Abstract
Alkaloid profiles in skin of poison frogs/toads (Dendrobatidae, Mantellidae, Bufonidae, and Myobatrachidae) are highly dependent on diet and hence on the nature of habitat. Extracts of the two species of toads (Melanophryniscus klappenbachi and M. cupreuscapularis) from similar habitats in the Corrientes/Chaco Provinces of Argentina have similar profiles of alkaloids, which differ considerably from profiles from other Melanophryniscus species from Brazil, Uruguay and Argentina. Structures of two major alkaloids 239Q (1) and 275I (2) were determined by mass, FTIR, and NMR spectral analysis as 5Z,9Z-3-(1-hydroxybutyl)-5-propylindolizidine and 6Z,10E-4,6-di(pent-4-enyl) quinolizidine, respectively. A third alkaloid, 249F (3), is postulated to be a homopumiliotoxin with an unprecedented conjugated exocyclic diene moiety.
Keywords: Alkaloids, ants, bufonidae, dietary arthropods, mass spectrometry, mites
1. Introduction
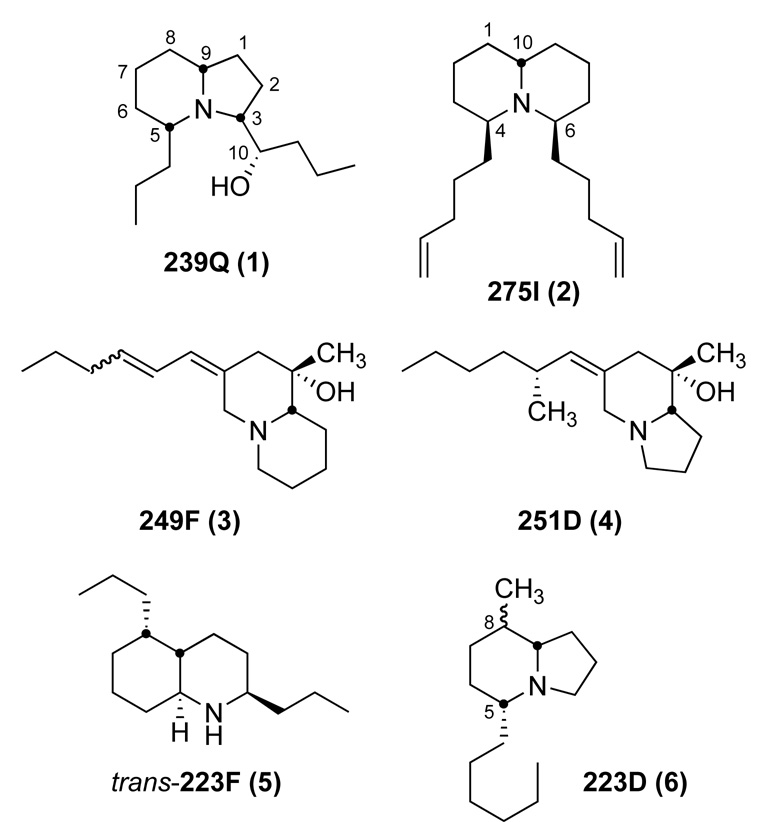
Alkaloids in skins of anurans were first discovered for a Colombian poison-dart frog (Phyllobates aurotaenia) of the neotropical family Dendrobatidae (Tokuyama et al., 1969). Following this discovery, numerous other alkaloids of diverse structural classes were subsequently identified from other species of dendrobatid frogs, and by 1978 they were collectively referred to as “dendrobatid alkaloids” (Daly et al., 1978). Then in 1984, such dendrobatid alkaloids were reported from a bufonid toad from South America (Melanophryniscus moreirae), two mantellid frogs from Madagascar, and a myobatrachid toadlet from Australia (Daly et al., 1984). Subsequently, a diverse array of alkaloids was found to be present in skin extracts of other species of Melanophryniscus toads (Garraffo et al., 1993; Mebs et al., 2005; Daly et al., 2007). It now appears that all the skin alkaloids of the dendrobatid, mantellid and bufonid anurans are sequestered from dietary alkaloid-containing arthropods (Daly et al., 2002; Jones et al., 1999; Saporito et al., 2004, 2006, 2007a, b; Takada et al., 2005), while the skin pseudophrynamines of the myobatrachid frogs (Pseudophryne) are produced by the frogs (Smith et al., 2002). Both dietary specialization and microphagy have been proposed as significant components in the ecology and evolution of sequestered defenses in poison frogs, and in dendrobatid poison frogs, multiple origins of dietary specialization have been proposed (see Toft, 1995; Caldwell, 1996; Vences et al., 1998; Santos et al., 2003; Darst et al., 2005). Recently, the over 800 alkaloids detected in amphibian skin extracts were summarized along with their occurrence in the four above-mentioned anuran families and, for some, the discovery of putative dietary arthropod sources (Daly et al., 2005). The present report documents the occurrence of alkaloids in two bufonid species (Melanophryniscus klappenbachi and M. cupreuscapularis) from neighboring locales in the Corrientes/Chaco Provinces of Argentina. Remarkably, three of the major alkaloids in these extracts had not been previously detected in any extracts of anuran skin and represent new alkaloids from poison frogs. Structures for the 3,5-disubstituted indolizidine 239Q (1) and the 4,6-disubstituted quinolizidine 275I (2) are proposed based on mass spectral and FTIR spectral analysis and in the case of 239Q, NMR spectral analysis. The structure of the third major alkaloid 249F remains uncertain, but is postulated to be that of an unusual homopumiliotoxin diene (3). Structures of 1, 2, and 3 and three other minor alkaloids (4, 5, 6) found in extracts of skins of these two bufonid species are shown in Figure 1.
Figure 1.
Structures of major and minor alkaloids detected from M. klappenbachi and M. cupreuscapularis.
2. Materials and Methods
2.1. Bufonid toads (Melanophryniscus)
Specimens of Melanophryniscus klappenbachi were collected in February 1999 in fields near the Aeropuerto Viejo, Resistencia, Chaco, Argentina. The eleven skins (3.3 g wet weight) were placed in methanol. Specimens of Melanophryniscus cupreuscapularis were collected also in February 1999 near Perichón, Corrientes, Argentina. The eleven skins (1.3 g wet weight) were placed in methanol.
The Melanophryniscus specimens were dissected and prey items were classified by taxon, to family or ordinal level, and life stage, using a binocular microscope. Items that could not be identified under any order but which were clearly of animal origin were classed only as individuals. Abundance of each of the various prey items was estimated from digestive tract contents. For each taxon, the frequency of occurrence (number of digestive tracts containing that particular taxon divided by the total number of digestive tracts analyzed) was calculated according to the formula of Lescure (1971).
2.2. Instrumentation
Mass spectral data [EI-MS and CI-MS (NH3)] were obtained with a Finnigan GCQ instrument, having a Restek RTX-5MS capillary column (30 m, 0.25 mm i.d.) programmed from 100° to 280° C at 10°C per min. GC-FTIR and EI-MS spectra were obtained in series with a Hewlett-Packard model 5890 gas chromatograph, having an HP-5 fused silica-bonded capillary column (30 m, 0.32 mm i.d.) programmed from 100° to 280° C at a rate of 10°C per min and interfaced with a Hewlett-Packard model 5971 Mass Selective Detector and a Model 5965B IRD with a narrow band (4000-750 cm−1) detector. A Hewlett-Packard ChemStation was used to generate EI-MS and FTIR spectra. High resolution GC-MS data was generated with a Waters GCT instrument. The 1H-NMR spectra were measured with a Varian VXR-500S spectrometer. An Agilent Model 1100 LC with a binary pump and UV detection at 260nm was used for the alkaloid work and purification. This LC with a DAD detector (Thermo-Finnigan UV6000LP) was used interfaced with a Thermo-Finnigan LCQ mass spectrometer in the APCI mode for the bufadienolide / cardenolide work. Typically a vaporizer heater at 480°C and capillary heater at 150°C and a flow rate of 0.5 mL / min was used.
2.3. Isolation and Analysis
The methanol extracts were subjected to acid-base partitioning as described (Garraffo et al., 1993). The resultant alkaloid fractions were investigated by GC-MS and GC-FTIR spectral analysis (see Results). About one-half of the alkaloid fraction from Melanophryniscus klappenbachi in methanol was concentrated to 125 µL and 25 µL portions were subjected to HPLC fractionation. A reversed-phase column (Phenomenex column AQUA-125A, C18, 250 mm × 4.6 mm i.d. with particle size 5 µm) was used with CH3CN (0.1% HOAc)-H20 (0.1% HOAc) and a gradient from 10:90 to 90:10 over a 30 min period with a flow rate of 0.5 mL/min. Thirty fractions of 0.5 mL were collected, and alkaloid content assayed by GC-MS. The fractions containing nearly pure 239Q were combined and used for NMR spectral analyses. APCI mass spectrometry with a Thermoquest-Finnigan LCQ LC-MS and the above solvent system and program yielded purified 239Q with a protonated molecular ion of m/z 240 and a single fragment showing loss of water from m/z 240.
3. Results
The alkaloids detected and identified by GC-MS and GC-FTIR spectral analysis are documented in Table 1.
Table 1.
Occurrence of alkaloids in skin extracts of two Argentinian species of Melanophryniscus.a
| Alkaloid | M. klappenbachi | M. cupreuscapularis | |
|---|---|---|---|
| Pumiliotoxins | |||
| 251D (4) | ++ | ---- | |
| 277B | ---- | + | |
| 307F | ---- | +,+ | |
| 307G | ---- | + | |
| 323A | + | ---- | |
| Homopumiliotoxins | |||
| 249F (3) | ++,+ | +++,++,+ | |
| 265N | + | ---- | |
| Decahydroquinolines | |||
| 223F (5) | ++,+ | --- | |
| 269AB | + | + | |
| 269B | + | ---- | |
| 271D | + | ---- | |
| 3,5-Disubstituted Indolizidines | |||
| 195B | + | ---- | |
| 5Z,9Z-223AB | + | + | |
| 239Q (1) | +++ | +++ | |
| 5,8-Disubstituted Indolizidines | |||
| 207A | + | ---- | |
| 223D (6) | ++ | ---- | |
| 259B | + | ++,++ | |
| Dehydro-5,8-Disubstituted Indolizidines | |||
| 219G | + | ---- | |
| 249L | + | ---- | |
| 1,4-Disubstituted Quinolizidines | |||
| 259E | ---- | + | |
| 4,6-Disubstituted Quinolizidines | |||
| 275I (2) | +++ | ++ | |
| 279H | ---- | + | |
| Izidines | |||
| 211C | ---- | + | |
| Tricyclics | |||
| 193C | ---- | + | |
| 205H | ---- | + | |
| 205I | + | + | |
| 235M | ---- | + | |
| Unclassified | |||
| 193K | ---- | + | |
| 207S | + | ++ | |
| 209R | ---- | + | |
| 223EE | + | ---- | |
| 223T | + | + | |
| 235S | + | ++ | |
| 293H | ---- | + | |
| 305H | ---- | + | |
| 307J | + | ---- | |
Major (+++, > 50 µg/100 mg skin); Minor (++, <50 and >5 µg per 100 mg skin); Trace (+, <5 µg per 100 mg skin). Two or more entries for an alkaloid in any species indicate isomers. Other trace alkaloids were present, but data was not sufficient to assign a code number and letter. For structures of some of the above alkaloids, including tentative structures, see Figure 1 and Daly et al., 2005. The postulated structure of 259B requires revision (Nelson et al., 2008), as does that of 207S.
The GC-MS chromatograms are shown in Figure 2A and 2B. The FTIR spectra for the three major new alkaloids (239Q, 275I, and 249F) are shown in Figure 3A,3B and 3C. The spectral data are as follows:
Figure 2.
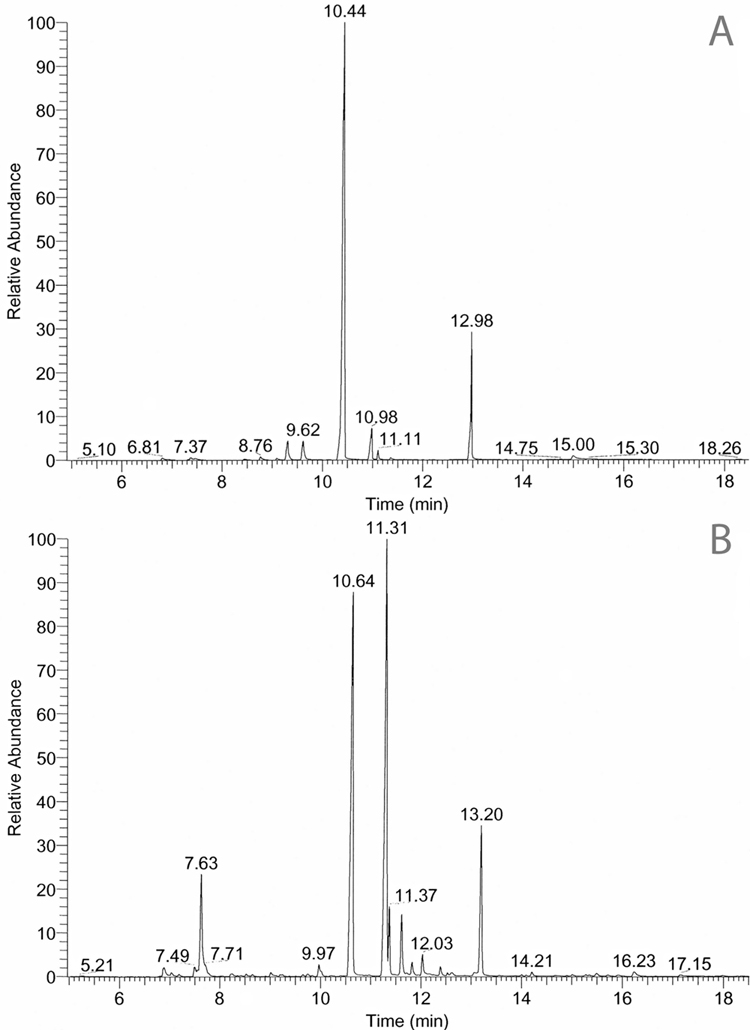
GC-MS chromatograms for A) Melanophryniscus klappenbachi and B) Melanophryniscus cupreuscapularis. Alkaloids were identified from retention times (Rt) and spectral data that are documented in Daly et al., 2005. Major and minor alkaloids are as follows: A: 223F (5), 9.20 min; 223D (6), 9.62 min; 239Q (1), 10.44 min; 249F (3), 10.98 min; 251D (4), 11.11 min; 275I (2), 12.98 min. B: 207S, 7. 63min, (see text); 235S, 9.97 min; 239Q (1), 10.64 min; 249F (3), three isomers, 11.31, 11.83, 12.03 min; 259B, two isomers, 11.37, 11.62 min; 275I (2), 13.20 min. Rts for 1, 2, and 3 differ between A and B, as the analyses were made several years apart. The x’s indicate non-alkaloid impurities.
Figure 3.
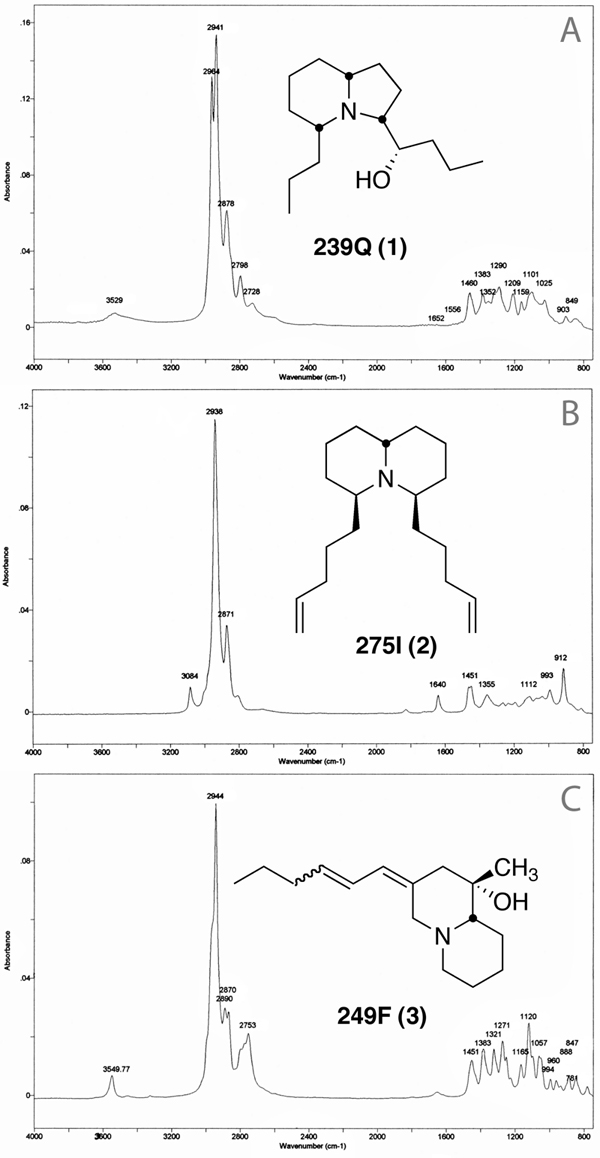
FTIR spectra for A) 3,5-disubstituted indolizidine 239Q (1); B) 4,6-disubstituted quinolizidine 275I (2); and C) the putative homopumiliotoxin diene 249F (3).
239Q (1)
The major GC peak at rt 10.70 min had a MW of 239 as determined with CI-MS (NH3) and had one exchangeable hydrogen as indicated by m/z 242 using ND3. The molecular formula was C15H29NO by HR-MS (mass measured on m/z 238 (M-H)) with a base peak at m/z 166, mass measured as C11H20N indicating a C4H9O loss. The exchangeable hydrogen is in this fragment as CIMS (ND3) still had a detectible EI fragment (22%) at m/z 166. A minor mass fragment at m/z 196 (ca. 10%) by HR-MS fits C12H22NO, indicating a propyl fragment is cleaved. In the structure we propose for 239Q (see Fig. 1), this propyl loss can occur from both the 5- position by α-cleavage and from the 3-(1-hydroxybutyl) group by cleavage at the 1′-position. There was also a significant (10%) fragment ion at m/z 70. LC-MS with APCI indicated a major ion at m/z 240 with only a loss of 18 amu (H2O) producing an ion at m/z 222 (6%). The presence of a hydroxyl group was confirmed by GC-FTIR where the vapor phase infrared spectrum indicated a very broad absorption, centered at 3531 cm−1 suggesting a hydrogen-bonded hydroxyl group (νO-H). Another absorption at 1209 cm−1 (νC-O) is also seen. The FTIR spectrum with a moderate Bohlmann band at 2798 cm−1 (see Fig. 3A) was typical of a 3,5-disubstituted indolizidine with the 5Z, 9Z configuration. Quantitation by GC-MS with a standard of another 3,5- disubstituted indolizidine, 239AB (3 µg/µL) indicated 239Q was present in the M. klappenbachi extract at a concentration of 16.5 µg/µL.
Thus the mass and IR spectral data indicated an indolizidine ring structure with α-propyl and α–hydroxybutyl side-chains and the three α-hydrogens being on the same molecular face. Frog skin alkaloid 239AB has a 4′-hydroxybutyl substituent at C-3 and a propyl substitutent at the C-5 position. We assumed ab initio that the propyl and butyl side-chains in 239Q would likewise be at C-5 and C-3 respectively, as usually (but not invariably) the odd-carbon side-chain is at C-5 in frog skin or arthropod indolizidines, and the even-carbon side-chain is at C-3. The position of the hydroxyl group in the butyl side-chain of 239Q remained unassigned as did conclusive proof of the ring position of the side-chains. The IR spectrum indicated the hydroxyl group was hydrogen-bonded to nitrogen and consequently was likely at either the 1′ or 2′ position of the butyl group and not the terminal position as seen previously with 239AB (Daly et al. 2005). Another naturally occurring regioisomer, 239CD, also had a terminal hydroxyl in the propyl group.
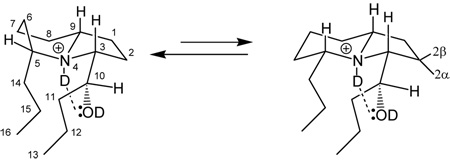
An 1H-NMR spectrum on HPLC purified 239Q was surprisingly complex. The deuterochloride in d6-acetone revealed two methyl triplets (δ 0.93, 0.89), not in the expected 1:1 ratio, but rather in ca. a 2:1 ratio, respectively. The hydrogen on the carbon bearing the hydroxyl group is likewise seen as a pair of doublets (δ 4.19, 4.20) however in ca. a 3:2 ratio. Three downfield signals (δ 3.1–3.8) are assigned to the α-CH-ND⊕ protons and δ 4.2 to the CH-OD proton. Proton H-3 shows up as a single dd signal at δ 3.73 (J = 10.4, 2.6 Hz). This was shown by a clear NOESY cross peak to be coupled with the downfield CH-OD (H-10) signal, even though no H-3—H-10 scalar coupling is detected. The broad triplet signal assigned to H-5 shows no such cross peak but only methylene hydrogen cross peaks (δ 1.7–2.5) indicating that the hydroxybutyl group is at C-3 as anticipated. Molecular modeling and energy-minimized structures with an imposed hydrogen bond (⊕N-D⋯O(D)-C-10) was consistent with the hydroxyl group being adjacent to the five-membered ring (i.e. the 1′-butyl position) and having a dihedral angle (θ3–10) approaching 100° and J ≈ 0 Hz if the R* configuration is assigned to C-10. Consequently H-3 shows only coupling with two hydrogens with large and small coupling constants assigned to 2β- and 2α-ring protons, respectively. On 1D irradiation, the H-10 proton showed coupling only with multiplets at δ 1.37 and 1.50, assigned to side-chain H-11 protons. Other 1D- and 2D H-H (TOCSY and Relay-H) couplings are reported in Table 2 as well as several relevant NOESY cross peaks.
Table 2.
1H-NMR Assignments for 239Q (1) in d6-acetone.
 | |||||
|---|---|---|---|---|---|
| H-# | δ (m, J (Hz))a | 1D-irradiation at H-# affects signal at δ |
1D-irradiation at δ affects signal at H-# |
TOCSY Cross-peaks |
NOESY Cross-peaks |
| 1 | 2.32 J=lg. | ||||
| 2 | 1.78 J=lg ; 2.32, J=sm. (2β, 2α respectively) | ||||
| 3 | 3.73 dd (10.4, 2.6) | 1.83, 1.88–1.93; 2.16–2.21 2.32 (sm. J removed) | 1,52–1.56; 1.77, 1.88–1.89; 2.16–2.23; 2.32 | 1.9 [1.95, 2.0, 2.2, 2.32 3.13] | H3-H9; H3-H10 |
| 4 NH, 6d | 10.7 broad s | ||||
| 5 | 3.12 dddd (12.0, 10.8, 3.0, 1.5) appears as br. t | 2.26–2.35 removes lg. J (t→d), 1.91, 1.74–1,82 (removes lg. J) | 1.75–1.77, 1.89, 2.16–2.23. | 2.32 [1.6, 1.8, 1.9, 1.95, 2.0, 2.2] | |
| 6 | 1.78, 2.32 | 1.91–1.94; 2.28–2.34 | 1.89; 2.16–2.23 | 1.8, 2.2 | |
| 7/8 | 2.32 | 1.95, 2.20 | |||
| 9 | 3.25 dddd (11.6, 11.3, 3.0, 2.8) | 2.21–2.26, sm. J with H-3 removed at δ 2.17–2.21 [dd→d; “U”-type coupling?] 2.32 two lg. Js removed. | 1.76–1.81; 1.92; 2.17–2.23; | 1.95, 2.22 [1.6, 1.9, 2.04, 2.32] | H9-H3 |
| 10 | 4.19, 4.2 (3:2, d, J=8.5) | 1.33–1.38 (→s), 1.48–1.5 (dd→d) | 1.33–1.38; 1.46–1.52; | 1.4 [1.5] | H10-H3 |
| 11 | 1.36–1.4; 1.49–1.5 | 1.48–1.52 | |||
| 12 | 1.48–1.52 (A) 1.28–1.31 (B) | ||||
| 13 | 0.93 (A, B); J=7.3 | 1.52–1.55 | 1.52–1.55 | 1.55 | |
| 15 | 1.40 | ||||
| 16 | 0.89 (A); 0.93 (B) 1:2, t J=7.3 | 1.37–1.38 | 1.47–1.52 | 1.5, 1.78, 2.12 | |
| OH, 6 d | 5.75; 5.8 3:2 (J=5,1) | ||||
s, singlet; d, doublet; t, triplet; br., broad; 6d, spectra on back-exchanged alkaloid after 6 days.; lg,, large J; sm,, small J; tocsy data in brackets are for the longest mixing time (0.05 sec); other data is for 0.002 sec.
The complexity of the NMR spectrum, we think, arises from a conformational equilibrium between the conformers A and B, in which the six-membered ring is significantly in a boat conformation to minimize the butyl-propyl side-chain steric interactions resulting from the R* configuration at C-10. If the six-membered ring exists in a 5:3 boat:chair proportion and if the terminal methyls (C-13, C-16) overlap in the chair but not in the boat, where the terminal methyl (C-16) of the propyl group is shielded and slightly upfield, then a 2:1 overall proportion of methyls at C-13 and C-16 arises. Also the two signals observed for HO in approximately a 3:2 ratio, most clearly and directly seen in the back-exchanged spectrum discussed below (but also for H-10 in the initial spectra), are explained (63%A : 37%B). See Figure with Table 2. The five-membered ring is locked into the envelope conformation in both A and B by the H-bond. Because H-3 is a simple, uncomplicated dd signal, we do not ascribe the complexity of H-10 to two different hydroxyl C-10 diastereomers for the two resulting dihedral angles of H3-H10 cannot both have Js of zero. Furthermore, the GC chromatogram of 239Q shows only a single homogeneous peak, not two peaks as typically seen with a diastereomeric pair. Invoking however a conformational equilibrium between 5 parts A and 3 parts B does explain the observed 1H-NMR spectrum. The α- proton at H-5 is a complex multiplet resembling a triplet with two large Js and several small or medium Js. We think this reflects conformational equilibria involving the six-membered ring only. Minimized energies are 36.5 and 29.8 kcal/mol for A and B, respectively.
Additional complications arose when the sample was reexamined after standing in a capped tube for one week and several new signals appeared. These were ascribed to a slow exchange occurring with the OD and ND groups when ambient H2O in the vapor above the acetone in the NMR tube exchanged for those deuteriums in 239Q•DCl in d6-acetone. The signals for H-3, H-5 and H-9 had all become more complex as additional coupling with ⊕N-H now exists. New signals at δ 5.78 and 5.80 (3:2 parts respectively) now are seen which we assign to the HO-C(10) signals, A J≈ 5 Hz is seen with the H-10—OH doublet in each of the two conformers. The new N-H signal is seen as a very broad singlet at δ 10.7 and on irradiation, the H-3, H-5 and H-9 signals all simplify as a trans coupling with ⊕NH is removed. On irradiation of the HO signals at C-10, H-10 becomes a narrower but still complex multiplet, indicating that the H-10 signals are a composite now of HOCH- and the original CH(10)–CH(11) couplings. Irradiation of H-11 at δ 1.37 removes the J10–11 coupling and with higher power, collapses H-10 to a singlet and also the upfield methyl triplet at δ 0.89 becomes a doublet indicating that all the additional complications seen in the 1H-NMR on standing one week can all be explained by a back-exchange. Irradiation of H-10 removes a small J (ca. 1 Hz) from HO coupling with H-10, leaving a doublet of ca. 5 Hz. On reexamination of the NMR sample by GC-MS, no change was seen in the mass spectrum or retention time, indicating all the NMR changes are due to proton back-exchange. On additional standing, signals for H2O (δ 2.85) and acetone (δ 2.05) are observed. Reexamination of 1D irradiations affecting the H-3, H-5, H-9 and H-10 protons in the back-exchanged spectra were unchanged from the original irradiations indicating the structure was unchanged.
Because of the complexity of the proton NMR spectrum, we found 2D spectra less useful than 1D irradiations. We focused mostly on those involving the four downfield signals and the upfield methyls. Irradiation at δ 1.35–1.37 (H-11) perturbs both the doublets at H-10 to a nearly overlapping pair of signals approximating broad singlets and collapses the upfield methyl triplet (H-16, δ 0.89) to a doublet. Here one of the H-15 protons in the boat configuration is affected as well as H-11 in both conformers A and B. Molecular modeling indicates that one H10-H11 has a J approaching zero. This proton at H-11 in A/B is shown to fall at δ 1.50 on irradiation of the pair of doublets of H-10 (δ 4.19) where a change is seen at both δ 1.50 and δ 1.36–1.40. Irradiation at δ 1.60 changes the downfield triplet at H-5 to a doublet-like signal. We think that the signal for H-5 (a broad triplet) is complex because of the conformational equilibrium and this is reflected in the couplings of H-5 with protons at δ 1.77, 1.90–1.93, 2.26–2.35. Additional assignments in Table 2 reflect these 1D-irradiations. The 1H-NMR data for 239Q•DCl is tabulated in Table 2 below.
Thus, the most likely structure for 239Q is that of a 3-(1-hydroxybutyl)-5-propyl indolizidine (1) (Figure 1), but because of certain anomalies in the NMR spectra, further studies are warranted if additional pure compound can be isolated. The postulation of the conformational equilibrium would, for example, be tested by spectra taken at various temperatures.
275I (2)
The proposed structure of major alkaloid 275I (2), shown in Figure 1, is based on MS and FTIR spectral analyses. High resolution MS indicated an empirical formula of C19H33N. The mass spectrum was dominated by loss of C5H9 to afford a base peak at m/z 206. A McLafferty rearrangement of this ion with concerted loss of C5H8 afforded a diagnostic fragment at m/z 138 (20) that was mass measured and fits C9H16N. A fragment ion at m/z 84 (5) indicated a quinolizidine rather than an indolizidine, where m/z 70 would be expected instead. No exchangeable hydrogens were detected using CIMS (ND3). On LC-MS with an APCI interface, an ion at m/z 276 was seen with no other significant fragments. The FTIR spectrum also indicated a quinolizidine with a weak Bohlmann band at 2813 cm−1 (Figure 3B). That the side-chain double bonds are terminal is indicated by a C=CH2 absorption at 3084 cm−1, a νC=C at 1641 cm−1 and absorptions at 993 and 912 cm−1 in the FTIR spectrum.
Thus, the most likely structure is that of a 4,6-dipent-4-enyl quinolizidine (2) with the relative configuration shown being based on that of another 4,6-disubstituted quinolizidine, 195C, from amphibian skin, whose Bohlmann bands are similar to those of 275I (Jones et al., 1999). However, NMR spectral data on isolated 275I will be required to confirm the proposed relative configuration.
249F (3)
A major alkaloid, 249F, in Melanophryniscus cupreuscapularis did not exhibit a mass spectral fragmentation diagnostic for any of the structural classes of alkaloids from amphibian skin and it was left unclassified in Daly et al., 2005. The empirical formula is C16H27NO. There is a significant loss of ethyl to yield a fragment at m/z 220, while the base peak is at m/z 84. The FTIR spectrum has a moderate, broad Bohlmann band at 2755 cm−1 and an absorption at 3550 cm−1 indicating a hydrogen-bonded OH (Figure 3C) falling in the characteristic range of the tertiary hydroxyl absorption in homopumiliotoxins. A possible structure for 249F (3), shown in Figure 1, is that of an unusual homopumiliotoxin with a conjugated exocyclic diene. The FTIR spectrum shows a weak absorption at 1650 cm−1 (likely νconj. C=C) not seen in homopumiliotoxins but a fingerprint region nearly identical with another homopumilotoxin 265N. Integration of the νCH asymm region for methyls and methylenes indicates two methyl groups are present (Garraffo, unpublished results) indicating that the usual side-chain allylic methyl of the pumiliotoxins and homopumiliotoxins is missing and is replaced by a double bond. That structure must be considered tentative until isolated compound can be subjected to NMR spectral analysis.
The gastrointestinal contents for M. klappenbachi and M. cupreuscapularis are presented in Table 3 and Table 4. Ants represented the major components that were detected.
Table 3.
Main prey taxa encountered in gastrointestinal tracts of Melanophryniscus klappenbachia
| PREY TYPES | n | f (%) | fa | FO (%) |
|---|---|---|---|---|
| ARTHROPODA | ||||
| INSECTA | ||||
| HYMENOPTERA | ||||
| Apidae | ||||
| Apis melifera | 2 | 0.17 | 2 | 5.9 |
| Formicidae | ||||
| Ecitoninae | ||||
| Labidus praedator | 27 | 2.3 | 10 | 29.4 |
| Myrmicinae | ||||
| Crematogaster quadriformis | 67 | 5.8 | 21 | 61.8 |
| Wasmannia auropunctata | 101 | 8.7 | 21 | 61.8 |
| Pheidole sp. | 22 | 1.9 | 7 | 20.6 |
| Pheidole subaberrans | 78 | 6.7 | 14 | 41.2 |
| Solenopsis sp. | 14 | 1.2 | 6 | 17.6 |
| Acromyrmex hispidus (inmature instars) | 778 | 67.2 | 27 | 79.4 |
| DIPTERA | ||||
| Culicidae | 2 | 0.17 | 2 | 5.9 |
| Ephedridae | 1 | 0.08 | 1 | 2.9 |
| COLLEMBOLA | 7 | 0.60 | 6 | 17.6 |
| ARACHNIDA | ||||
| ARANAE | ||||
| Araneomorphae | ||||
| Thomisidae | 7 | 0.60 | 5 | 14.7 |
| (n.i) | 4 | 0.34 | 4 | 11.8 |
| ACARI, Opilioacarida | 11 | 0.94 | 7 | 20.6 |
| SCORPIONIDAE (immature instar) | ||||
| Buthidae | ||||
| Tityus trivittatus | 4 | 0.34 | 3 | 8.8 |
| PLANTS | ||||
| ANGIOSPERMS | ||||
| Poaceae | ||||
| seeds | 17 | 1.46 | 7 | 20.6 |
| leaf | 9 | 0.77 | 6 | 17.6 |
| Polygonaceae (seeds) | 1 | 0.08 | 1 | 2.9 |
| Compositae | ||||
| Taraxum officinale (seeds) | 6 | 0.51 | 5 | 14.7 |
| ANIMAL FRACTION (n.i) | X | X | 26 | 76.5 |
| TOTAL NUMBER OF PREY | 1158 |
35 Individual toads, were examined including the 11 analyzed for alkaloids). n = Number of individuals per taxon, f (%) = percent of the total number of prey accounted for by that particular prey type, fa = number of gastrointestinal tracts with that prey category and FO (%) = frequency of occurrence. X = not quantified, n.i = not identified. In addition, many items, presumably of animal origin, occurred in 26 of the 35 gastrointestinal tracts but could not be identified.
Table 4.
Main prey items encountered in gastrointestinal tracts of Melanophryniscus cupreuscapularisa
| PREY TYPES | n | f (%) | fa | FO (%) |
|---|---|---|---|---|
| ARTHROPODA | ||||
| INSECTA | ||||
| HYMENOPTERA | ||||
| Formicidae | ||||
| Ecitoninae | ||||
| Labidus praedator | 1 | 0.41 | 1 | 8.3 |
| Eciton sp. | 5 | 2.1 | 2 | 16.6 |
| Myrmicinae | ||||
| Crematogaster quadriformis | 168 | 69.4 | 12 | 100 |
| Wasmannia auropunctata | 1 | 0.41 | 1 | 8.3 |
| Pheidole subaberrans | 20 | 8.3 | 4 | 33.3 |
| Acromyrmex ambiguus | 10 | 4.1 | 2 | 16.6 |
| COLEPTERA | ||||
| Melodidae | 2 | 0.82 | 1 | 8.3 |
| Crysomelidae | 1 | 0.41 | 1 | 8.3 |
| COLLEMBOLA | 2 | 0.82 | 1 | 8.3 |
| PROTURA | 11 | 4.5 | 3 | 25 |
| ARACHNIDA | ||||
| ARANAE | ||||
| Araneomorphae | 17 | 7.0 | 4 | 33.3 |
| SCORPIONIDAE (immature instar) | ||||
| Buthidae | ||||
| Tityus trivittatus | 2 | 0.82 | 2 | 8.3 |
| PLANTS | ||||
| POACEAE | ||||
| leaf | 1 | 0.41 | 1 | 0.83 |
| ANIMAL FRACTION (n.i) | X | X | 8 | 66.6 |
| TOTAL NUMBER OF PREY | 242 |
Twelve toads, were examined including the 11 analyzed for alkaloids. n = Number of individuals per taxon, f (%) = percent of the total number of prey accounted for by the particular prey type, fa = number of gastrointestinal tracts with the prey category and FO (%) = frequency of occurrence. X = not quantified, n.i = not identified. In addition, many items, presumably of animal origin, occurred in 8 of the 12 gastrointestinal tracts, but could not be identified. It should be noted that the prey detected in the present study and others do not represent the long term prey items that over the course of the toad’s life have given rise to the alkaloids stored in the skin glands
4. Discussion
Bufonid toads of the genus Melanophryniscus now have been found to have over 80 different alkaloids in skin extracts (Daly et al., 2005). The amounts and profiles differ considerable in different species. The Brazilian species Melanophryniscus moreirae, collected in 1979 from a cloud forest on Serra da Mantiquerira, 19 km NW of Itatiaia in the state of Rio de Janeiro, contained pumiliotoxin 267C as a major alkaloid and allopumiliotoxin 323B as a minor alkaloid (Daly et al., 1984). Subsequently, Melanophryniscus montevidensis collected from La Coronilla, Depto. Rocha, Uruguay was also shown to contain pumiliotoxin 267C as the major alkaloid and allopumiliotoxin 323B as a trace alkaloid (Garraffo et al., 1993). Recently, pumiliotoxin 251D was reported as a major alkaloid in some, but not all populations of Melanophryniscus montevidensis from Depto. Rocha, Uruguay (Mebs et al., 2005). Other pumiliotoxins were reported to be present, along with some unidentified indolizidines.
Two populations of Argentinian Melanophryniscus stelzneri collected in Tanti, Córdoba in May 1989 and Las Alpacas, Córdoba in November, 1987 from montane fields with spring-fed streams contained similar profiles of alkaloids (Garraffo et al., 1993). Major alkaloids included decahydroquinolines, 3,5-disubstituted indolizidines, 3,5- disubstituted pyrrolizidines and a 5,6,8-trisubstituted indolizidine. All of these alkaloids are suspected to have an ant or mite dietary origin (Daly et al., 2005; Takada et al., 2005, Saporito et al., 2007a, b). The two populations come from similar habitats and 21 of the 27 alkaloids were shared by both populations. In a recent study, the alkaloid profile from the 1989 collection of M. stelzneri was compared to the alkaloid profile for a 1999 collection from the same site (Daly et al., 2007). The profiles were similar except that the decahydroquinolines of the 1989 collection were all absent from the 1999 collection. Such decahydroquinolines are presumed to be obtained from dietary ants (Jones,et al.,1999).
Four populations of Argentinian Melanophryniscus rubriventris, collected in the provinces of Salta and Jujuy, all contained pumiliotoxins as major alkaloids (Daly et al., 2007). One population also contained the tricyclic alkaloid precoccinelline (193C) as a major alkaloid. The pumiliotoxins are highly toxic (Daly and Myers, 1967; Daly et al., 2003) and were proposed to be of mite origin. The precoccinelline probably originates from coccinellid beetles, but a mite source is also possible (Takada et al., 2005; Saporito et al., 2007b). The major pumiliotoxins in the M. rubriventris toads varied by population, but included PTXs 251D, 291G, 307G and 323A (Dalyet al.,2007).
The current study on two species of Melanophryniscus from similar habitats in fields near the cities of Corrientes, Corrientes and Resistencia, Chaco, Argentina, documents the presence of alkaloids in both species. Only three alkaloids were present as major or minor alkaloids in both species: 3,5-disubstituted indolizidine 239Q (1), 4,6- disubstituted quinolizidine 275I (2), and an alkaloid, 249F (3) postulated to be a homopumiliotoxin (Table 1 and Figure 1). However, while pumiliotoxin 251D (5) was present as a minor alkaloid in M. klappenbachi, it was absent in M. cupreuscapularis. Alkaloid 207S, tentatively proposed to have an indolizidine structure (Daly et al., 2005), is present as a trace alkaloid in M. klappenbachi and as a minor alkaloid in M. cupreuscapularis. It has an FTIR indicating a terminal double bond (νHC=, 3082 cm−1) very likely disubstituted (significant 1642, 893 cm−1; overtone 1790 cm−1) and an intense Bohlmann band at 2791 cm−1 typical of an indolizidine with three trans anti-parallel hydrogens. The structure is still under investigation, and will probably require revision. There are 30 other alkaloids detected in trace amounts, 24 of which were found in only one of the two species.
Even though both Melanophryniscus klappenbachi and M. cupreuscapularis are distributed exclusively in the Gran Chaco, their geographic ranges do not overlap. The former species is broadly distributed in the eastern Chacoan region, but also in parts of the western or dry Chaco. The latter species is restricted to the north-western province of Corrientes, on the eastern shore of the Paraná river, where it inhabits an environment known as “levee and northeastern subconcave plain” (Carnevali, 1994).
Melanophryniscus klappenbachi is slightly larger (SVL (mm) mean = 27. 7, range = 22.2–32.4, n = 76, Baldo, personal obs.) than M. cupreuscapularis (SVL mean = 23.9, 15 range = 21.825.8, n = 15, Baldo, personal obs.). The specimens included in this study were collected in localities quite close to each other, with similar climatic regimes and biotic compositions. The specimens of M. cupreuscapularis were collected in seasonally flooded grassy savannahs, where the tree Schinopsis balansae is the originally dominant element (Carnevali, 1994). Specimens of M. klappenbachi were collected in temporary marshes in remnants of deciduous xerophilous forest, with a predominance of grasses, cacti, and terrestrial bromeliads (Cabrera, 1976; Cabrera & Willink, 1980).
The first observations on the diet composition of species of Melanophryniscus extend back to the end of the 19th and the beginning of the 20th century, when earlier workers noticed the predominance of dietary ants in species of Melanophryniscus (Jiménez de la Espada, 1875 on an unidentified species of the genus; Fernández, 1926 on M. stelzneri stelzneri). Subsequently, more detailed studies corroborated a predominance of ants (mainly of the genus Crematogaster), and mites (mostly Oribatidae) in the diet of M. moreirae from the planalto of Itatiaia, in the state of Rio de Janeiro, Brazil (Bokerman, 1967). Filipello and Crespo (1992) indicate a preponderance of ants (no taxonomic information provided), followed by mites and termites in adults of M. stelzneri from San Luis, Argentina, whereas in the juveniles, the diet has a preponderance of Collembolans. A non-quantitative analysis of feces of M. montevidensis revealed the presence of ants (Pheidole sp.) and mites (Mebs et al., 2005). Skin alkaloids and stomach contents of four populations of M. rubriventris from NW Argentina were recently reported (Daly et al., 2007). Two of the populations had ants (38 and 41%) and mites (36 and 40%) as the major prey items, while one had ants (79%) as the only major prey items; the fourth had hemipterans (39.2%) followed by collembolans (22%) and mites (20%) as the major prey items. Pumiliotoxins, now presumed to be of mite origin (Saporitoet al., 2006; Takada et al., 2005) were the major alkaloids from all four populations. Indeed, a putative ant alkaloid, namely the 3,5-disubstituted indolizidine 223AB, was detected only from one population and then only in trace amounts.
The present analysis of contents of the intestinal tracts of M. cupreuscapularis and M. klappenbachi revealed that both contained a preponderance of ants (Table 3 and Table 4) of two subfamilies, Ecitoninae and Myrmicinae; these mymicines were collectively much more abundant in terms of frequency of occurrence. Ant remains were followed in preponderance by unidentified mites (order Opilioacarina), in M. klappenbachi, that were undetected in M. cupreuscapularis. In M. klappenbachi, the most frequently detected prey items were immature instars of Acromyrmex hispidus (61 %) followed by Wasmannia auropunctata (9 %), Pheidole subaberrans (7 %), Crematogaster quadriformis (6%), Labidus praedator, Pheidole sp., and Solenopsis sp. (see Table 3 for quantitative data). Of these genera, only the Solenopsis are known to contain alkaloids (T.H. Jones, personal communication).
In M. cupreuscapularis the most frequently detected prey items were Crematogaster quadriformis (69 %) followed by Pheidole subaberrans (8 %), Acromyrmex ambiguus (4 %), Eciton sp, Labidus praedator, and Wasmannia auropunctata (see Table 4 for quantitative data).
It now appears that mites will prove to be the dietary source of the pumiliotoxins and of the izidines, containing branch points in their carbon skeletons (Saporito et al., 2007b; Takada et al., 2005). However, mites were detected in only 20% and 33% of gastrointestinal tracts of M. klappenbachi and M. cupreuscapularis, respectively (Table 3 and Table 4). To our knowledge, mites of the order Opilioacarida have not been examined for alkaloids. Certain pumiliotoxins and related homopumiliotoxins were either minor (251D and 249F in M. klappenbachi) or major (249F in M. cupreuscapularis) alkaloid constituents (Table 1). There were other pumiliotoxin/ homo-pumiliotoxins and branched chain izidines (207A, 223D, 219G, 249L, 259E), but all in only trace amounts. It should be noted that pumiliotoxins 307A and 323A have been reported in extracts of two species of formicine ants of the genera Brachymyrmex and Paratrechina from Panama (Saporito et al., 2004). The unprecedented occurrence of such alkaloids in a formicine ant must perhaps be reconsidered in view of the detection of pumiliotoxins in mites and the possibility that mites have been prey items for these ants. Myrmicine ants have been well accepted as the probable source of the decahydroquinolines and izidines with linear carbonchain skeletons (Jones et al., 1999). Decahydroquinolines (four in M. klappenbachi and one in M. cupreuscapularis) were detected only in trace amounts (Table 1). None of these decahydroquinolines have been reported from an arthropod, although closely related alkaloids of this class have been found in ants of the Solenopsis, subgenus Diplorhoptrum (Jones et al., 1999). The 3,5-disubstituted indolizidines 195B and 223AB found in the present Melanophryniscus species as trace alkaloids also are well known from myrmicine ants, Monomorium pharaonis and Solenopsis (Diplorhoptrum) species, respectively. One of the major alkaloids 239Q of both Melanophriniscus species is a 3,5-disubstituted indolizidine, which is unusual in having a side-chain hydroxyl group. Such an indolizidine has not been detected in an ant, but an isomeric 3,5-disubstituted indolizidine with a ring hydroxyl group has recently been reported from a myrmicine ant Myrmicaria melanogaster from Brunei in the South Pacific (Jones et al., 2007). The other major alkaloid of M klappenbachi is the 4,6-disubstituted quinolizidine 275I. It is minor in M. cupreuscapularis. The only 4,6-disubstituted quinolizidine as yet reported from an arthropod is 195C from a myrmicine ant Solenopsis (Diplorhoptrum) species, picea group (Jones et al., 1999). Although ants, including immature instars of the myrmicine ant Acromyrmex hispidus represent major prey items, none are of genera known to contain decahydroquinoline or izidine alkaloids. Ants were collected at the M. klappenbachi site in November, 2001. These were Solenopsis tridens, a Pheidole species (perhaps containing some Solenopsis), and Crematogaster quadriformis. Methanol extracts of the Solenopsis and Pheidole confirmed several 2-methyl-6-substituted piperidines (data not shown), alkaloids known to occur in fire ants of the genus Solenopsis (MacConnell et al., 1971). Such alkaloids were not detected in the M. klappenbachi or M. cupreuscapularis, nor have they been found in prior studies of alkaloids of Melanophryniscus. The Crematogaster extract had no alkaloids.
Further studies on other species of Melanophryniscus toads are in progress and should provide additional evidence that habitat and thus availability of dietary alkaloid-containing prey are major factors in defining profiles of alkaloids in such bufonid toads. It appears almost certain that all of the alkaloids found in skin extracts from Melanophryniscus species are sequestered without change from dietary arthropods. Thus, it is not surprising that toads from distant locales share few alkaloids, while toads from close and similar habitats share a large number of alkaloids.
Bioactive molecules and phylogenetic relationships of Bufonids
Melanophryniscus is a very distinctive group of toads that currently comprises twenty described species. Several non-described species are also known. So far only 5 of these 20 species are known to have been screened for alkaloids, all with positive results. No lipophilic alkaloids have so far been reported in any of the other remaining bufonids.
Until 1980, toxic bufadienolides were known to occur in amphibians only in the widespread bufonid toads of the genus Bufo. Bufadienolides (Steyn and van Heerden, 1998) have an unusual C17 steroidal nucleus with an α-pyrone ring at the C-17 position and usually A–B and C–D cis-fused rings with 3β, 14β hydroxyl groups and often additional oxygenation, such as epoxide, ketone, acetoxyl or more hydroxyl groups. These are neutral lipophilic materials. If the C-3-alcohol is esterified with a C2-C8 dicarboxylic acid and this in turn has the other carboxyl group in an amide link with an amino acid such as arginine, the hydrophilic (polar) bufotoxin class results. If the lactone at C-17 is a butenolide, then the cardenolide class results with the equivalent conjugate at C-3 being referred to as a cardenobufotoxin. All four classes have been found in bufonids but bufadienolides and bufotoxins greatly predominate. Polar 3-O sulfates of bufadienolides and cardenolides have also been reported in bufonids. In plants, there are conjugates of bufadienolides and cardenolides with sugars.
In 1980 bufadienolides were reported in skin extracts of Atelopus with telocinobufagin and bufotalin being tentatively identified in extracts of A. ignescens (Flier et al., 1980). In addition, polar bufotoxin-like substances were detected in skin extracts of Melanophryniscus moreirae and Dendrophryniscus minutus (Flier et al., 1980).
Our own unpublished work (TFS and JWD) indicates that the aqueous methanol fraction, remaining after extraction of alkaloids and other lipophilic compounds with chloroform from Melanophryniscus moreirae, after storage in a freezer for 14 years, now shows by HPLC-UV-MS, at least 5 bufadienolide-like (UV λmax ≈ 300 nm) and three cardenolide-like (UV λmax ≈ 220 nm) non-polar substances with 1–2 exchangeable hydrogens in hydroxyl groups, one of which is acetylatable but in only one bufadienolide. These appear, from the MWs (354–384 amu), to be in the range of cardiotonic steroids. All are neutral, forming both [M + H]⊕, and [M + NH4]⊕ ions with APCI mass spectrometry and HPLC-acetonitrile-water solvent systems. Alkaloids typically form only [M + H]⊕ ions under these conditions. One or two losses of H2O and frequently one CO are seen from [M+H]⊕ for both classes while CO2 losses are seen only with the bufadienolides. The ammonium ion adducts are base peaks with the cardenolides but significantly weaker with bufadienolides. Additional work is underway to obtain more structural information on these presumed elimination products that have lost functionality at C-3. The structures of the polar bufadienolide-like toxins have, as yet, likewise not been elucidated.
Recently, bufadienolides were identified in skin extracts of Ansonia inthanon (arenobufagin, hellebrigenin, telocinobufagin, marinobufagin and two unidentified bufadienolides, Leptophryne borbonica (two unidentified bufadienolides), and Pedostibes hossi (arenobufagin, cinobufotalin, gamabufotalin, hellebrigenin, telocinobufagin and one unidentified bufadienolide (Daly et al., 2004). The bufadienolides were shown to be synthesized by the toad in the case of Rhinella schneideri (formerly Bufo paracnemis) (Porto and Gros, 1971), R. arenarum (formerly Bufo arenarum) (Garraffo and Gros, 1986) and appear to be synthesized by the toad Atelopus varius (Daly et al., 1997). The genus Bufo is now undergoing partition into several genera (see review of literature by Frost et al., 2006). This has added further genera, all of which were formerly Bufo, to those that have been reported to have bufadienolides. The former Bufo group that have been reported to contain bufadienolides are as follows: Amietophrynus regularis; Anaxyrus americanus, A. fowlerii, A. terrestris, A. woodhousii; Bufo bankorensis, B. bufo, B. gargarizans, B. japonicus; Cranopsis alvarius, C. valliceps; Dutaphrynus melanostictus; Ingerophrynus biporcatus, I. parvus; Peltophryne peltocephala; Phrynoidis asper; Pseudoepidalea raddei, P. viridis; Rhaebo blombergi; Rhinella arenarum, R. crucifer, R. granulosa, R. icterica, R. marina, R. schneideri, R. rubescens, R. spinulosa.
The taxonomic distribution of bufadienolides within bufonids also requires further research, but current data indicates the presence of bufadienolides, in assays of exemplars of the following genera: Amietophrynus (Flier et al., 1980), Anaxyrus (Shimada and Nambara, 1980), Ansonia (Daly et al., 2004), Atelopus (Flier et al., 1980), Bufo (Schröter et al., 1958), Dendrophryniscus (Flier et al., 1980), Duttaphrynus (Iseli et al., 1964), Ingerophrynus (Daly et al., 2004), Leptophryne (Daly et al., 2004), Melanophyrniscus (Flier et al., 1980; see above), Mertensophryne (Daly, unpublished results), Phrynoides (Shimada, et al., 1985; Daly et al., 2004), Pseudoepidalea (Shimada & Nambara, 1986), Peltophryne (Barbier et al., 1961), Ollotis (Barbier et al., 1961), Rhaebo (Barbier et al. 1961) and Rhinella, formerly Chaunus, Bufo (Schröter et al., 1958; Flier et al., 1980). This taxonomic distribution suggests that the presence of bufadienolides is a synapomorphy of Bufonidae.
Various phylogenetic studies with disparate taxonomic samplings have suggested that Melanophryniscus is the sister taxon of all remaining bufonids (Graybeal, 1997; Darst & Cannatella, 2004; Faivovich et al., 2005; Frost et al., 2006; Grant et al., 2006) or in a similar position forming a clade with a few other bufonid genera (Pramuk, 2006). Although the taxonomic distribution of sequestration within Melanophryniscus requires further studies, in the context of the phylogenetic hypothesis of Frost et al. (2006), the ability to sequester dietary alkaloids clearly optimizes as a synapomorphy of Melanophryniscus.
Alkaloids have so far not been reported in any of the bufonids except Melanophryniscus. Such lipophilic alkaloids have not been detected in the following bufonid genera: Amietophrynus, Ansonia, Atelopus, Dendrophryniscus, Ingerophrynus, Leptophryne, Mertensophryne, Pedostibes, Phrynoidis, Rhaebo, and Rhinella (Daly et al., 2004 and unpublished results). Related to this is the interesting observation of two types of skin glands in Melanophryniscus cupreuscapularis (Delfino, et al. 1998); one similar to the secretory glands of bufonids, the other thought to secrete “proteinaceous products.”
Other putatively defensive molecules present in bufonids include biogenic amines (Cei et al., 1968, 1972) and the tetrodotoxins (Daly et al., 1994, 1997, Kim et al., 1975, Mebs et al., 1995) and the related chiriquitoxin (Yotsu, et al., 1990) and zetekitoxin (Yotsu-Yamashita et al., 2004). The tetrodotoxins occur in at least four families of frogs including most species of Atelopus in Bufonidae (Daly, 2004), however they are not present in all Atelopus species. The fact that no tetrodotoxin-like inhibitory activity of [3H] saxitoxin binding test or presence of tetrodotoxin was recorded in ethanolic extracts of several other bufonids (Daly et al., 1994; Mebs et al., 1995; Daly, 2004) suggests that the ability to incorporate tetrodotoxins is likely a synapomorphy of at least some species of Atelopus. Other anurans known to have tetrodotoxins are (1) at least three of the eleven species of Brachycephalus (family Brachycephalidae, Sebben et al., 1986; Pires et al, 2005), (2) Colostethus inguinalis (family Dendrobatidae, Daly et al., 1994), and (3) an unidentified species of Polypedates (family Rhacophoridae, Tanu et al., 2001). These three are quite distant phylogenetically from bufonids (Frost et al., 2006) so the most parsimonious explanation is that 23 during the evolutionary history of anurans there have been at least four independent origins of the presence of tetrodotoxin, regardless of its specific mechanism of sequestration.
Bufadienolide-like compounds and biogenic amines (Cei et al., 1968) are present in Melanophryniscus (Flier et al, 1980). A recent study (Mebs et al., 2007) did not detect bufadienolides in extracts of M. atroluteus, M. devincenzii, and M. montevidensis, but the possible presence of the more polar bufadienolide-like toxins reported to be present in M. moreirae by Flier et al. (1980) was not unambiguously excluded from these three toads since no bioassays were reported. Current studies indicate that the polar bufadienolide-like compounds, present in extracts of Melanophryniscus moreirae hydrolyze over time to bufadienolides and cardenolides (unpublished results, TFS and JWD, see above) and it seems likely that polar bufadienolides are also present in M. montevidensis and make a major contribution to the toxicity of all toads of the genus Melanophryniscus. Indeed, in terms of interaction with the target Na/K ATPase responsible for toxicity, the levels of bufadienolide-like compounds in Melanophyrniscus moreirae exceeds the levels of bufadienolides in Amietophrynus regularis, Rhinella granulosa and R. marina (Flier et al., 1980).
Acknowledgements
We thank Ralph Saporito for valuable suggestions and references. Work at NIH was supported by intramural funds of NIDDK. PMP, JDB, and JF thank Rafael C. Lajmanovich, CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) and PICT (Proyecto de Investigación Científica y Tecnológica) 2006 223. JF thanks FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo) processes 2005/56756-0 and 2006/562088-5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbier M, Bharucha M, Chen KK, Deulofeu V, Iseli E, Jäger H, Kotake M, Rees R, Reichstein T, Schindler O, Weiss EK. Papierchromatographische Prüfung weiterer Krötensekrete. Helv Chim Acta. 1961;44:362–367. [Google Scholar]
- Bokermann WCA. Observacões sobre Melanophryniscus moreirae (Mir. Rib.) (Amphibia-Brachycephalidae) Anais da Academia Brasileira de Ciências. 1967;39:301–306. [Google Scholar]
- Cabrera AL. Regiones fitogeográficas argentinas. Encicl. Arg. Agric. Jard. 1976;2(1):1–85. [Google Scholar]
- Cabrera AL, Willink A. Biogeografía de América Latina. Serie de Biología, monografía N° 13, O.E.A. 2a edición corregida. 1980 [Google Scholar]
- Caldwell JP. The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae) Journal of Zoology (London) 1996;240:75–101. [Google Scholar]
- Carnevali R. Fitogeografía de la provincia de Corrientes. Gobierno de la Provincia de Corrientes. INTA. 1994 [Google Scholar]
- Cei JM, Erspamer V, Roseghini M. Taxonomic and evolutionary significance of biogenic amines and polypeptides occurring in amphibian skin. II. Toads of the genera Bufo and Melanophryniscus. Syst. Zool. 1968;17:232–245. [PubMed] [Google Scholar]
- Cei JM, Erspamer V, Roseghini M. Biogenic amines. In: Blair F, editor. Evolution in the Genus Bufo. Austin London: University of Texas Press; 1972. pp. 233–243. [Google Scholar]
- Daly JW, Myers CW. Toxicity of Panamanian poison frogs (Dendrobates): some biological and chemical aspects. Science. 1967;156:970–973. doi: 10.1126/science.156.3777.970. [DOI] [PubMed] [Google Scholar]
- Daly JW, Brown GB, Mensah-Dwumah M, Myers CW. Classification of skin alkaloids from Neotropical poison-dart frogs (Dendrobatidae) Toxicon. 1978;16:163–188. doi: 10.1016/0041-0101(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Daly JW, Highet RJ, Myers CW. Occurrence of skin alkaloids in non-dendrobatid frogs from Brazil (Bufonidae), Australia (Myobatrachidae) and Madagascar (Mantellinae) Toxicon. 1984;22:905–919. doi: 10.1016/0041-0101(84)90182-x. [DOI] [PubMed] [Google Scholar]
- Daly JW, Gusovsky F, Myers CW, Yotsu-Yamashita, Yasumoto T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis) with further reports for the bufonid genus Atelopus. Toxicon. 1994;32:279–285. doi: 10.1016/0041-0101(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett WL, Saunders RL, Cover JF., Jr. Absence of tetrodotoxins in a captive-raised riparian frog, Atelopus varius. Toxicon. 1997;35:705–709. doi: 10.1016/s0041-0101(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Daly JW, Kaneko T, Wilham J, Garraffo HM, Spande TF, Espinosa A, Donnelly MA. Bioactive alkaloids of frog skin: Combinatorial bioprospecting reveals that pumiliotoxins have an arthropod source. Proc. Natl. Acad. Sci. USA. 2002;99:13996–14001. doi: 10.1073/pnas.222551599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JW, Garraffo HM, Spande TF, Clark VC, Ma J, Ziffer H, Cover JF., Jr. Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid frogs of the genus Dendrobates. Proc. Natl. Acad. Sci. USA. 2003;100:11082–11092. doi: 10.1073/pnas.1834430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JW. Marine toxins and nonmarine toxins: convergence or symbiotic organisms? J. Nat. Prod. 2004;67:1211–1215. doi: 10.1021/np040016t. [DOI] [PubMed] [Google Scholar]
- Daly JW, Noimai N, Kongkathip B, Kongkathip N, Wilham JM, Garraffo HM, Spande TF, Nimit Y, Nabithabhata J, Chan-ard T. Biologically active substances from amphibians: preliminary studies on anurans from twenty-one genera of Thailand. Toxicon. 2004;44:805–815. doi: 10.1016/j.toxicon.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Daly JW, Spande TF, Garraffo HM. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]
- Daly JW, Wilham JM, Spande TF, Garraffo HM, Gil RR, Silva GL, Vaira M. Alkaloids in bufonid toads (Melanophryniscus): temporal and geographic determinants for two Argentinian species. J. Chem. Ecol. 2007;33:871–887. doi: 10.1007/s10886-007-9261-x. [DOI] [PubMed] [Google Scholar]
- Darst CR, Cannatella DC. Novel relationships among hyloid frogs inferred from 12S and 16S mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2004;31:462–475. doi: 10.1016/j.ympev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Darst CR, Menendez-Guerrero PA, Coloma LA, Cannatella DC. Evolution of dietary specialization and chemical defense in poison frogs (Dendrobatidae): A comparative analysis. American Naturalist. 2005;165(1):56–69. doi: 10.1086/426599. [DOI] [PubMed] [Google Scholar]
- Delfino G, Brizzi R, Kracke-Berndorff R, Alvarez B. Serous gland dimorphism in the skin of Melanophrniscus stelzneri (anura: bufonidae) J. of Morphology. 1998;237:19–32. doi: 10.1002/(SICI)1097-4687(199807)237:1<19::AID-JMOR2>3.0.CO;2-J. (This toad, M. cf. stelzneri was recognized later as a new species, M. cupreuscapularis) [DOI] [PubMed] [Google Scholar]
- Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheeler WC. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull. Am. Mus. Nat. Hist. 2005;294:1–240. [Google Scholar]
- Fernández K. Sobre la biología y reproducción de batracios argentinos Segunda Parte. Boletín de la Academia Nacional de Ciencias de Córdoba, Córdoba. 1926;29:271–320. [Google Scholar]
- Filipello AM, Crespo FA. Alimentación en Melanophryniscus stelzneri (Anura: Bufonidae) Cuadernos de Herpetología. 1992;8:18–24. [Google Scholar]
- Flier J, Edwards MW, Daly JW, Myers CW. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Science. 1980;208(4443):503–505. doi: 10.1126/science.6245447. [DOI] [PubMed] [Google Scholar]
- Frost DR, Grant T, Faivovich J, Haas A, Haddad CFB, Bain R, de Sá RO, Donnellan SC, Raxworthy CJ, Wilkinson M, Channing A, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green D, Wheeler WC. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006;297:1–370. [Google Scholar]
- Garraffo HM, Gros EG. Biosynthesis of bufadienolides in toads. XI. Experiments with [1,2-3H] cholesterol, [21-14C] coprostanol and 5-β-[21-14C] pregnanolone in the toad Bufo arenarum. Steroids. 1986;48:251–257. doi: 10.1016/0039-128x(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Garraffo HM, Spande TF, Daly JW, Baldessari A, Gros EG. Alkaloids from bufonid toads (Melanophryniscus): Decahydroquinolines, pumiliotoxins and homopumiliotoxins, indolizidines, pyrrolizidines, and quinolizidines. J. Nat. Prod. 1993;56:357–373. doi: 10.1021/np50093a008. [DOI] [PubMed] [Google Scholar]
- Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means BD, Noonan BP, Schargel WE, Wheeler WC. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae) Bull. Am. Mus. Nat. Hist. 2006;299:1–262. [Google Scholar]
- Graybeal A. Phylogenetic relationships of bufonid frogs and tests of alternate macroevolutionary hypotheses characterizing their radiation. Zool. J. Linn. Soc. 1997;119:297–338. [Google Scholar]
- Iseli E, Weiss E, Reichstein T, Chen KK. Papierchromatographische Prüfung der Sekrete von Bufo melanostictus Schneider und Bufo asper Gravenhorst. Helv. Chim. Acta. 1964;47:116–119. [Google Scholar]
- Jiménez de la Espada M. Tomo I: Batracios. Madrid: Imprenta de Miguel Ginesta; 1875. Vertebrados del viaje al Pacífico; 208 pp. [Google Scholar]
- Jones TH, Gorman JST, Snelling RR, Delabie JHC, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF. Further alkaloids common to ants and frogs: Decahydroquinolines and a quinolizidine. J. Chem. Ecol. 1999;25:1179–1193. [Google Scholar]
- Jones TH, Voegtle HL, Miras HM, Weatherford RG, Spande TF, Garraffo HM, Daly JW, Davidson DW, Snelling RR. Venom chemistry of the ant Myrmicaria melanogaster from Brunei. J. Nat. Prod. 2007;70:160–168. doi: 10.1021/np068034t. [DOI] [PubMed] [Google Scholar]
- Kim Y, Brown GB, Fuhrman F. Tetrodotoxin: Ocurrence in atelopid frogs of Costa Rica. Science. 1975;189(4197):151–152. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- Lescure J. L’alimentation du crapaud Bufo regularis Reuss et de la grenouille Dicroglossus ocipitalis (Gunther) au Senégal. Bull. de II.F.A.N. 1971;33(2):446–466. [Google Scholar]
- MacConnell JG, Blum MS, Fales HM. The Chemistry of fire ant venom. Tetrahedron. 1971;26:1129–1139. [Google Scholar]
- Mebs D, Yotsu-Yamashita M, Yasumoto T, Lötters S, Schluter A. Further report of tetrodotoxin in Atelopus species (Family Bufonidae) Toxicon. 1995;33:246–249. doi: 10.1016/0041-0101(94)00149-3. [DOI] [PubMed] [Google Scholar]
- Mebs M, Pogoda W, Maneyro R, Kwet A. Studies on the poisonous skin secretions of individual red bellied toads, Melanophryniscus montevidensis (Anura, Bufonidae), from Uruguay. Toxicon. 2005;46:641–650. doi: 10.1016/j.toxicon.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Mebs M, Wagner MG, Pogoda W, Maneyro R, Kwet A, Kauert G. Lack of bufadienolides in the skin secretions of red bellied toads, Melanophryniscus spp. (Anura, Bufonidae), from Uruguay. Comp. Biochem. Physiol. part C. 2007;144:398–402. doi: 10.1016/j.cbpc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Nelson A, Garraffo HM, Spande TF, Daly JW, Stevenson PJ. Facile synthesis of two diastereomeric indolizidines corresponding to the postulated structure of alkaloid 5,9E-259B from a bufonid toad (Melanophryniscus) Beilstein J. Org. Chem. 2008;4(No6) doi: 10.1186/1860-5397-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto AM, Gros E. Biosynthesis of the bufadienolide marinobufagin in toads Bufo paracnemis from cholesterol-20-14C. Experientia. 1971;27:506. doi: 10.1007/BF02147562. [DOI] [PubMed] [Google Scholar]
- Pramuk JB. Phylogeny of South American Bufo (Anura: Bufonidae) inferred from combined evidence. Zool. J. of the Linnean Soc. 2006;146:407–452. [Google Scholar]
- Santos JC, Coloma LA, Cannatella DC. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl. Acad. Sci. USA. 2003;100:12792–12797. doi: 10.1073/pnas.2133521100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT. Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid poison frogs. Proc. Natl. Acad. Sci. USA. 2004;101:8045–8050. doi: 10.1073/pnas.0402365101. A. 101(21), 8045-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW. Geographic and seasonal variation in alkaloid-based chemical defenses of Dendrobates pumilio from Bocas del Toro, Panama. J. Chem. Ecol. 2006;32:795–814. doi: 10.1007/s10886-006-9034-y. [DOI] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Jain P, Garraffo HM, Spande TF, Daly JW. Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon. 2007a;50:757–778. doi: 10.1016/j.toxicon.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW. Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc. Natl. Acad. Sci. USA. 2007b;104:8885–8890. doi: 10.1073/pnas.0702851104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H, Tamm C, Reichstein T. Beitrag zur Konstitutionsermittlung des Bufotalinins. Helv. Chim. Acta. 1958;41:720–735. [Google Scholar]
- Shimada K, Nambara T. Isolation and characterization of a new type of bufotoxin from the skin of Bufo americanus. Chem. Pharm. Bull. 1980;28:1559–1562. doi: 10.1248/cpb.28.1559. [DOI] [PubMed] [Google Scholar]
- Shimada K, Ohishi K, Nambara T. Studies on steroids CCVIII. Bufadienolides from Bufo asper. J. Nat. Prod. 1985;48:159. [Google Scholar]
- Shimada K, Ishi N, Nambara T. Occurrence of bufadienolides in the skin of Bufo viridis Laur. Chem. Pharm. Bull. 1986;34:3454–3457. doi: 10.1248/cpb.34.3454. [DOI] [PubMed] [Google Scholar]
- Smith BP, Tyler MJ, Kaneko T, Garraffo HM, Spande TF, Daly JW. Evidence for biosynthesis of pseudophrynamine alkaloids by an Australian myobatrachid frog (Pseudophryne) and sequestration of dietary pumiliotoxins. J. Nat. Prod. 2002;65:439–447. doi: 10.1021/np010506a. [DOI] [PubMed] [Google Scholar]
- Steyn PS, van Heerden FR. Bufadienolides of plant and animal origin. Nat. Prod. Rep. 1998;15:397–413. doi: 10.1039/a815397y. [DOI] [PubMed] [Google Scholar]
- Takada W, Sakata T, Shimano S, Enami Y, Mori N, Nishida R, Kuwahara Y. Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J. Chem. Ecol. 2005;31:2403–2415. doi: 10.1007/s10886-005-7109-9. [DOI] [PubMed] [Google Scholar]
- Toft CA. Evolution of Diet Specialization in Poison-Dart Frogs (Dendrobatidae) Herpetologica. 1995;51(2):202–216. [Google Scholar]
- Tokuyama T, Daly JW, Witkop B. The structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog Phyllobates aurotaenia and partial synthesis of batrachotoxin and its analogs and homologs. J. Am. Chem. Soc. 1969;91:3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]
- Vences M, Glaw F, Bohme W. Evolutionary correlates of microphagy in alkaloid-containing frogs (Amphibia : Anura) Zoologischer Anzeiger. 1998;236(4):217–230. [Google Scholar]
- Yotsu M, Yasumoto T, Kim YH, Naoki H, Kao CY. The structure of chiriquitoxin from the Costa Rican frog Atelopus chiriquiensis. Tet. Lett. 1990;31:3187–3190. [Google Scholar]
- Yotsu-Yamashita M, Kim YH, Dudley SCC, Jr., Chaudhary G, Pfahnl A, Oshima Y, Daly JW. The structure of zetekitoxin AB, a saxitoxin analog from the Panamanian golden frog Atelopus zeteki: A potent sodium-channel blocker. Proc. Natl. Acad. Sci. USA. 2004;101:4346–4351. doi: 10.1073/pnas.0400368101. [DOI] [PMC free article] [PubMed] [Google Scholar]