Abstract
Molecular motors drive key biological processes such as cell division, intracellular organelle transport, and sperm propulsion and defects in motor function can give rise to various human diseases. Two dimeric microtubule-based motor proteins, kinesin-1 and cytoplasmic dynein can take over one hundred steps without detaching from the track. In this review, we discuss how these processive motors coordinate the activities of their two identical motor domains so that they can walk along microtubules.
Introduction
Kinesin-1 and cytoplasmic dynein (herein referred to as kinesin and dynein) are two-headed motor proteins that use ATP-derived energy to transport a variety of intracellular cargoes toward the plus-ends and minus-ends of microtubules (MTs), respectively [1,2]. Kinesin and dynein can take many consecutive steps along their MT tracks without dissociating [3-8,9••], allowing them to shuttle cargoes over long distances spanning between a cell’s center and periphery. Such continuous movement (termed ‘processivity’) requires head–head coordination to prevent premature MT dissociation and futile cycling of ATP that does not give rise to productive steps. The molecular basis by which one motor domain might ‘sense’ and respond to the nucleotide/conformational state of its identical partner constitutes a major area of study (including processive myosin motors although they are not covered in this review).
A conceptual framework for thinking about intramolecular communication is that one motor domain stalls in a particular mechanochemical intermediate until the partner proceeds through a particular step. This has been coined a ‘gating mechanism’, since one motor domain has to wait until the partner head opens a ‘gate’ that allows it to proceed through the next steps in its mechanochemical cycle. Deciphering the molecular nature of the gate constitutes an important quest for the field. In principle, the gate could operate by controlling the rate at which the motor domain binds or releases from the MT filament (here termed a ‘polymer gate’) or by controlling the rate of a particular chemical transition in the ATPase cycle (here termed a ‘nucleotide gate’). As is true of many regulatory mechanisms in biology, motors may employ more than one type of gating mechanism. There must also be a ‘gatekeeper’ (e.g., a specific conformation of the motor or a chemical transition in the enzymatic site) that controls the opening of the gate. In this review, we first give short and very general overviews of the kinesin and dynein mechanisms. We then discuss recent findings that provide new insights into gating mechanisms that allow the head domains to communicate during processive motion. This topic also has received attention in other recent reviews [10-13].
Overview of the kinesin mechanism
Kinesin is composed of two identical heavy chains (HCs) and two associated light chains (Figure 1a) [1]. Each HC includes an N-terminal motor domain that houses catalytic activity followed by a coiled-coil stalk that facilitates dimerization. While stepping, ATP hydrolysis is coupled to an 8 nm center-of-mass displacement (Figure 2a) [14,15], the distance between adjacent tubulin heterodimers. While kinesin predominantly takes forward steps along a single MT protofilament [16,17,18••], an opposing force increases the probability of rearward stepping, and kinesin can walk backward at loads exceeding 7 pN [18••,19].
Figure 1. Schematic representations of kinesin and dynein and associated chains.
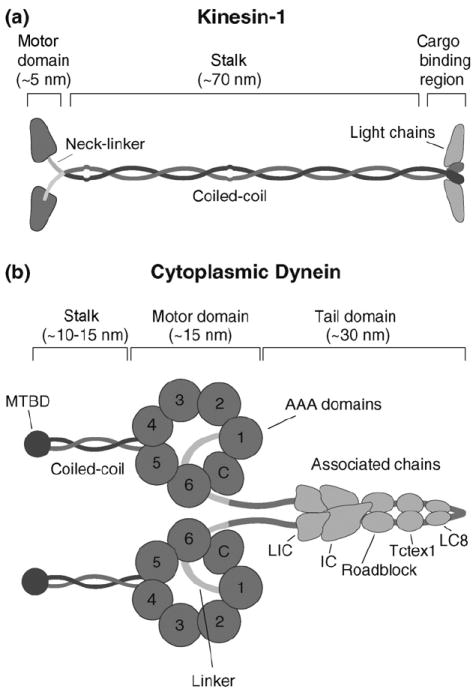
(a) Kinesin structure. Kinesin is composed of two identical heavy chains and two light chains. The heavy chain contains an N-terminal globular motor domain that possesses catalytic and MT-binding activity, a neck-linker element that connects the motor domain to the common coiled-coil dimerization domain, and a C-terminal light chain and cargo-binding region. (b) Cartoon representation of dynein. Dynein is composed of two identical heavy chains and several associated chains. The heavy chain forms a C-terminal catalytic motor domain that consists of multiple AAA ATP-binding sites (1–4) and a coiled-coil stalk with the microtubule-binding domain (MTBD) at its tip; AAA domains 5 and 6 do not contain sequences associated with nucleotide binding and the C-terminus (C) does not contain sequences characteristic for AAA proteins. The motor and dimerization domains are joined by a linker element. Multiple associated chains bind to dynein’s tail domain (LIC, light intermediate chain; IC, intermediate chain; Roadblock, Tctex1 and LC8, light chains).
Figure 2. Models for kinesin and dynein stepping.
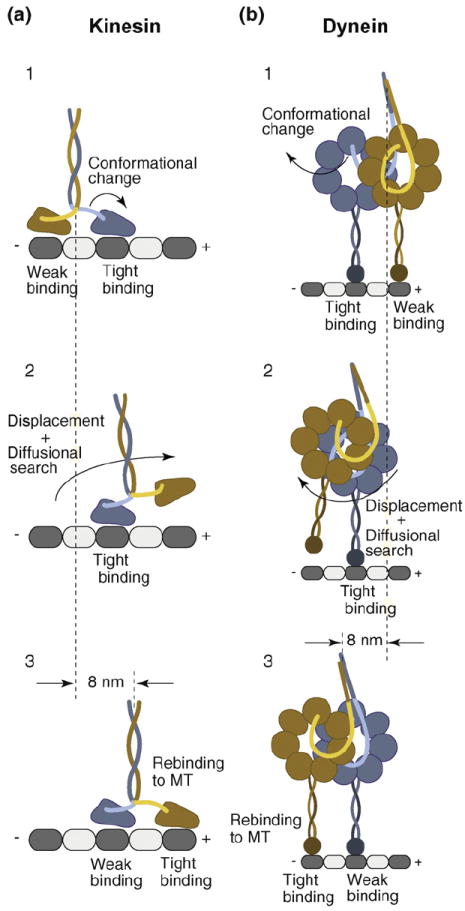
(a) Consensus stepping sequence of kinesin (for a detailed description, see text). A nucleotide-driven conformational change in the tightly MT-interacting front head displaces the weakly MT-interacting rear head toward the MT plus-end, biasing its diffusional search and rebinding to the next available MT-binding site in front of its partner head. While the rear head undergoes a 16 nm displacement, kinesin’s center-of-mass advances 8 nm. (b) Possible dynein stepping sequence. Dynein’s head domains are MT-bound with partially overlapping AAA rings, aligned parallel to the long MT axis. A nucleotide-dependent conformational change of the linker element in the tightly MT-binding front head displaces the weakly MT-interacting partner head toward the MT minus-end (opposite to the direction of kinesin movement). The displaced head then undergoes a rapid diffusional search and rebinds to the MT, resulting in a center-of-mass movement of 8 nm. Although dynein takes predominantly 8 nm steps, it has a considerable diffusional component to its step, resulting in different sized center-of-mass steps (4–24 nm).
Kinesin walks in a ‘hand-over-hand’ manner [e.g. [10]], with each head taking alternating 16 nm ‘steps’ (Figure 2a) [20]. This mechanism requires tight coupling between the biochemical cycles of both heads so that the front head remains bound to the MT while the rear head detaches. A conformational change in the front head then moves the detached head forward. This conformational change most probably occurs in a ~13 aa peptide that links the motor domain to the coiled-coil stalk called the neck linker (Figure 1a) [21]. Specifically, ATP binding to the MT-bound front head immobilizes the neck linker along the catalytic core [21], which shifts the rear head toward the MT plus-end (Figure 2a, state 1 and 2). Recent work also has suggested that the kinesin N-terminus may stabilize this docked conformation of the neck linker [22••]. After a rapid diffusional search, the detached head rebinds to the MT in front of its partner. Evidence is accumulating for a neck-linker conformational change [23-26], but how neck-linker docking is affected by load and the precise role of neck-linker conformational changes in head–head coordination are still open questions.
Overview of the dynein mechanism
Unlike kinesin, relatively little is known about the molecular mechanism of dynein (Figure 1b). Dynein, which is a member of the AAA+ family (AAA: ATPase associated with various cellular activities), is involved in diverse processes in eukaryotic cells, such as spindle formation, chromosome segregation, and the trafficking of organelles and mRNA [2]. Dynein is a large protein complex (1.2 MDa) composed of two identical heavy chains and several associated chains [1]. The heavy chain contains six AAA domains arranged in a ring [27]. The first four domains (AAA1–AAA4) have conserved ATP binding/ hydrolysis motifs. AAA1 is essential for dynein motility, while the other sites may play regulatory roles [28-30]. In contrast to kinesin and myosin, whose MT interfaces are located on the surface of their ATPase cores, dynein’s MT-binding domain (MTBD) is located at the end of a ~15 nm long coiled-coil stalk [27,31] that emerges between AAA4 and AAA5.
Single-molecule studies suggest that dynein walks in a hand-over-hand-like fashion (Figure 2b), although its stepping behavior and directionality are more irregular than kinesin’s [6-8,9••]. Evidence has shown that dynein advances via coordination of its two motor domains [8,32], but the details of cytoplasmic dynein’s step size, stall force, and directionality remain controversial [6-8,9••,33]. Observations of variable step sizes (4–32 nm) and directionality suggest a considerable diffusional component to its step, making dynein stepping more akin to that of myosin VI (21–51 nm) [34] than kinesin. A ~10 nm long ‘linker’ that connects the AAA ring to the dimerization domain has been suggested to power dynein motion (Figure 1b) [31]. The linker shifts its position relative to the catalytic ring during the ATPase cycle [31,35] and is believed to facilitate dynein motility (Figure 2b, steps 1 and 2) [8,32]. However, with no atomic structure for dynein in hand, a structural model for dynein’s motility is less advanced than that for kinesin.
Potential pathways for head–head communication
A gating mechanism requires that one motor domain can influence the action of its partner. Before we discuss detailed studies for kinesin and dynein, we consider some general models for how this might occur. Possible models for gating are discussed separately in this review. However, many of these mechanisms are not mutually exclusive and it is likely that motors employ more than one gating strategy.
One possibility is that the two motor domains are interacting with one another directly during an intermediate in the ATPase cycle. An example of such a model was proposed by Alonso et al. [36••] (Figure 3a). In their model, kinesin waits in-between steps as a one-head-bound intermediate; the detached tethered head is parked against the MT-bound head toward the MT plus-end and this interaction creates a ‘polymer gate’ by preventing the tethered head from binding to tubulin. This gate is opened when ATP binds to the MT-bound head (the ‘gatekeeper’ is ATP binding), which relieves the interaction between the two motor domains, thus allowing the tethered head to rebind to the MT and take a step. However, a new study suggests that the ‘stepping’ head is not parked in front of the MT-bound head [37••]. Nevertheless, one cannot rule out head–head interaction contributing to gating, perhaps as a back-up mechanism. Direct interactions between the motor domains also is plausible for dynein, where the two AAA rings are likely to be very close to one another in the MT-bound state (Figure 2b).
Figure 3. Potential head–head coordination mechanisms.
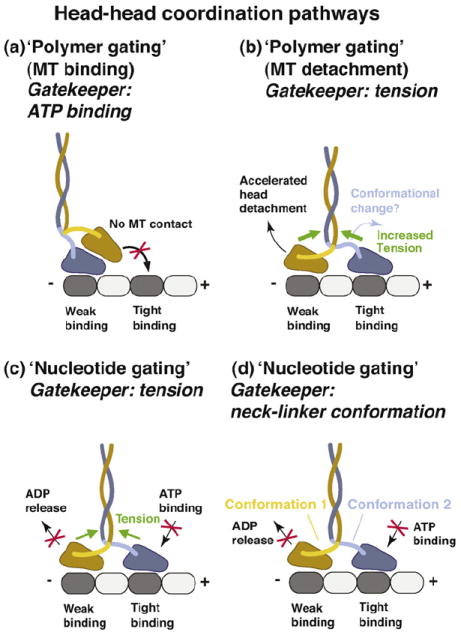
(a) In this tension-independent, ‘polymer gate’ pathway, the tethered head is detached from the MT and parked against the MT-bound head toward the MT plus-end. This interaction prevents the tethered head from binding to tubulin. The gate is opened when ATP binds to the MT-bound head (the ‘gatekeeper’ is ATP binding). (b) Internal tension might affect the MT-affinity of the head domains (‘polymer gating’). In this pathway, forward tension on the rear head is suggested to weaken its MT-affinity and promote its detachment. Acceleration of rear head detachment might be enhanced by the initiation of neck-linker docking in the front head (the ATP-driven conformation change). However, to be consistent with models in which the front head cannot bind ATP until the rear head detaches [50], the ADP-bound rear head must be sufficiently mobile to relieve the block on the front head. (c) Internal tension (the ‘gatekeeper’), derived from the simultaneous binding of both motor domains to the MT, controls the binding/release of nucleotides (‘nucleotide gating’). Backward tension on the front head transmitted via the neck-linker elements is suggested to prevent ATP binding, while the forward tension on the rear head is proposed to inhibit ADP release, thus helping to keep both heads out-of-phase. (d) Neck-linker conformation-based mechanism. In this pathway, the neck-linker elements might act as ‘control levers’ to regulate the binding/release of nucleotides allosterically. The backward-pointing/backward-docked orientation in the front head might prevent ATP binding and the forward-pointing/ forward-docked orientation in the trailing head might inhibit ADP release. Although tension might assist in creating and/or maintaining these conformations, the neck-linker conformation-based mechanism discussed here envisions the neck linkers as active regulatory elements, in contrast to the tension-based gating mechanisms described in parts (b) and (c) of this figure, in which the neck-linker elements serve as passive intermediaries that relay tension to the nucleotide and polymer binding sites.
Motor domains also might influence each other without being in direct contact. Such ‘action-at-a-distance’ models require simultaneous MT-binding of both motor domains (Figure 3b-d). One way in which this could be accomplished is through intramolecular tension, which could develop when the motor’s solution structure distorts to allow the heads to separate and to simultaneously bind to the MT. The resulting rearward tension on the front head and the forward tension on the rear head could affect the nucleotide or MT affinity of both heads asymmetrically (Figure 3b and c), creating gating mechanisms that will be discussed in more detail below. Two MT-bound motor domains also could communicate on the basis of the conformations of the mechanical elements that connect both heads rather than the magnitude of tension applied to these elements (Figure 3d) [37••,38]. Finally, the MT itself might serve as a communication pathway, since motor binding to the MT can induce structural changes in the MT lattice [39] (not covered further in this review). The next section focuses on recent studies on kinesin and dynein, which provide support for these ‘action-at-a-distance’ communication mechanisms.
Coordinating kinesin movement
Does intramolecular tension facilitate coordination of kinesin’s two motor domains?
Most models for tension sensing in head–head communication require that both kinesin heads are bound simultaneously to adjacent tubulin binding sites. In this two-head-bound waiting state, the kinesin neck linker in the front head must extend backward and the neck linker in the rear head must extend forward (Figure 3b). Does such a state exist? The alternating 16 and 0 nm steps observed by single-molecule fluorescence microscopy at rate-limiting ATP concentration [20] are most consistent with a two-head-bound waiting state. A recent single-molecule FRET study also supports a two-head-bound waiting state when kinesin moves processively at physiological ATP concentrations (at which the trailing head’s transition into the weakly MT binding ADP state is limited by ATP hydrolysis and phosphate release) [37••], which is in agreement with previous results [38]. However, the study of Mori et al. also suggested that there is a one-head-bound waiting state at very low ATP concentrations, when the rear head can enter a low MT affinity ADP state before ATP binds to the nucleotide-free front head. Unlike the Alonso et al. model [36••], their data indicates that the ‘detached’ head is positioned behind the MT-bound head. Even though ‘detached’, the rear head might weakly interact with the MT and might make brief transitions into the two-head-bound state [37••]. MT interaction of the rear head is in agreement with biochemical data from Hackney [40]. Thus, in summary, there is evidence for a two-head-bound waiting state when kinesin walks at physiological ATP concentrations. However, the nature of the waiting state at low ATP requires clarification, particularly with regard to the position and MT interactions of the rear head.
Tension sensing also has received support from experiments with mutant kinesins that had flexible spacers introduced between the neck linkers and the coiled-coil, which is predicted to reduce tension between the two motors domains. In a study by Hackney et al. [41], it was found that the chemical kinetic processivity (number of calculated ATP molecules hydrolyzed per MT encounter) of such kinesin mutants was greatly reduced. In a more recent single-molecule study, Yildiz et al. [18••] showed that kinesins with neck-linker extensions display normal processivity but have slow velocities owing to an impaired coupling of ATP hydrolysis forward stepping. This coupling defect could be reversed by recovery of tension by chemical crosslinking of the neck linkers or by applying external tension with an optical trap. These experiments strongly suggest that tension plays an important role in the coordination of kinesin’s head domains and that the dimensions and flexibility of the neck linkers are key to tension sensing. Where does the tension come from and what is its magnitude? The tension may derive simply from the MT-binding by the front head, since this alone would stretch out both neck linkers (Figure 3b). The magnitude of this tension can be estimated from ‘worm-like chain’ models that treat the neck-linker peptide as an entropic spring, but calculations for the forces required to stretch out the neck linkers differ considerably (between 4 pN [18••] and 12–15 pN [42•]). The tension also might be enhanced by the initiation of neck-linker docking in the front head (the ATP-driven conformation change) [18••].
A polymer gating mechanism?
As discussed earlier, one mechanism by which tension sensing could facilitate communication is through a ‘polymer gate’. Here we consider the idea of a gate in which the MT detachment of the rear head is facilitated by tension from the front head (with tension being the ‘gatekeeper’) (Figure 3b). This model suggests that the kinesin-MT interface is influenced by strain, a possibility that is receiving experimental support. This idea was first evoked by Hancock and Howard [43] who, on the basis of the studies of ATPase activity of monomeric and dimeric kinesin constructs, proposed that internal tension accelerates rear head detachment. More direct evidence was obtained by Uemura et al. [44], who showed that the unbinding force of a kinesin head is lower under external forward load than under backward load in all nucleotide states tested and concluded that kinesin’s rear head (ADP-Pi or ADP-bound state) is more likely to detach than the front head (nucleotide-free or ATP-bound state). In addition, recent optical trapping experiments show that kinesin steps processively along MTs under an external load in the presence or absence of nucleotides, with less force required for plus-end-directed than for minus-end-directed stepping [18••]. Earlier studies concluded that backward and forward stepping requires ATP binding [11,19]. However, the result from Yildiz et al. [18••] suggests that tension alone can drive repetitive nucleotide-independent MT attachment/detachment cycles of kinesin, emphasizing the role of tension-facilitated rear head detachment.
Tension-mediated release of the rear head might also act in synchrony with the ATPase cycles. Yildiz et al. showed that high forces (~9 pN) are required to detach the rear kinesin head and induce processive stepping in the presence of AMPPNP (a non-hydrolysable ATP analog that induces a tight binding state) but much lower forces (1–2 pN) are required with ADP, which promotes a weak binding state. If the internal tension between the heads is less than 9 pN but greater than 2 pN, then the detachment of the rear head would only occur at favorable rates when it proceeds through ATP hydrolysis and phosphate release to the reach the ADP state [18••]. Notably, however, Subramanian and Gelles [45••] recently reported that kinesin can take several consecutive forward steps along MTs with one head continuously bound to AMPPNP. In addition, Thoresen and Gelles [46••] showed that a kinesin heterodimer in which the ATPase activity of one head was blocked by a point mutation in the catalytic domain moved processively along MTs (as expected, however, a mutant homodimer capable of ATP binding but incapable of ATP hydrolysis, bound strongly to MTs but did not move). These studies indicate that a strongly MT-interacting rear head can be detached and moved forward by a front head capable of ATP binding and neck-linker docking, supporting the possibility that neck-linker docking can enhance the internal tension in the two-head bound waiting state. However, further experiments will be needed to determine definitely how dimeric motors with only one functional head walk along MTs.
A nucleotide gating mechanism?
The idea of nucleotide gating first emerged from a study by Hackney [47], who showed that dimeric kinesin released only one ADP when it bound to the MT; the release of the second ADP then required ATP binding to the MT-bound head [47,48]. This result suggests that ADP release of one head is gated until the partner head undergoes an ATP-driven neck-linker conformational change. The initial interpretation was that the ‘gated’ head was detached and unable to bind to the MT, but more recent experiments suggest that this head may be interacting with tubulin but still unable to release its ADP [37••,40]. One interpretation of this result is that forward tension on the neck linker inhibits ADP release (Figure 3c). By decreasing the rate of ADP release, the rear head also will remain in a weak MT binding state. In support of such a rear head nucleotide gating mechanism, elegant experiments by Uemura and Ishiwata [49] showed that the affinity of a kinesin head for ADP increases under forward tension while it decreases under backward tension.
Another model (not mutually exclusive) is that nucleotide gating takes place on the front head at the step of ATP binding. Specifically, ATP will not bind to the nucleotide-free front head because the neck linker is under backward tension (Figure 3c); after rear head detachment, this tension is relieved and then ATP can bind and induce neck-linker docking. In addition to allowing coordination of the chemical cycles, this gating mechanism increases the likelihood of rear versus front head detachment by maintaining the front head in a nucleotide-free, tight MT-bound state. Much of the evidence for this model comes from ATPase kinetic measurements of monomeric versus dimeric kinesin, the later assumed to reflect the cycles of processively moving kinesins [50,51]. However, front head nucleotide gating has also recently gained support by a compelling single-molecule study by Guydosh and Block [52]. The authors report that kinesin must first take an obligatory backward step to escape from a block created by the binding of a non-hydrolysable nucleotide to one of the two kinesin heads, suggesting that nucleotide release occurs preferentially in the front head. This result supports the notion that backward tension on the front head inhibits ATP binding but also lends support to a rear head gating mechanism in which forward tension on the rear head inhibits nucleotide release.
Something more than tension? Conformational states of the neck linker and gating
The simplest tension sensing mechanism envisions the neck linker as a stretched spring that relays tension to the nucleotide and polymer binding sites. However, it is possible that the neck linkers in the front and back heads adopt very specific and different conformations that allosterically control the enzymatic core. For example, the neck linker of the front head might not just be passively stretched backward but might adopt a specific ‘backward docked’ conformation (by forming bonds with the catalytic core), as has been seen in the crystal structure of Kinesin-5 (a mitotic kinesin) [53]. Alternatively, changes in the angle at which the neck linker emerges from the catalytic core could act allosterically on the nucleotide site. For example, the neck linker of the front head might be rotated backward in the two-head bound state (tension could assist in creating and/or maintaining this conformation). In such models, the neck linkers act as ‘control levers’ that keep both heads out-of-phase, the backward-pointing orientation in the front head possibly preventing ATP binding and the forward-pointing orientation in the trailing head inhibiting ADP release (Figure 3d). This possibility is supported by the finding that kinesin mutants with diminished internal tension move as processively as wild-type kinesin albeit at lower speed [18••], suggesting that the opposite-pointing neck-linker orientations in the leading and trailing mutant heads still allow for processivity albeit with less efficient coupling of ATP hydrolysis and forward stepping. However, although such a possibility seems plausible, it will require more experimental proof.
Coordinating dynein movement
There is general agreement that dynein moves processively [5-8,9••,33,54,55] but the underlying mechanism remains unknown. Some form of interhead coordination must exist in dynein, since dynein processivity requires two heads [8,32]. Furthermore, a truncated single-headed dynein spends less time MT-attached during its ATPase cycle than a head of a walking two-headed dynein [32], suggesting that mechanochemical steps in one head are affected by the presence of the second head. Hence, some form of nucleotide gating also is probably operational in walking dynein, but the mechanism is not known.
A role for tension and a ‘polymer gate’ in the dynein mechanism comes from recent experiments showing that dynein moves processively under a constant force in the absence of nucleotides [9••], similar to the results subsequently described for kinesin [18••]. The interpretation of this experiment is that the pulling force detaches the rear head and then biases it to pass the partner head before it rebinds to the MT. This result also suggests that both MTBDs are simultaneously bound to the MT before the rear head detaches, although direct evidence for this is lacking. Interestingly, a pulling force causes dynein to take processive steps in either direction along the MT, with less force required for minus-end-directed than for plus-end-directed stepping. This result demonstrates that dynein’s MT affinity is asymmetrically affected by load, with less force required to break the dynein-MT bond when pulled toward the MT minus-end (the normal direction of dynein movement). The lowering of dynein’s MT affinity under forward tension (which might be increased by a conformational change in the front head) may facilitate rear head detachment during processive movement [9••].
In contrast to the detailed information available on the kinesin neck linker, the type of the structural elements that interconnect the two dynein heads is not known. However, dynein’s step size and response to load might provide some clues. Dynein displays variable step sizes (4–32 nm) [6,8,9••] and the motor frequently takes backward steps [8,9••]. Dynein’s average center-of-mass step size of 8 nm [7,8,9••] is small compared with the dimensions of the dynein ring (13–15 nm) and stalk (10–15 nm). Dynein thus may need to adopt a compact form to constrain itself to take 8 nm steps [8]. Such a conformation could be achieved by overlapping AAA rings, aligned parallel to the long MT axis, a conformation similar as seen for axonemal dyneins [56]. The larger (e.g., 24 nm) steps of dynein, however, may require the heads to splay apart. This possibility gains support from a recent optical trapping study that showed that an opposing force from an optical trap can activate a ‘non-advancing’ stepping mode of dynein, characterized by large (12–24 nm) forward–backward displacements [9••]. This result suggests that an applied force can promote the ‘splayed’ conformation of the two rings. In support of this conclusion, shortening of the linker elements that connect both AAA rings decreased the occurrence and step size of non-advancing stepping [9••]. An important goal for the future is to measure the relative positions of the two dynein AAA rings during processive motion.
Recent single-molecule experiments suggest that dynein’s associated chains might regulate dynein’s interhead coordination and directionality. Ross et al. [33] showed that dynein–dynactin complexes can move continuously over micrometer distances toward the MT plus-end (opposite to the normal direction of dynein movement). Since there is small energy barrier difference for dynein stepping in the forward and backward directions under low loads [9••], it is possible that this energy barrier might be subject to modifications by dynein regulatory proteins.
Conclusions
Single-molecule and ensemble-based kinetic experiments have provided evidence that the structural elements that interconnect the two motor domains (neck linkers for kinesin and unknown structural linker elements in the case of dynein) are crucial for head–head coordination. These structural elements are under tension in case of kinesin, allowing a very tight coupling between ATP hydrolysis and forward stepping. The connection between the heads may be ‘more flexible’ for dynein, resulting in variable stepping and looser coupling (e.g., more backward steps). Interestingly, relaxing the connection between the kinesin heads results in a motor that displays stepping behavior that is more akin to dynein [18••]. The physiological significance of why these motors differ in their head–head coupling and stepping behavior is an interesting topic that is beginning to be explored [57•,58,59].
There is increasing evidence for ‘polymer gating’ for both kinesin and dynein, as demonstrated by the fact that both can be induced to move processively along their tracks in the absence of ATP [9••,18••]. Interestingly, both motors show asymmetric responses to strain: the polymer-binding site of kinesin more easily detaches when pulled toward the plus-end, while the opposite is true for dynein (consistent with their normal directions of travel).
For kinesin, there is compelling evidence for nucleotide gating although deciphering the exact mechanism(s) remains a challenge for the future. Good cases have been made for nucleotide gating in the rear head and the front head; potentially both might be correct. Direct measurement of chemical steps at the single molecule level, as recently demonstrated for myosin V [60••], might provide important new tools for this question. For dynein, nucleotide gating remains an unexplored issue awaiting future experimentation.
Acknowledgments
The authors would like to thank Nick Guydosh and Michael Diehl for helpful comments on the manuscript.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 2.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 3.Howard J, Hudspeth AJ, Vale RD. Movement of microtubules by single kinesin molecules. Nature. 1989;342:154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- 4.Block SM, Goldstein LS, Schnapp BJ. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Khan S, Sheetz MP. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys J. 1995;69:2011–2023. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;12:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 7.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc Natl Acad Sci U S A. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;29:335–348. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131:952–965. doi: 10.1016/j.cell.2007.10.016.. This study shows that external force (applied by an optical trap) can drive the processive bidirectional stepping of cytoplasmic dynein in the absence of nucleotides, with less force required for minus-end-directed than for plus-end-directed movement. The study also shows that an opposing force from an optical trap can activate a ‘non-advancing’ stepping mode of dynein, characterized by large (12–24 nm) forward–backward displacements that might reflect a ‘splayed’ conformation of the dynein rings.
- 10.Asbury CL. Kinesin: world’s tiniest biped. Curr Opin Cell Biol. 2005;17:89–97. doi: 10.1016/j.ceb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Carter NJ, Cross RA. Kinesin’s moonwalk. Curr Opin Cell Biol. 2006;18:61–67. doi: 10.1016/j.ceb.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Block SM. Kinesin motor mechanics: binding, stepping, tracking, gating, and limping. Biophys J. 2007;92:2986–2995. doi: 10.1529/biophysj.106.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentine MT, Gilbert SP. To step or not to step? How biochemistry and mechanics influence processivity in Kinesin and Eg5. Curr Opin Cell Biol. 2007;19:75–81. doi: 10.1016/j.ceb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnitzer MJ, Block SM. Kinesin hydrolyses one ATP per 8-nm step. Nature. 1997;388:386–390. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- 15.Hua W, Young EC, Fleming ML, Gelles J. Coupling of kinesin steps to ATP hydrolysis. Nature. 1997;388:390–393. doi: 10.1038/41118. [DOI] [PubMed] [Google Scholar]
- 16.Kuo SC, Gelles J, Steuer E, Sheetz MP. A model for kinesin movement from nanometer-level movements of kinesin and cytoplasmic dynein and force measurements. J Cell Sci Suppl. 1991;14:135–138. doi: 10.1242/jcs.1991.supplement_14.27. [DOI] [PubMed] [Google Scholar]
- 17.Ray S, Meyhoöfer E, Milligan RA, Howard J. Kinesin follows the microtubule’s protofilament axis. J Cell Biol. 1993;121:1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Yildiz A, Tomishige M, Gennerich A, Vale RD. Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell. 2008;134:1030–1041. doi: 10.1016/j.cell.2008.07.018.. This study demonstrates that kinesin mutants with artificially elongated neck linkers that lack internal tension advance processively, albeit at lower speed owing to an uncoupling of ATP hydrolysis and forward stepping. However, speed recovers to nearly normal levels when external tension is applied by an optical trap. In addition, the authors show that kinesin steps processively along MTs under an external load in the absence of nucleotides, with less force required for plus-end-directed than for minus-end-directed stepping.
- 19.Carter NJ, Cross RA. Mechanics of the kinesin step. Nature. 2005;435:308–312. doi: 10.1038/nature03528. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 21.Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 22••.Hwang W, Lang MJ, Karplus M. Force generation in kinesin hinges on cover-neck bundle formation. Structure. 2008;16:62–71. doi: 10.1016/j.str.2007.11.008.. Molecular dynamics simulations suggest that the conformational change of the neck linker is aided by the nine-residue-long N-terminal region, the ‘cover strand’
- 23.Rosenfeld SS, Jefferson GM, King PH. ATP reorients the neck linker of kinesin in two sequential steps. J Biol Chem. 2001;276:40167–40174. doi: 10.1074/jbc.M103899200. [DOI] [PubMed] [Google Scholar]
- 24.Skiniotis G, Surrey T, Altmann S, Gross H, Song YH, Mandelkow E, Hoenger A. Nucleotide-induced conformations in the neck region of dimeric kinesin. EMBO J. 2003;22:1518–1529. doi: 10.1093/emboj/cdg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asenjo AB, Weinberg Y, Sosa H. Nucleotide binding and hydrolysis induces a disorder-order transition in the kinesin neck-linker region. Nat Struct Mol Biol. 2006;13:648–654. doi: 10.1038/nsmb1109. [DOI] [PubMed] [Google Scholar]
- 26.Tomishige M, Stuurman N, Vale RD. Single-molecule observations of neck linker conformational changes in the kinesin motor protein. Nat Struct Mol Biol. 2006;13:887–894. doi: 10.1038/nsmb1151. [DOI] [PubMed] [Google Scholar]
- 27.Asai DJ, Koonce MP. The dynein heavy chain: structure, mechanics and evolution. Trends Cell Biol. 2001;11:196–202. doi: 10.1016/s0962-8924(01)01970-5. [DOI] [PubMed] [Google Scholar]
- 28.Reck-Peterson SL, Vale RD. Molecular dissection of the roles of nucleotide binding and hydrolysis in dynein’s AAA domains in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2004;101:1491–1495. doi: 10.1073/pnas.2637011100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Takahashi Y, Edamatsu M, Toyoshima YY. Multiple ATP-hydrolyzing sites that potentially function in cytoplasmic dynein. Proc Natl Acad Sci U S A. 2004;101:12865–12869. doi: 10.1073/pnas.0403429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 31.Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- 32.Shima T, Imamula K, Kon T, Ohkura R, Sutoh K. Head–head coordination is required for the processive motion of cytoplasmic dynein, an AAA+ molecular motor. J Struct Biol. 2006;156:182–189. doi: 10.1016/j.jsb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur EL. Processive bidirectional motion of dynein–dynactin complexes in vitro. Nat Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- 34.Rock RS, Rice SE, Wells AL, Purcell TJ, Spudich JA, Sweeney HL. Myosin VI is a processive motor with a large step size. Proc Natl Acad Sci U S A. 2001;98:13655–13659. doi: 10.1073/pnas.191512398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kon T, Mogami T, Ohkura R, Nishiura M, Sutoh K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nat Struct Mol Biol. 2005;12:513–519. doi: 10.1038/nsmb930. [DOI] [PubMed] [Google Scholar]
- 36••.Alonso MC, Drummond DR, Kain S, Hoeng J, Amos L, Cross RA. An ATP gate controls tubulin binding by the tethered head of kinesin-1. Science. 2007;316:120–123. doi: 10.1126/science.1136985.. This ensemble-based kinetic study shows that only one head of the kinesin dimer binds free tubulin in solution in the absence of ATP. A gaiting model is put forward that proposes that the ADP-containing tethered head in the waiting state is parked against the MT-bound head toward the MT plus-end and sterically/allosterically inhibited from contacting the MT surface. ATP binding to the tubulin-attached head is required to unblock the tubulin-binding site on the other head.
- 37••.Mori T, Vale RD, Tomishige M. How kinesin waits between steps. Nature. 2007;450:750–754. doi: 10.1038/nature06346.. This single-molecule FRET study suggests that kinesin waits for ATP in a one-head-bound waiting state but also indicates that the tethered head makes transitions to a two-head-bound intermediate. The authors provide evidence that the tethered head is positioned behind the strongly bound front head.
- 38.Asenjo AB, Krohn N, Sosa H. Configuration of the two kinesin motor domains during ATP hydrolysis. Nat Struct Biol. 2003;10:836–842. doi: 10.1038/nsb984. [DOI] [PubMed] [Google Scholar]
- 39.Krebs A, Goldie KN, Hoenger A. Complex formation with kinesin motor domains affects the structure of microtubules. J Mol Biol. 2004;335:139–153. doi: 10.1016/j.jmb.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Hackney DD. The tethered motor domain of a kinesin-microtubule complex catalyzes reversible synthesis of bound ATP. Proc Natl Acad Sci U S A. 2005;102:18338–18343. doi: 10.1073/pnas.0505288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackney DD, Stock MF, Moore J, Patterson RA. Modulation of kinesin half-site ADP release and kinetic processivity by a spacer between the head groups. Biochemistry. 2003;42:12011–12018. doi: 10.1021/bi0349118. [DOI] [PubMed] [Google Scholar]
- 42•.Hyeon C, Onuchic JN. Internal strain regulates the nucleotide binding site of the kinesin leading head. Proc Natl Acad Sci U S A. 2007;104:2175–2180. doi: 10.1073/pnas.0610939104.. In this theory study the authors estimate that the internal tension that develops in the two-head bound state of kinesin is 12–15 pN.
- 43.Hancock WO, Howard J. Processivity of the motor protein kinesin requires two heads. J Cell Biol. 1998;140:1395–1405. doi: 10.1083/jcb.140.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uemura S, Kawaguchi K, Yajima J, Edamatsu M, Toyoshima YY, Ishiwata S. Kinesin-microtubule binding depends on both nucleotide state and loading direction. Proc Natl Acad Sci U S A. 2002;99:5977–5981. doi: 10.1073/pnas.092546199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Subramanian R, Gelles J. Two distinct modes of processive kinesin movement in mixtures of ATP and AMP-PNP. J Gen Physiol. 2007;130:445–455. doi: 10.1085/jgp.200709866.. This single-molecule study provides evidence that kinesin can take several consecutive forward steps along MTs with one head continuously bound to the non-hydrolysable ATP analog AMPPNP, which suggests that a strongly MT-interacting rear head can be detached and moved forward by a front head capable of ATP binding and neck-linker docking. The study suggests that alternating-site catalysis is not essential for weak processive kinesin stepping.
- 46••.Thoresen T, Gelles J. Processive movement by a kinesin heterodimer with an inactivating mutation in one head. Biochemistry. 2008;47:9514–9521. doi: 10.1021/bi800747e.. This single-molecule study demonstrates that a kinesin heterodimer with one active head and one inactive mutant head can move processively along MTs with near-wild-type ATPase and motor activity. The authors suggest that internal strain in the kinesin heterodimer either complements the kinetic defect of the mutant head and allows it to hydrolysis ATP or is capable of detaching a strongly MT-interacting mutant head.
- 47.Hackney DD. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91:6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma YZ, Taylor EW. Interacting head mechanism of microtubule-kinesin ATPase. J Biol Chem. 1997;272:724–730. doi: 10.1074/jbc.272.2.724. [DOI] [PubMed] [Google Scholar]
- 49.Uemura S, Ishiwata S. Loading direction regulates the affinity of ADP for kinesin. Nat Struct Biol. 2003;10:308–311. doi: 10.1038/nsb911. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld SS, Fordyce PM, Jefferson GM, King PH, Block SM. Stepping and stretching. How kinesin uses internal strain to walk processively. J Biol Chem. 2003;278:18550–18556. doi: 10.1074/jbc.M300849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klumpp LM, Hoenger A, Gilbert SP. Kinesin’s second step. Proc Natl Acad Sci U S A. 2004;101:3444–3449. doi: 10.1073/pnas.0307691101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guydosh NR, Block SM. Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proc Natl Acad Sci U S A. 2006;103:8054–8059. doi: 10.1073/pnas.0600931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner J, Anderson R, Guo J, Beraud C, Fletterick R, Sakowicz R. Crystal structure of the mitotic spindle kinesin Eg5 reveals a novel conformation of the neck-linker. J Biol Chem. 2001;276:25496–25502. doi: 10.1074/jbc.M100395200. [DOI] [PubMed] [Google Scholar]
- 54.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 55.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 56.Nicastro D, McIntosh JR, Baumeister W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proc Natl Acad Sci U S A. 2005;102:15889–15894. doi: 10.1073/pnas.0508274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993.. This single-molecule study shows that kinesin and dynein bound to dynactin behave differently on tau-decorated MTs. While kinesin frequently dissociated from MTs when encountering patches of bound tau, dynein–dynactin complexes tended to reverse the direction of movement.
- 58.Ross JL, Shuman H, Holzbaur EL, Goldman YE. Kinesin and dynein–dynactin at intersecting microtubules: motor density affects dynein function. Biophys J. 2008;94:3115–3125. doi: 10.1529/biophysj.107.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vershinin M, Xu J, Razafsky DS, King SJ, Gross SP. Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic. 2008;9:882–892. doi: 10.1111/j.1600-0854.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Sakamoto T, Webb MR, Forgacs E, White HD, Sellers JR. Direct observation of the mechanochemical coupling in myosin Va during processive movement. Nature. 2008;455:128–132. doi: 10.1038/nature07188.. This single-molecule study demonstrates the visualization of the binding and dissociation of single fluorescently labeled nucleotides to and from myosin-V motors, while simultaneously observing the motor’s stepping motion along actin filaments. This work provides direct evidence for the tight coupling between forward stepping and the binding and dissociation of nucleotides.


