fitted to the Boltzmann equation, I/Imax= 1/(1+exp((Vm−V1/2)/k)) to determine the membrane potential for half-maximal inactivation V1/2 and the slope factor k. For all ICa recordings, the interpulse interval was 25 s to ensure full recovery and availability of channels. Membrane currents were analyzed with Clampfit 9 software (Axon Instruments, Foster City, CA). Results from pooled data are presented as Mean±SEM and n represents the number of cells in each experiment. Repeated measures ANOVA followed by Student-Newman-Keuls test or paired Student’s t-test was used as appropriate for comparing paired data and a p<0.05 value was considered statistically significant.
RESULTS
The proband (MMRL284), a 34 year old male, experienced a syncopal attack and was seen in the ER but was discharged later that day. The following day, the patient still felt lethargic and again lost consciousness and was once again rushed to the ER. A preliminary ECG of patient showed ST segment elevation and negative T-wave in lead V1 (Figure 1A) and a QTc of 428 ms. Blood work revealed that CPK was elevated at 290 Units/liter. Upon procainamide infusion, the ECG of the patient became more consistent with a Brugada pattern with ST segment elevation in leads V1 and V2 (Figure 1B) and the QTc prolonged to 488 ms. After 1 gram of procainamide, patient had inducible VF and a shorter runs of polymorphic VT upon stimulation as well (Figure 1C). The father of the proband died at age 47 of an MI although the exact cause of death is questionable because he had substernal chest pain and felt poorly prior to collapsing and hitting his head and bleeding. Based on these findings and family history, he was implanted with an ICD. Subsequent follow-up interrogation of the ICD revealed 2 brief episodes of very rapid ventricular tachycardia (VT). Interestingly, the patient was also given the clinical diagnosis of vasovagal syncope.
Figure 1.
Electrocardiograms of the patient. (A) Twelve-lead ECG of the patient at rest. ST-segment elevation and negative T-wave are present in one right precordial lead (V1). (B) After infusion of procainamide, a type ST segment elevation was apparent in V2 as well.. (C) Development of polymorphic ventricular tachycardia following programmed electrical stimulation (double extrastimuli).
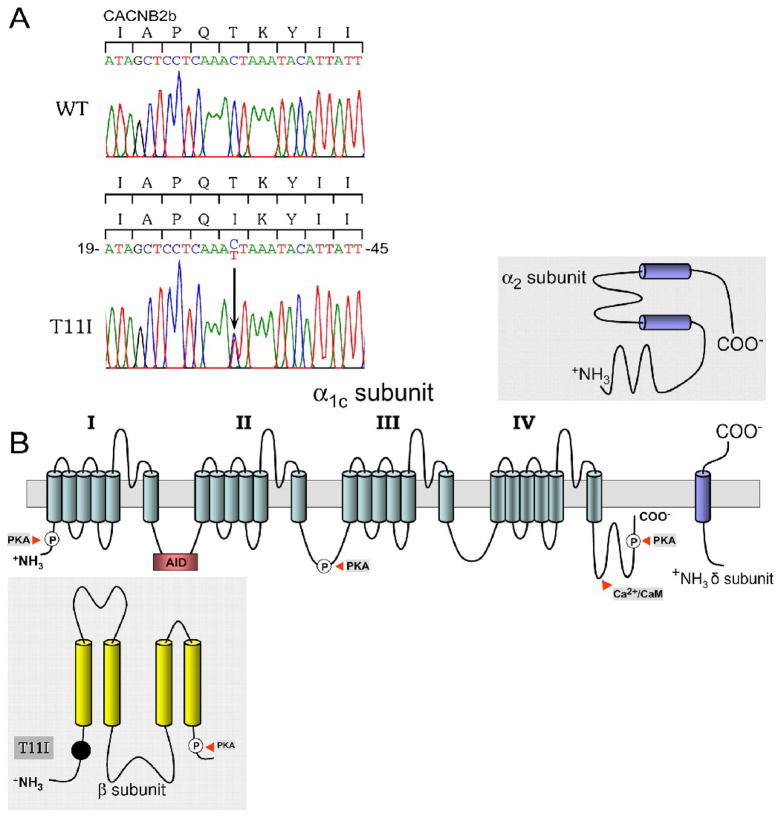
Analysis of the patient’s DNA showed a heterozygous C to T transition in exon 1 that predicted a substitution of threonine to isoleucine at position 11 (T11I) of CACNB2b, which was not present in 214 ethnically matched control alleles (Figure 2A). This mutation is located upstream of the -subunit interaction domain segment (Figure 2B), in variable domain 1 near the N-terminus [19].
Figure 2.
A: DNA sequencing analysis. C to T substitution in exon 1 of CACNB2b predicts an amino acid substitution of threonine for lysine at codon 11 (T11I). B: Location of the T11I in the $-subunit of Cav 1.2. The cardiac Ca2+ channel α-subunit consists of four domains each containing six transmembrane-spanning segments.
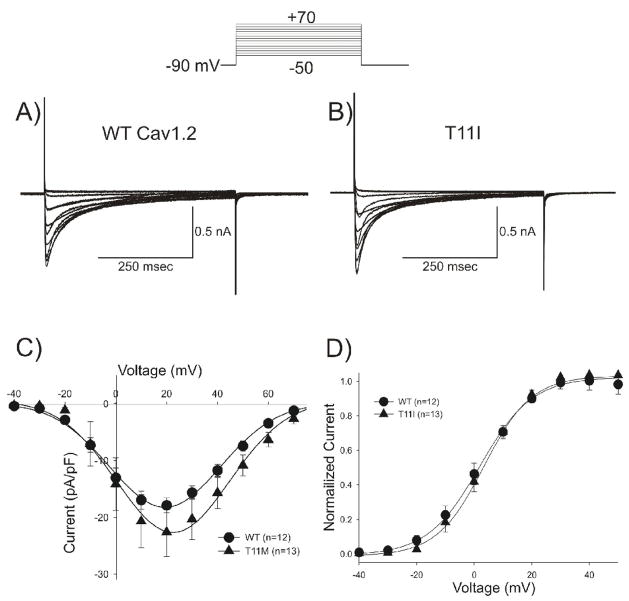
To determine how the mutation in CACNB2b altered the biophysical properties of Ca2+ current and contributed to the clinical phenotype, we expressed calcium channels in TSA201 cells and performed patch clamp experiments. To compare the current-voltage (I–V) relationship between WT and the mutant channels, depolarizing pulses were applied to the cells in 10 mV increments from a holding potential of −90 mV. Both WT (Figure 3A) and T11I mutant channels (Figure 3B) showed substantial current under these recording conditions. Analysis of the current-voltage (I–V) relation of peak ICa showed that the current density was not significantly different between WT and T11I mutant channels (Figure 3C). The activation threshold and voltage eliciting peak current were similar for the WT and T11I channels, suggesting there were minimal differences in the activation or availability (Figure 3C). This was confirmed by analysis of steady-state activation, which showed mid-activation voltages of +1.5±0.57 mV (n=12), and +3.5±0.49 mV, (n=13) for WT and T11I, respectively (p=NS, Figure 3D).
Figure 3.
Representative whole cell current recordings from a WT (A) and T11I mutant (B) expressed in TSA201 cells. Current recordings were obtained at test potentials between −50 and +60 mV in 10 mV increments from a holding potential of −90 mV. C: I–V relation for WT (n=12) and T11I (n=13) cells showing no statistically significant differences in peak calcium channel current density. D: Steady state-activation relation for WT and T11I. Chord conductance was determined using the ratio of current to the electromotive potential for the cells shown in Panel C. Data were normalized and plotted against their test potential.
To probe for differences in steady state gating parameters between WT and T11I mutant channels, steady state inactivation of ICa was evaluated using a standard prepulse-test pulse voltage clamp protocol (Figure 4, top). The peak current following application of a 10 s pre-pulse to voltages between −100 and +20 mV was normalized to the maximum current and plotted as a function of the prepulse voltage to obtain the availability of the channels. Data were fitted to a Boltzman equation to determine the membrane potential for half-maximal inactivation V1/2 and the slope factor k. Results showed a small but statistically significant shift in both steady-state inactivation and slope factor with mid-inactivation potentials of −24.8±0.46 mV, k=9.13±0.44 (n=13) for WT and −30.0±0.39 mV, k=6.25±0.31 (n=13) for T11I (p<0.05, Figure 4C). However, at normal cardiac membrane potentials both WT and mutant channels would exhibit full availability and it is unlikely that the difference in mid-inactivation would contribute to the clinical phenotype.
Figure 4.
Representative steady-state inactivation recordings for WT (A) and T11I (B) observed in response to the voltage clamp protocol shown at the top of the figure. C: Steady state-inactivation relation. Peak currents were normalized to their respective maximum values and plotted against the conditioning potential. T11I channels showed a mid-inactivation potential that was slightly but significantly hyperpolarized compared to WT channels.
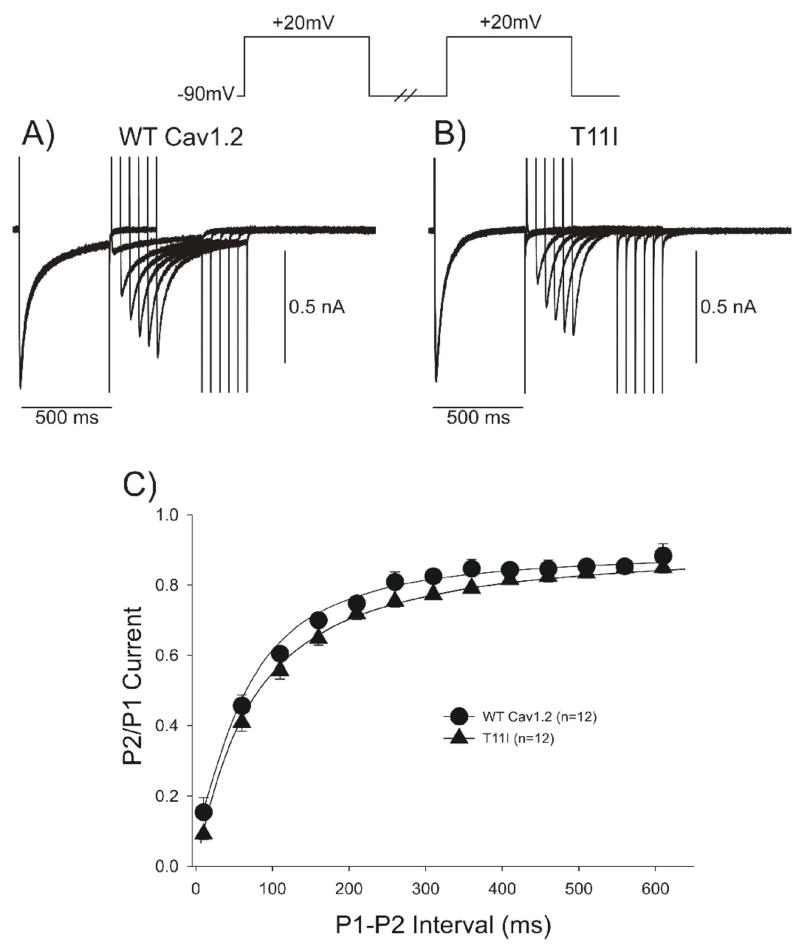
A double pulse protocol was used to examine recovery from inactivation (Figure 5). Representative traces of the frequency-dependent changes in ICa in WT and T11I channels are shown in Figure 5A and 5B. Recovery from inactivation of ICa at −90 mV was fit with a single exponential: τ = 101.4±3.7 ms for WT channels and τ =110.2±5.2 ms for T11I mutant channels (p=NS). The reactivation time course of ICa was similar for WT and mutant channels (Figure 5C).
Figure 5.
Representative traces recorded from a WT (A) and T11I mutant (B) showing recovery of ICa. Recovery was measured using two identical voltage clamp steps to +20 mV from a holding potential of −90 mV separated by selected time intervals. Recovery time-course fit to a single exponential showed no difference in recovery rate between WT and T11I channels (C).
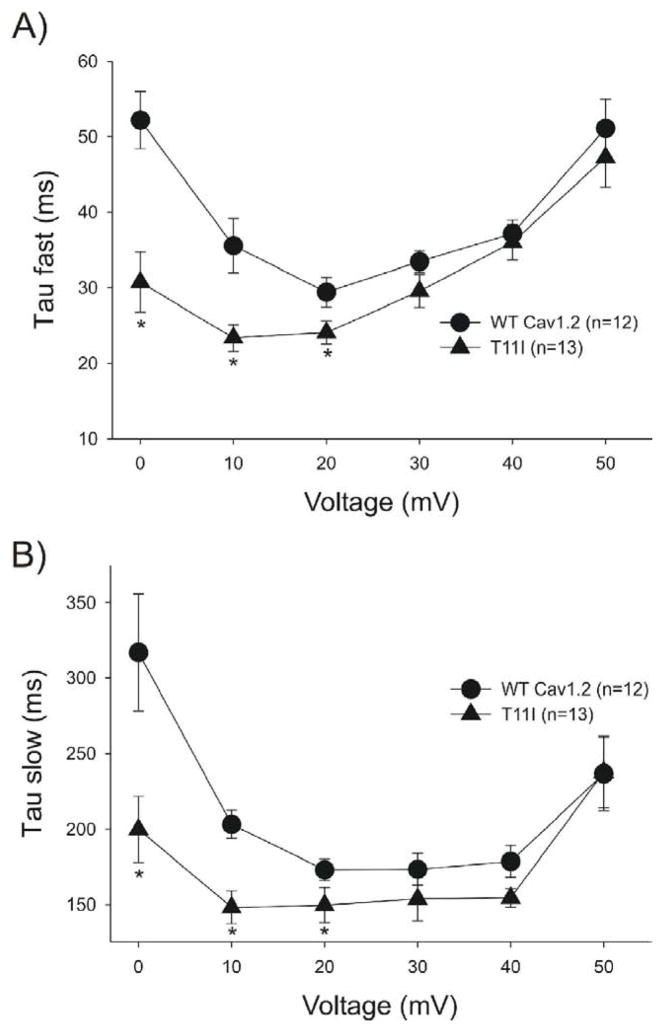
Although peak ICa density was not significantly affected by the T11I mutation, the inactivation of the current appeared to be accelerated. WT channels exhibited a residual pedestal current as previously described [20] which appeared to be smaller or absent in the mutant. To confirm, we next analyzed the decay of the current by analyzing the inactivation kinetics of ICa current and produced by either WT- CACNB2b or T11I - CACNB2b. The decay of ICa (traces shown in Figure 3) elicited by pulses positive to −10 mV was fit with a bi-exponential function. Both the fast (Figure 6A) and slow (Figure 6B) time constants (τ) of decay were shorter for the mutated vs. WT channels at potentials between 0 and +20 mV (p<0.05). These results demonstrate that the T11I mutation in CACNB2b accelerates the inactivation kinetics of ICa.
Figure 6.
A. Inactivation time constants (τ) for the fast phase of ICa decay as a function of voltage. Inactivation time constants (τ) values were measured by fitting a biexponential function to the current decay. *p<0.05 vs WT. B: Inactivation time constants (τ) for the slow phase of ICa decay as a function of voltage. *p<0.05 vs WT.
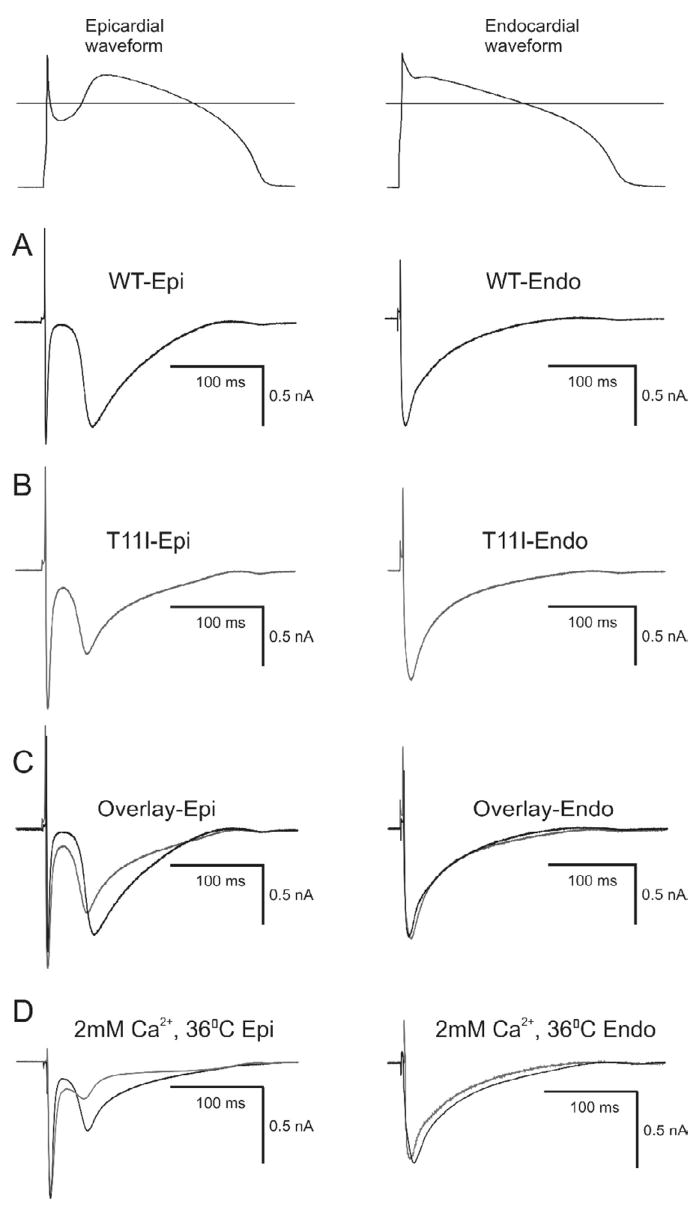
To assess whether the accelerated inactivation of the mutated channels leads to a reduction in total charge during the action potential, we evaluated total charge carried by ICa by integrating the area under the current trace elicited by an action potential clamp using waveforms previously recorded from canine epicardial (Epi) or endocardial (Endo) cells (Figure 7). In response to the Endo AP waveform, WT currents (Figure 7A) and T11I (Figure 7B) displayed no significant differences in the magnitude or decay of ICa. We next integrated the current during the dome of the Epi AP waveform. The integrated current was evaluated from the lowest of the notch to the end of the action potential waveform. The amount of total charge during the plateau of the Epi action potential was 42±2.3% less in the T11I mutant compared to WT channels (n=5, p<0.05). The use of elevated Ca2+ improved signal-to-noise ratio in our current recordings but may have affected Ca2+-dependent inactivation. Therefore, we performed the action potential clamp experiments under more physiological conditions (normal ionic concentrations at 36° C). The ICa recorded from WT channels following application of Epi and Endo waveforms (Figure 7D) closely resembled ICa recorded from myocytes under similar conditions [17,21]. At 36° C, WT and T11I currents displayed no significant differences in the magnitude or decay of ICa in response to the Endo AP waveform. However, the total charge during the plateau of the Epi action potential was 51±6.7% less in the T11I mutant compared to WT channels at 36° C (n=4, p<0.05). These observations confirm that the faster inactivation kinetics produced by T11I results in reduced depolarizing current contributing to the plateau of the epicardial action potential.
Figure 7.
Representative ICa currents elicited during action potential clamp experiments in TSA201 cells co-transfected with either WT or T11I CACNB2b. The action potentials were recorded from canine epicardial and endocardial cells which then served as the action potential clamp waveform (shown at top of figure). Horizontal line represents 0 mV. A: WT current traces following application of an epicardial and endocardial waveform. B: T11I currents following application of an epicardial and endocardial waveform. C: Superimposed current traces recorded from WT and T11I channels. Total charge during the plateau of the action potential was reduced by 42±2% (n=5) in T11I channels compared to WT when the epicardial waveform was applied. D: Superimposed current traces recorded from WT and T11I channels in 2 mM Ca2+ external and 36° C. Total charge during the plateau of the action potential was reduced by 51±6.7% (n=4, p<0.05) in T11I channels compared to WT when the epicardial waveform was applied.
DISCUSSION
Mutations in SCN5A are known to reduce Na+ current by a variety of mechanisms, leading to the development of BrS. One of the mechanisms includes accelerate inactivation of the cardiac Na+ current [2,11]. In the present study, we have identified a case of BrS in which the disease phenotype was observed as a result of accelerated inactivation of the L-type Ca2+ current without significantly affecting peak current. The accelerated inactivation was due to a mutation in CACNB2b, which encodes the β subunit of the cardiac L-type Ca2+ current. The carrier of this mutation exhibited ST-segment elevation in only one precordial lead which converted to a more typical BrS phenotype with a procainamide challenge. VT/VF was inducible and subsequently detected upon interrogation of the implanted ICD, corroborating the diagnosis of a potentially life-threatening syndrome.
Alterations in L-type Ca2+ current have been implicated in the development of BrS both clinically [9] and experimentally [8]. However, in both studies the BrS phenotype was the result of a loss in peak ICa. In the present study, the BrS phenotype appears to be loss of function occurring as a result of accelerated inactivation of ICa, similar to the mechanism described for INa [11]. Moreover, results from the action potential clamp experiments suggest that cells with a prominent spike and dome morphology (i.e., epicardial and midmyocardial) would be affected to a greater degree compared to cells lacking a prominent phase 1 and having a consistently high plateau (i.e., endocardial cells or left ventricular cells). These cell type-specific differences are explained by the fact that inactivation of T11I channels was significantly faster at potentials negative to +20 mV (Figure 6). The presence of a spike and dome morphology therefore results in a greater degree of inactivation during the early phases of the action potential.
In the majority of cases involving loss of function of ICa previously reported, a new clinical phenotype was observed in which BrS characteristics were combined with a shorter than normal QT interval [9]. The proband in the present study presented with the ECG and arrhythmic manifestation of BrS, but with a normal QT interval (QTc=428 ms). This is likely due to the fact that cells in the left ventricle, which generally lack a prominent spike and dome morphology, are little affected by the accelerated inactivation of ICa.
Arrhythmogenesis in BrS is believed to be the result of amplification of heterogeneities in action potential characteristics among the different transmural cell types in the right ventricular myocardium [22,8]. A decrease in INa [23] or ICa [9] or augmentation of any one of a number of outward currents including IKr [24] or Ito [7] can cause preferential abbreviation of the right ventricular epicardial action potential, leading to the development of spatial dispersion of repolarization and thus the substrate and trigger for VT, which is usually polymorphic and less frequently monomorphic [25]. In the present study, we demonstrate that the T11I channels displays faster inactivation of ICa preferentially in the right ventricular epicardium resulting in reduced depolarizing current.
In a small minority of patients, BrS presents in conjunction with vasovagal syncope, as in the case of our proband. Whether this particular genotype contributes to this phenotypic expression is not known and could be the subject of future studies.
The functional role of Cav subunits is to promote trafficking of the subunit to the membrane [26], change the voltage dependence of channel activation and alter channel gating [13,27]. Evidence suggests that a 1:1 ratio of and subunits is necessary to form a functional L-type Ca2+ channel [28]. The importance of the N-terminal region of the 2 subunit to Ca2+ channel gating has been described previously. Biophysical analysis of 5 Ca2+ channel 2 splice variants that differed only in their amino terminal revealed dramatic differences in the rate of channel inactivation. Consistent with that study, our mutation at position 11 of the 2 subunit resulted in accelerated inactivation of the Ca2+ current.
Recent PCR screening studies have uncovered a large diversity of Cav subunits in the human ventricle [29]. The 2b isoform is thought to be the major isoform [26] with smaller amounts of other isoforms such as 1d [30]. Immunocytochemical analysis of the localization and distribution of Cav2 in ventricular myocytes showed a predominant T-tubular staining with some staining at the surface sarcolemma [29]. A similar staining pattern has been observed in ventricular myocytes for L-type Ca2+ channels [31] further demonstrating a functional interaction between and subunits. The importance of 2b subunit in Ca2+ current inactivation was demonstrated by Gudzenko et al., who found that Ca2+-induced inactivation was 83% complete in the presence of the 2b subunit with a residual non-inactivating or pedestal current whereas in the absence of 2b, only 55% of the current inactivated [20]. WT current recorded in this study also exhibited a residual pedestal current which was absent in the T11I mutant. The absence of a pedestal current suggests less depolarizing current during the plateau of the action potential, an observation confirmed by AP clamp experiments (Figure 7).
Changes in action potential waveform and duration are known to affect excitation-contraction coupling [32,17,18]. In addition, it is well established that Ca2+ influx through L-type Ca2+ channels maintains the plateau of the action potential and initiates cardiac contractions [33]. However, the patients did not exhibit any impairment in cardiac output, presumably due to the fact that there was no reduction in QT interval or in peak ICa. In previous studies in which mutations in L-type Ca2+ channel were associated with a loss of current, no disruption in cardiac output was noted, suggesting compensatory mechanisms to preserve cardiac output [9]. For example, a greater proportion of the Ca2+ transient necessary for excitation-contraction coupling may be derived from the sarcoplasmic reticulum to compensate for reduced Ca2+ influx. Alternatively, there may be an upregulation of Ca2+ influx via other mechanisms, such as NCX [34,35].
Limitations of the Study
The results of our study show an acceleration of inactivation of L-type Ca2+ current. However, caution should be exercised when making the translation to the clinical setting. The TSA201 cells used in the present study expressed only the α, 2b, and α2δ subunits whereas cardiac myocytes likely have additional proteins which are not present in TSA201 cells. Some of these proteins include different β subunits as well as anchoring proteins, all of which combine to form the L-type Ca2+ channel [12]. It is likely that TSA201 cells are missing several key elements not present in the native myocytes. Although we clearly demonstrate that T11I produces accelerated inactivation of ICa, it is unclear to what extent the cardiac action potential duration would be affected due to the presence of other currents.
In summary, we have found a mutation in CACNB2b, the -subunit of the cardiac calcium channel in patient with BrS. Patch clamp analysis revealed that the mutation did not alter the magnitude of peak ICa but resulted in an acceleration of inactivation of L-type Ca2+ current. The accelerated inactivation of ICa translates into reduced depolarizing current preferentially in right ventricular Epi and M cells, which predisposes to the development of the Brugada phenotype. Thus, the results of the present study present a novel mechanism for the phenotypic expression of BrS.
Acknowledgments
We are grateful to Susan Bartkowiak for maintaining our Inherited Cardiac Arrhythmia Database and Judy Hefferon for assistance with the figures.
This study was supported by grants from the American Health Assistance Foundation (JMC), and US National Institutes of Health (NIH) grant HL47678 (CA).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Brugada, Brugada Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. Journal of American College of Cardiology. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Chen, Kirsch, Zhang, Brugada, Brugada, Brugada, Potenza, Moya, Borggrefe, Breithardt, Ortiz-Lopez, Wang, Antzelevitch, O’Brien, Schultze-Bahr, Keating, Towbin, Wang Genetic basis and molecular mechanisms for idiopathic ventricular fibrillation. Nature. 3191998;392:293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 3.Bezzina, Rook, Wilde Cardiac sodium channel and inherited arrhythmia syndromes. Cardiovascular Research. 212001;49:257–71. doi: 10.1016/s0008-6363(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 4.London, Michalec, Mehdi, Zhu, Kerchner, Sanyal, Viswanathan, Pfahnl, Shang, Madhusudanan, Baty, Lagana, Aleong, Gutmann, Ackerman, McNamara, Weiss, Dudley Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 11132007;116:2260–8. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Norstrand, Valdivia, Tester, Ueda, London, Makielski, Ackerman Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation. 11132007;116:2253–9. doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe, Koopmann, Le, Yang, Ingram, Schott, Demolombe, Probst, Anselme, Escande, Wiesfeld, Pfeufer, Kaab, Wichmann, Hasdemir, Aizawa, Wilde, Roden, Bezzina Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–8. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delpón, Cordeiro, Núñez, Thomsen, Guerchicoff, Pollevick, Wu, Kanters, Larsen, Burashnikov, Christiansen, Antzelevitch Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–18. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish, Antzelevitch Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1:210–7. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antzelevitch, Pollevick, Cordeiro, Casis, Sanguinetti, Aizawa, Guerchicoff, Pfeiffer, Oliva, Wollnik, Gelber, Bonaros, Burashnikov, Wu, Sargent, Schickel, Oberheiden, Bhatia, Hsu, Haissaguerre, Schimpf, Borggrefe, Wolpert Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 1152007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antzelevitch Brugada syndrome. Pacing and Clinical Electrophysiology. 2006;29:1130–59. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumaine, Towbin, Brugada, Vatta, Nesterenko, Nesterenko, Brugada, Brugada, Antzelevitch Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circulation Research. 10291999;85:803–9. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 12.Catterall, Perez-Reyes, Snutch, Striessnig International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacological Reviews. 2005;57:411–25. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 13.Morad, Soldatov Calcium channel inactivation: possible role in signal transduction and Ca2+ signaling. Cell Calcium. 2005;38:223–31. doi: 10.1016/j.ceca.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro JM, Marieb M, Pfeiffer R, Calloe K, Burashnikov E, Antzelevitch C. Accelerated inactivation of the L-type calcium due to a mutation in CACNB2b underlies the development of a Brugada ECG phenotype. Circulation. 10282008;118:S884–S885. doi: 10.1016/j.yjmcc.2009.01.014. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Splawski, Timothy, Sharpe, Decher, Kumar, Bloise, Napolitano, Schwartz, Joseph, Condouris, Tager-Flusberg, Priori, Sanguinetti, Keating Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 1012004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Splawski, Timothy, Decher, Kumar, Sachse, Beggs, Sanguinetti, Keating Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 672005;102:8089–96. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordeiro, Greene, Heilmann, Antzelevitch, Antzelevitch Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2004;286:H1471–H1479. doi: 10.1152/ajpheart.00748.2003. [DOI] [PubMed] [Google Scholar]
- 18.Cordeiro, Malone, Di Diego, Scornik, Aistrup, Antzelevitch, Wasserstrom Cellular and subcellular alternans in the canine left ventricle. Am J Physiol Heart Circ Physiol. 9282007 doi: 10.1152/ajpheart.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van, Clark, Chatelain, Minor Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 6102004;429:671–5. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudzenko, Shiferaw, Savalli, Vyas, Weiss, Olcese Influence of channel subunit composition on L-type Ca2+ current kinetics and cardiac wave stability. Am J Physiol Heart Circ Physiol. 2007;293:H1805–H1815. doi: 10.1152/ajpheart.01160.2006. [DOI] [PubMed] [Google Scholar]
- 21.Hund, Rudy Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 11162004;110:3168–74. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan, Antzelevitch Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 10121999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 23.Vatta, Dumaine, Varghese, Richard, Shimizu, Aihara, Nademanee, Brugada, Brugada, Veerakul, Li, Bowles, Brugada, Antzelevitch, Towbin Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Human Molecular Genetics. 212002;11:337–45. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Verkerk, Wilders, Schulze-Bahr, Beekman, Bhuiyan, Bertrand, Eckardt, Lin, Borggrefe, Breithardt, Mannens, Tan, Wilde, Bezzina Role of sequence variations in the human ether-a-go-go-related gene (HERG, KCNH2) in the Brugada syndrome1. Cardiovascular Research. 1212005;68:441–53. doi: 10.1016/j.cardiores.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch, Brugada, Borggrefe, Brugada, Brugada, Corrado, Gussak, LeMarec, Nademanee, Perez Riera, Shimizu, Schulze-Bahr, Tan, Wilde Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 1172005;111:659–70. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 26.Colecraft, Alseikhan, Takahashi, Chaudhuri, Mittman, Yegnasubramanian, Alvania, Johns, Marban, Yue Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 612002;541:435–52. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobrinsky, Kepplinger, Yu, Harry, Kahr, Romanin, Abernethy, Soldatov Voltage-gated rearrangements associated with differential β-subunit modulation of the L-type Ca2+ channel inactivation. Biophys J. 2004;87:844–57. doi: 10.1529/biophysj.104.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalton, Takahashi, Miriyala, Colecraft A single CaVbeta can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol. 9152005;567:757–69. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foell, Balijepalli, Delisle, Yunker, Robia, Walker, McEnery, January, Kamp Molecular heterogeneity of calcium channel β-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 4132004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- 30.Cohen, Foell, Balijepalli, Shah, Hell, Kamp Unique modulation of L-type Ca2+ channels by short auxiliary beta1d subunit present in cardiac muscle. Am J Physiol Heart Circ Physiol. 2005;288:H2363–H2374. doi: 10.1152/ajpheart.00348.2004. [DOI] [PubMed] [Google Scholar]
- 31.Brette, Salle, Orchardm Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circulation Research. 792004;95:e1–e7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
- 32.Sah, Ramirez, Oudit, Gidrewicz, Trivieri, Zobel, Backx Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)) J Physiol. 112003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bers Cardiac excitation-contraction coupling. Nature. 1102002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 34.Levi, Spitzer, Kohmoto, Bridge Depolarization-induced Ca entry via Na-Ca exchange triggers SR release in guinea pig cardiac myocytes. Am J Physiol. 1994;266:H1422–H1433. doi: 10.1152/ajpheart.1994.266.4.H1422. [DOI] [PubMed] [Google Scholar]
- 35.Litwin, Li, Bridge Na-Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–71. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]