Abstract
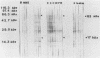
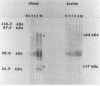
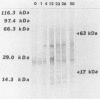
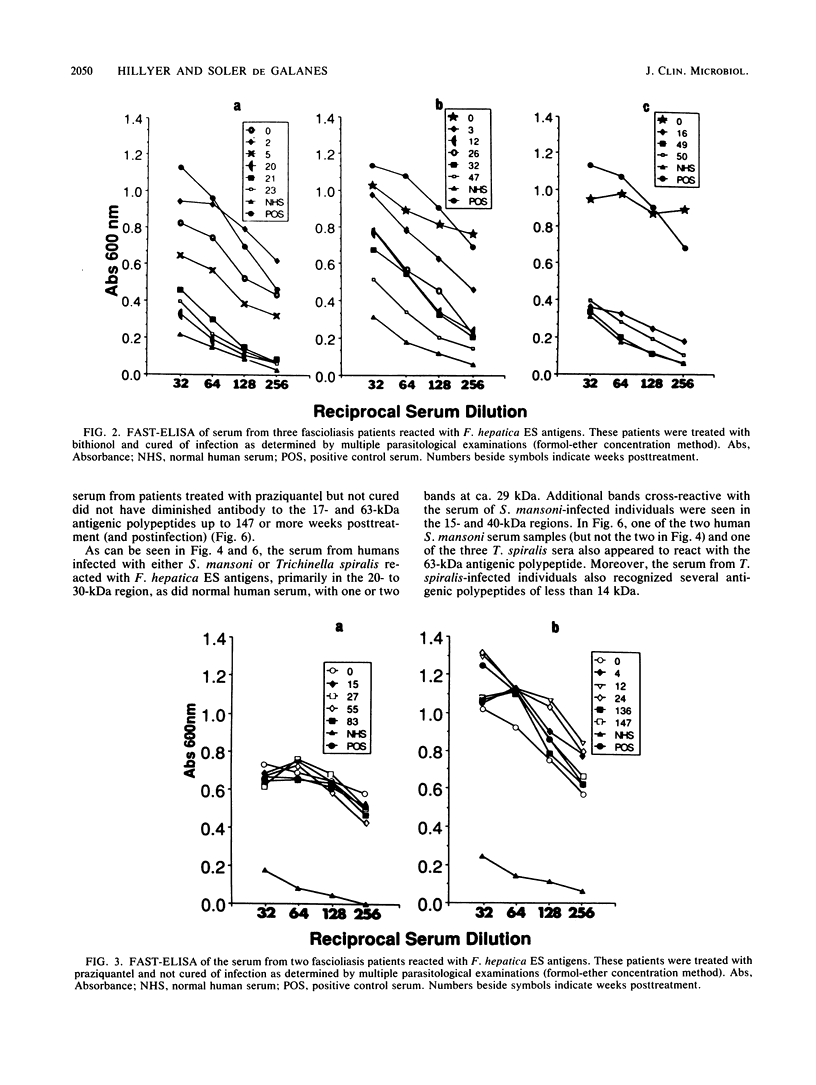
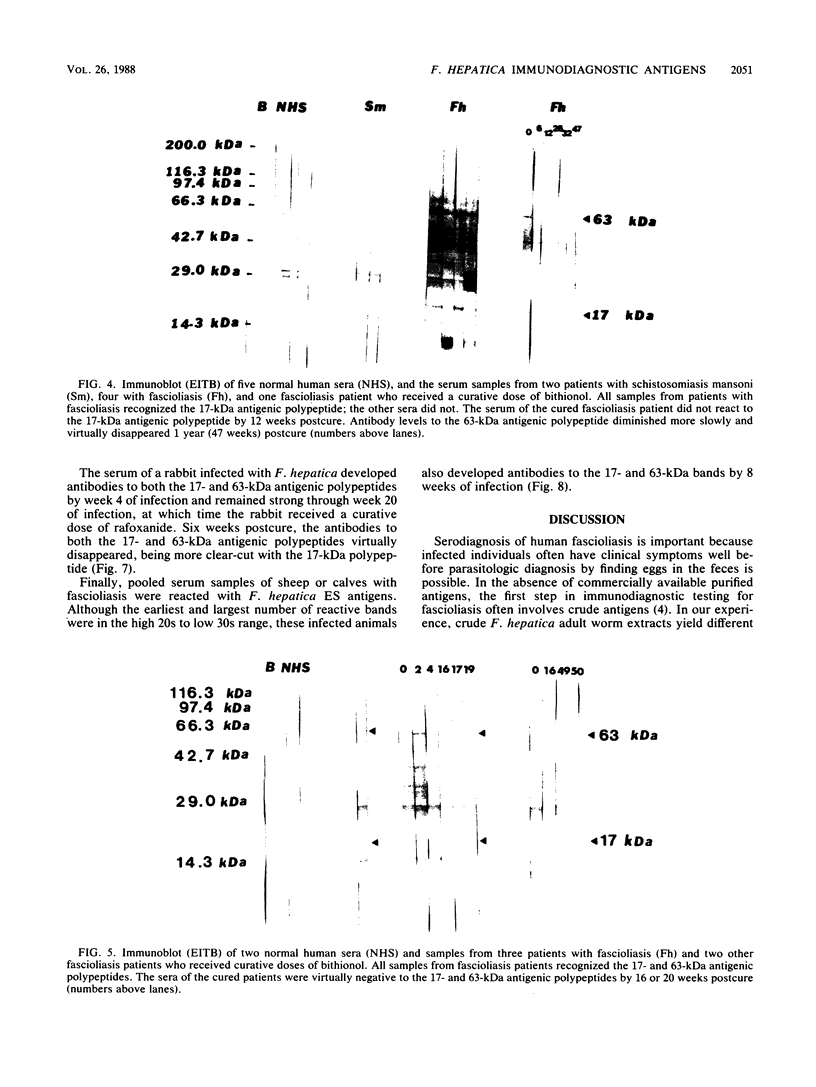
Sera obtained from human patients, calves, sheep, and rabbits infected with Fasciola hepatica were tested by the Falcon assay screening test enzyme-linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) techniques with Fasciola hepatica excretory-secretory antigens in order to evaluate their immunodiagnostic potential. The study included sera from 13 patients infected with F. hepatica or a history suggesting fascioliasis, 5 patients infected and treated with bithionol or praziquantel (3 were cured with bithionol), 10 patients infected with Schistosoma mansoni, 6 infected with Trichinella spiralis, and 13 controls and sera from calves, sheep, and rabbits with a primary F. hepatica infection. By FAST-ELISA with F. hepatica excretory-secretory antigens, the serum samples from fascioliasis patients gave the highest absorbance values, and the schistosomiasis patient sera gave intermediate values compared with a normal human serum control. Also by FAST-ELISA, the values for serum from patients with fascioliasis decreased steadily after cure, reaching normal levels 20 to 47 weeks postcure. In contrast, the serum from two patients who had been treated but were not yet cured had high levels of antibodies for up to 3 years of infection. By EITB, the serum samples from humans, rabbits, cattle, and sheep with fascioliasis recognized two antigenic polypeptides of 17 and 63 kilodaltons (kDa) in the form of sharp bands. For humans, this recognition lasted for at least 3 years of infection. Sera from individuals with schistosomiasis mansoni or trichinosis or from normal controls did not recognize the 17-kDa F. hepatica antigenic polypeptide. However, serum from one human with S. mansoni and one with T. spiralis infection has slight bands in the 63-kDa region, suggesting cross-reactivity. Reactivity to the 17-kDa polypeptide was absent in fascioliasis patients at 1 year postcure. Reactivity to the 63-kDa polypeptide was significantly diminished in fascioliasis patients at 1 year postcure. The sera from rabbits with a primary F. hepatica infection also recognized both the 17- and 63-kDa antigenic polypeptides by week 4 of infection. Reactivity to both antigens diminished significantly 6 weeks postcure and disappeared by 8 weeks postcure. The sera from infected cattle and sheep recognized these two antigenic polypeptides by week 8 of infection. These studies suggest that the 17-kDa F. hepatica excretory secretory antigen is an excellent candidate for the immunodiagnosis of acute and chronic fascioliasis. Purification of this antigen and its application to quantitative serologic tests will permit further analysis of its predictive value to evaluate cure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Espino A. M., Duménigo B. E., Fernández R., Finlay C. M. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg. 1987 Nov;37(3):605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. Development and optimization of the FAST-ELISA for detecting antibodies to Schistosoma mansoni. J Immunol Methods. 1986 Sep 27;92(2):167–176. doi: 10.1016/0022-1759(86)90162-6. [DOI] [PubMed] [Google Scholar]
- Hillyer G. V., Bermúdez R. H., Ramírez de Arellano G. Use of immunologic techniques to predict success of therapy in human fascioliasis: a case report. Bol Asoc Med P R. 1984 Mar;76(3):116–119. [PubMed] [Google Scholar]
- Hillyer G. V., Ruiz Tiben E., Knight W. B., Gómez de Rios I., Pelley R. P. Immunodiagnosis of infection with Schistosoma mansoni: comparison of ELISA, radioimmunoassay, and precipitation tests performed with antigens from eggs. Am J Trop Med Hyg. 1979 Jul;28(4):661–669. [PubMed] [Google Scholar]
- Hillyer G. V., del Llano de Díaz A. Use of immunologic techniques to detect chemotherapeutic success in infections with Fasciola hepatica. I. Rabbit infections. Am J Trop Med Hyg. 1976 Mar;25(2):307–311. doi: 10.4269/ajtmh.1976.25.307. [DOI] [PubMed] [Google Scholar]
- Irving D. O., Howell M. J. Characterization of excretory-secretory antigens of Fasciola hepatica. Parasitology. 1982 Aug;85(Pt 1):179–188. doi: 10.1017/s003118200005424x. [DOI] [PubMed] [Google Scholar]
- Knight W. B., Hiatt R. A., Cline B. L., Ritchie L. S. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni eggs. Am J Trop Med Hyg. 1976 Nov;25(6):818–823. doi: 10.4269/ajtmh.1976.25.818. [DOI] [PubMed] [Google Scholar]
- Reddington J. J., Leid R. W., Wescott R. B. A review of the antigens of Fasciola hepatica. Vet Parasitol. 1984 Jun;14(3-4):209–229. doi: 10.1016/0304-4017(84)90092-x. [DOI] [PubMed] [Google Scholar]
- Santiago N., Hillyer G. V., Garcia-Rosa M., Morales M. H. Identification of functional Fasciola hepatica antigens in experimental infections in rabbits. Am J Trop Med Hyg. 1986 Jan;35(1):135–140. doi: 10.4269/ajtmh.1986.35.135. [DOI] [PubMed] [Google Scholar]
- Santiago N., Hillyer G. V. Isolation of potential serodiagnostic Fasciola hepatica antigens by electroelution from polyacrylamide gels. Am J Trop Med Hyg. 1986 Nov;35(6):1210–1217. doi: 10.4269/ajtmh.1986.35.1210. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]