Abstract
Several instruments for diagnosing substance use disorders (SUD) have been developed, but to date none has emerged as the standard for community-based clinical studies. To select the most suitable SUD diagnostic instrument for its clinical trials, the National Drug Abuse Treatment Clinical Trials Network (CTN) implemented a procedure in which 36 university-based addiction researchers and 62 community-based addiction treatment providers evaluated and ranked five widely recognized diagnostic instruments: (1) the SUD section of the Structured Clinical Interview for DSM-IV (SCID); (2) the SUD section of the Composite International Diagnostic Interview, 2nd ed. (CIDI-2); (3) the SUD section of the Diagnostic Interview Schedule for DSM-IV Diagnosis (DIS-IV); (4) the Diagnostic Statistical Manual-IV Checklist (DSM-IV Checklist); and (5) the Substance Dependence Severity Scale (SDSS). To assist the evaluation and ranking process, key characteristics of each instrument were presented in tabular and narrative formats. Participants ranked each instrument from 1 (most preferred) to 5 (least preferred). The SCID received the best overall mean score (2.24) followed by the CIDI-2 (2.59), DIS (2.94), DSM Checklist (3.40) and the SDSS (3.83). After discussing the pragmatic and scientific advantages and disadvantages of each instrument, the CTN Steering Committee selected the CIDI-2. The selection of the CIDI-2 standardizes the collection of diagnostic data and provides a common diagnostic tool for practitioners and clinical researchers in the CTN. Implications for practice/research collaboration and initiatives are explored.
Keywords: Substance use disorder, Assessment, Diagnosis, Clinical trials
1. Introduction
The diagnosis of substance use disorders (SUD) is based primarily on the findings of an interview rather than the interpretation of laboratory results or other biological measures. Dependence upon an interview as the basis for making a diagnosis is not unique to SUD but rather is common to the majority of psychiatric conditions (Manley, 2000). While laboratory measures such as urine drug toxicology provide clinically useful information (Allen, Litten, Anton, & Cross, 1994; Anton, 2001; Chiang & Hawks, 1986), the interview process remains the primary source of data for SUD diagnosis. At the present time, ICD-10 (World Health Organization, 1992) and DSM-IV (American Psychiatric Association, 2000) are the primary nosologic systems used for establishing an SUD diagnosis.
To guide SUD diagnostic interviews, several structured and semi-structured instruments have been developed and shown to have good reliability and validity. Semi-structured interviews are designed to be administered by experienced clinical interviewers because they require interpretation of the interviewees’ responses. Fully structured interviews record the interviewee’s responses to items and can be administered by trained lay interviewers. Diagnostic instruments for SUD utilize a series of standardized questions to elicit symptoms from the interviewee and determine whether diagnostic criteria are met. The use of standardized probes, followup questions, and anchor points reduces information variance and potential interviewer bias.
There is currently no consensus as to which diagnostic instrument is best suited for making a SUD diagnosis, nor is there a clear basis for selecting one instrument over another. The use of different SUD diagnostic instruments complicates the comparison of patients, the conduct of meta-analytic research studies, and the evaluation of treatment programs because resulting diagnoses are made using different interviews and items. Further exacerbating the challenges arising from this variety of diagnostic instruments is the disparity between how clinicians and researchers determine a SUD diagnosis. While researchers tend to use one of five instruments, community-based addiction treatment providers (CTP) rarely use any standardized instrument to make an SUD diagnosis (Roman & Blum, 1997). This difference in how clinicians and researchers arrive at a diagnosis introduces an additional level of heterogeneity which is particularly important when conducting community-based clinical trials.
Many factors contribute to the challenge of researchers and practitioners achieving a consensus on a diagnostic instrument for use in community-based studies. Although several authors have compared the most commonly used SUD diagnostic measures (Cottler et al., 1997; Hasin et al., 1997; Hasin, 2003; Janca, Robins, Bucholz, Early, & Shayka, 1992; Pull et al., 1997; Ustun et al., 1997), the focus of these articles was primarily on the reliability and validity of the SUD diagnostic instruments under consideration. Although reliability and validity of SUD diagnostic instruments is of paramount importance in choosing a measure, a variety of other factors may also contribute to preference for one instrument over another. These considerations include time for administration, cost, computerization, interviewer qualifications, training burden, and diagnostic nosology.
The characteristics of the five most commonly used diagnostic instruments in drug abuse clinical research are summarized in Table 1, with 26 criteria for evaluating their suitability for use in practice and research. These criteria were developed by the authors to address a broad range of potential concerns that investigators and clinicians might have in adopting a SUD diagnostic instrument.
Table 1.
Diagnostic instruments comparison table
| DSM-IV Checklist1 | SDSS | SCID1 | DIS-IV1 | CIDI-21 | |
|---|---|---|---|---|---|
| Full Name | Diagnostic Statistical Manual-IV Checklist | Substance Dependence Severity Scale |
Structured Clinical Interview for DSM-IV |
Diagnostic Interview Schedule-IV | Composite International Diagnostic Interview-2 |
| Diagnostic Instrument Characteristics | |||||
| Provides SUD Diagnosis by DSM-IV criteria | X | X | X | X | |
| Assesses Lifetime SUD Symptoms | X | X | X | ||
| Assesses Past 30 day SUD Symptoms | X | X | X | X | X |
| Provides SUD Severity Index | X | X | |||
| Assesses SUD remission2 | X | X | X | ||
| Assesses Onset of Symptoms | X | X | X | ||
| Measures Frequency of Drug Use | X | X | |||
| Measures Amount of Drug Use | X | X | |||
| Psychometric Properties3 | |||||
| Reliability studies | X | X | X | X | X |
| Validity studies | X | X | X | X | X |
| Valid as SUD Treatment Outcome Measure | X | ||||
| Instrument Administration | |||||
| Time to Administer (minutes) | 10–15 | 30–45 | 20–30 | 15–25 | 20–30 |
| Interviewers with clinical background preferred | X | X | |||
| Challenge to Administerr4 | X | XX | XX | X | X |
| Public acceptability | |||||
| Prior use in published clinical trials | X | X | X | ||
| Prior use in published epidemiological studies | X | X | |||
| Accepted in first-tier journal publications | X | X | X | X | |
| Prior use by CTPs | |||||
| Automatization | |||||
| Computer data entry available | X | X | X | X | X |
| Computer scoring available | X | X | X | X | X |
| Computerized clinical summary | X | ||||
| Training considerations | |||||
| 2–3 days of training required | X | X | X | X | X |
| Training supervision requirements5 | X | XX | XX | X | X |
| Financial Considerations | |||||
| Proprietary Fees | X6 | X7 | X8 | ||
| International Considerations | |||||
| Provides SUD diagnosis by ICD-10 criteria | X | X | |||
| Translated into other languages | X | X | X | ||
Only the substance use sections of these instruments were included in this review.
Partial or full remission, early or sustained remission, with or without physiological dependence, on agonist therapy in a controlled environment.
There are no peer-reviewed articles comparing the relative reliability/validity of these five instruments; this table only notes whether reliability/validity studies have been published.
This row presents the reviewers assessment of the challenge of administering these instruments for interviewers. X = light to moderate challenge to administer; XX = moderate-to-heavy challenge to administer.
This row presents the reviewers assessment of the training supervision requirements for new interviewers using these instruments. X = light to moderate supervisory requirements; XX = moderate-to-heavy supervisory requirements.
$50 for unlimited use.
$2,000 per project or $15,000 for unlimited use.
$500 per project.
The purpose of this paper is to describe the process employed by the National Drug Abuse Clinical Trials Network (CTN) in selecting the diagnostic instrument it would use in its community-based, multi-site clinical trials. The CTN is a multi-center practice and research infrastructure linking university-based investigators and community-based treatment programs. Initiated by the National Institute on Drug Abuse in 1999, the mission of the CTN is to: (a) test empirically based medications and psychosocial innovations for the treatment of substance use disorders in diverse community treatment settings, with diverse patient populations; and (b) facilitate the transfer of these evidence-based innovations to the larger community of CTPs (NIDA, 1999). In 2001, the CTN consisted of 14 practice/research partnerships or “nodes” that joined approximately 100 community-based treatment organizations with a national network of university-based addiction research centers. A Steering Committee composed of the 14 principal investigators (PI) who were university-based researchers, 14 CTP representatives, plus the CTN Director and Deputy Director, makes policy decisions for the CTN and governs its ongoing operations.
The Director of the CTN requested that the first author facilitate a process to select the diagnostic instrument that would be used in all of the CTN’s studies. The expectation was that the use of one standardized diagnostic instrument in the CTN would allow reliable and valid comparisons across multiple settings and patient populations. Similarly, this standardization would facilitate communication among all parties that make SUD diagnoses or use those diagnoses to make policy and funding decisions. Finally, responsive to the CTN’s mission of transferring evidence-based innovations to CTPs, the selection of a single SUD diagnostic measure could provide an opportunity for treatment providers and researchers to learn about each other priorities in selecting a SUD diagnostic instrument.
The advantages of each diagnostic instrument considered in the CTN ranking process are presented below:
1.1. Diagnostic Interview Schedule for DSM-IV (DIS-IV)
The DIS was developed for psychiatric epidemiology research (Robins, HelzerF, Croughan, & Ratcliff, 1981). It is a fully structured interview that can be administered by lay interviewers, reducing training and supervision costs. The DIS-IV assesses for presence of a DSM-IV lifetime history of symptoms as well as current (past 12 months) symptoms. It records age of onset and time of most recent symptom occurrence. All responses are pre-coded and the interview has been adapted for computerization (Erdman et al., 1992). The substance use disorders section requires 15–25 min to administer and has demonstrated good reliability and validity (Malgady, Rogler, & Tryon, 1992). It has been translated into other languages and has been used in major epidemiological studies.
1.2. Composite International Diagnostic Interview-Second Edition (CIDI-2)
The CIDI was originally developed by the World Health Organization (Robins et al., 1988) to standardize the collection of psychiatric symptoms in its epidemiological studies, and then the Substance Abuse Module was modified and expanded (Cottler, 1991). The CIDI-2 (Kessler et al., 1998) is a fully structured, standardized instrument for assessment of substance abuse/dependence and other psychiatric disorders. It was developed for use in epidemiological studies and can be administered by trained lay interviewers who do not have a clinical background. It provides lifetime diagnoses (past and current) for substance use disorders according to DSM-IV and ICD-10 nosologies. In epidemiological research, the CIDI has demonstrated good reliability and validity for SUD diagnoses (Robins et al., 1989). The alcohol and drug use sections of the CIDI-2 typically require 20–30 min to administer. Computerized as well as paper-and-pencil systems have been developed for CIDI-2 administration, as well as data entry and scoring (Peters, Clark, & Carroll, 1998). Interviewer training on these sections typically requires 2 days.
1.3. DSM-IV Checklist
The DSM-IV Checklist is a semi-structured diagnostic instrument that provides current diagnoses for SUDs based on DSM-IV diagnostic criteria. The DSM Checklist was originally designed for the DSM-III (Helzer, Stoltzman, et al., 1985; Helzer, Robins, et al., 1985; Helzer, Spitznagel, & McEvoy, 1987) and subsequently has been updated for the DSM-IIIR (Hudziak et al., 1993) and the DSM-IV (Helzer, personal communication, 2002). Multiple versions of the DSM-IV Checklist have been developed for use in clinical research and community-based practice, but little is presently known about the reliability and validity of these updated instruments. DSM-IV Checklist requires only 10–15 min to administer and training requires 2–3 days. Typically, a background in psychiatric diagnosis is helpful, but the structured nature of the interview facilitates probing even in the absence of former clinical experience. There are no costs associated with use of the DSM-IV Checklist.
1.4. Structured Clinical Interview for DSM-IV (SCID)
The SCID is a semi-structured diagnostic instrument that provides lifetime diagnoses (past and current) for substance use disorders following DSM-IV nosology. In clinical research studies, the SCID has demonstrated good reliability and validity for SUD diagnoses (Albanese, Bartel, Bruno, Morgenbesser, & Schatzberg, 1994; Spitzer & Williams, 1985; Spitzer, Williams, Gibbon, & First, 1992; Williams et al., 1992). The SCID assesses age of onset for diagnostic symptoms and summarizes SUD remission status for patients with a past history of the disorder. The SCID is one of the most frequently used diagnostic instruments in clinical trials. It typically requires 20–30 min to administer the SUD module. Computerized systems have been developed for data entry and scoring. Interviewers are expected to have clinical interview skills and familiarity with diagnostic terminology, enabling training to be completed generally in 2–3 days.
1.5. Substance Dependence Severity Index (SDSS)
The SDSS was derived from the Psychiatric Research Interview for Substance and Mental Disorders (Hasin et al., 1996), a semi-structured interview for substance use and mental disorders. The SDSS assesses current (past 30 days) substance dependence and abuse symptoms (Miele et al., 2000a) and can be used to monitor changes in symptom severity. The SDSS can assess the current (past 30 days) symptoms of DSM-IV and ICD-10 drug and alcohol dependence and abuse, but not the past year. The instrument was designed specifically to measure changes in diagnostic severity over time. It measures quantity and frequency of recent drug use and is therefore sensitive to short-term variation in patient clinical status. Studies have shown good reliability and validity (Miele et al., 2000a, 2000b). The SDSS requires 30–45 min to administer. Training typically requires 2–3 days if trainees have background and expertise in diagnostic terminology. Computerized data entry and scoring programs are available.
2. Methods
A workgroup composed of clinical investigators and CTPs had originally been tasked with selecting the SUD diagnostic instrument for use in all CTN studies but after considerable effort the group was unable to come to a consensus. Initially, three instruments were considered (the SCID, the DSM-IV Checklist, and SDSS); later, as it became apparent that many CTN research technicians had limited clinical experience, two measures that had been used by trained lay interviewers in epidemiological studies—the DIS and CIDI—were added for consideration. To facilitate the resolution of the instrument selection process, the authors initiated the following procedures:
CTN Investigators and CTPs from each of the 14 nodes were invited to review and rank each of the five instruments mentioned above.1 The criteria for participation was being a member of the CTN and being “willing and able to take the time to carefully evaluate each instrument.” To facilitate the review process, the authors prepared and distributed to the node PIs a table comparing the five instruments along eight dimensions and 26 unique characteristics (see Table 1). In addition, brief summaries highlighting key features of each instrument were prepared and distributed. To minimize bias, nationally recognized experts in the development and use of diagnostic measures (see acknowledgements) were identified by the authors and asked to review the table and instrument summaries for accuracy and ease of interpretation. Based upon input from these experts, revisions were made to the table and instrument summaries. Nodes were given 6 weeks to complete their evaluation of the instruments and fax their rankings to the lead author.
An Individual Rank Order Form for capturing instrument rankings was distributed to each node with instructions for its completion. Respondents were instructed to write the number ’1’ next to the instrument they would most like to see adopted for use in the CTN, the number ’2’ next to their second choice. . . and so on. The form advised respondents that there could be no “tie rankings.” The form also instructed respondents to indicate whether they were a CTP, investigator or staff member (e.g. research technician or research coordinator) by placing a check next to the appropriate option.
A Node Rank Order Form was designed to capture all of individual rankings within each node as well as the overall ranking from the node PI. A section of this form provided for transposing the individual rankings from each completed Individual Rank Order Form on to the Node Rank Order Form in sections designated for the Investigator CTP or Staff rankings. In addition, this form included a section for the node PI to record an overall Node Ranking, using “whatever process he or she deemed reasonable and in the best interests of the CTN.”
The authors mailed the node PIs a letter describing the review and ranking procedures as well as copies of the five SUD instruments under consideration. Node PIs were asked to have the instruments and Individual Rank Order Forms duplicated and distributed within their node to co-investigators, CTPs and staff members. Rankings were entered by participants onto the Individual Rank Order Forms and then transferred by node staff to the Node Rank Order Form. In addition to these individual rankings, the node PIs were asked to enter their overall node ranking of the five instruments. Completed Node Rank Order Forms were faxed to the lead author, and rankings were entered into a database for analysis.
After the ranking procedures had been completed, the results were presented at a meeting of the CTN Steering Committee. The presentation of instrument rankings was followed by presentations by advocates for each of the five instruments. Members of the Steering Committee then questioned the presenters and discussed the relative merits of each instrument. Following this discussion, each of the 30 members of the CTN Steering Committee cast a vote to select the SUD diagnostic instrument that would be used in the CTN.
3. Results
Twelve of the 14 nodes submitted Node Rank Order Forms; from these forms, individual rank order data were obtained from 62 CTP representatives and 36 investigators.2 Actual response rates are unavailable since the total number of eligible CTP representatives and investigators could not be determined due to the subjectivity of eligibility criteria (i.e. “willing and able to take the time to carefully evaluate each instrument”). Using an estimate of six eligible CTP representatives and four eligible investigators per node yields an estimated response rate of 74% of CTPs and 64% of the investigators.
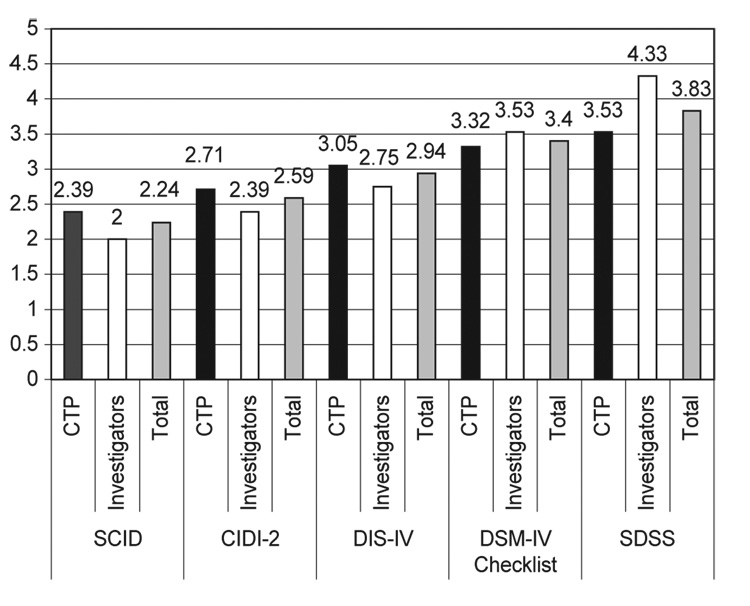
Mean rank order scores were calculated separately for investigators only, CTPs only, and CTPs and investigators combined. As seen in Table 2, the SCID received the best ranking from both the investigators and CTPs (combined), followed by the CIDI, DIS-IV, DSM-IV Checklist, and the SDSS. A repeated measures analysis of variance (investigator vs. CTP was the between subjects factor, and instrument was the within subjects factor) indicated that there were significant differences among the ratings of the instruments, F(4,384) = 20.89, p < .0001. Specific contrasts revealed that the SCID received lower (i.e. better) ratings than the DIS-IV, DSM Checklist, and SDSS. In addition, the CIDI and the DIS-IV received significantly lower ratings than the SDSS. It should be noted that the two instruments with the two best ratings, the SCID and CIDI, did not differ significantly from one another. As seen in Fig. 1, the provider and investigator mean rankings of the instruments were ordered similarly. Analyses indicated that provider and investigator rankings differed significantly only for one comparison, F(1,96) = 14.862, p < .0001. Specifically, investigators made a greater distinction between the SCID and SDSS than did providers.
Table 2.
Combined sample mean ratings of each instrument
| Instrument | |||||
|---|---|---|---|---|---|
| SCID | CIDI-2 | DIS-IV | DSM-IV checklist | SDSS | |
| M | 2.24a | 2.59ab | 2.94b | 3.40bc | 3.83c |
| SD | 1.03 | 1.52 | 1.09 | 1.65 | 1.11 |
Note: Means with different letters are significantly different from one another.
p < .025 (Bonferroni correction).
(n = 98)
Fig. 1.
Ranking order means of diagnostic instruments by investigators and community—based CTPs. Contrasts indicated a significant instrument × rater effect for ratings of the SCID relative to the SDSS, F(1,96) = 14.862, p < .0001. N = 62 CTPs, 36 Investigators; 98 Total. 1 = highest ranking, 5 = lowest ranking
Although both investigators and CTPs gave the SCID the highest mean ranking, after the CTN Steering Committee discussed the advantages of the top-ranked instruments, they ultimately selected the CIDI-2 with an overwhelming majority (26 to 4). Despite the higher ranking of the SCID, there were three reasons why the CTN Steering Committee chose the CIDI-2 over the SCID. First, SCID interviewers should be clinically experienced and preferably hold at least a masters degree in a mental health discipline. Since many of the CTN nodes employed research technicians who did not have prior clinical experience or hold a masters degree, adoption of the SCID was problematic. Second, during the Steering Committee discussion, CTP representatives noted that several states were going to require ICD-10 coding of SUD diagnoses. If the selected instrument was to have utility for CTPs, then it would need to be able to generate both DSM-IV and ICD-10 coded diagnoses. Only the SDSS and CIDI-2 provide diagnoses for both of these nosologies; the SCID does not. Finally, the SDSS was developed to measure change in symptom severity within a 30-day timeframe but did not provide the past year symptoms required for DSM-IV and ICD-10 diagnoses—an important consideration for CTPs. With these practical considerations in the balance, the CIDI-2 became the instrument of choice.
4. Discussion
The selection of the SUD diagnostic instrument for the CTN is a pragmatic example of the kind of practice/research collaboration called for in the 1998 Institute of Medicine report, Bridging the Gap (Lamb, Greenlick, & McCarty, 1998). In reviewing, ranking, and selecting the diagnostic instrument the CTN would use, investigators and treatment providers weighed the scientific and practical considerations that were important to them and came to a near unanimous agreement by selecting the CIDI-2. In light of what has been reported about the depth and breadth of the practice/research gap, it is remarkable that investigators and CTPs not only agreed on this instrument, but also arrived at the same relative ranking of the five instruments under consideration. While the highest and lowest rankings of investigators tended to be more extreme than those of CTPs, the mean ranks of both groups were identical.
Given the importance of diagnosis for both practice and research—and the absence of a definitive biologically based diagnostic procedure—the selection of a common diagnostic instrument is a potentially significant advance for addiction practice and research. The adoption of a common diagnostic instrument by both clinicians and researchers in the CTN is a meaningful step towards improving communications between leading addiction practitioners and clinical researchers by elevating the precision with which both parties can now assess the effectiveness of diagnosis-specific treatments. By selecting a research instrument that addresses many of the scientific and real world requirements of investigators and providers in the CTN, the needs of both constituencies were accommodated.
In order to resolve the SUD instrument selection process within three months, scientific rigor was sacrificed in favor of expediency. One consequence of this approach was that the eligibility criteria used for participation in the instrument evaluation process was left to the subjective determination of each CTN members; all CTN investigators and CTPs who were “willing and able to take the time to carefully evaluate each instrument” were invited to participate. Similarly, two of the 14 nodes did not complete the ranking forms within the time allotted and the level of participation across the remaining 12 nodes varied. As a result, the rankings presented in this study are not representative of the CTN or the larger community of practitioners and clinical researchers.
The procedures used in this ranking process did not control for how carefully participants studied the instruments, interacted with each other, or considered their rankings. Similarly, there was no effort to limit participation in the study only to those with a strong background in assessment, instrument development, or clinic management. On the contrary, the 98 respondents were self-selected members of the CTN who may or may not have had a background or particular interest in diagnostic procedures. Limitations of our procedures make it impossible to know whether rankings were made independently or how carefully the instruments were ranked. We do not know what processes were used within nodes to review the instruments or arrive at rankings. Anecdotal reports from several nodes indicate that CTPs and investigators met to discuss the instruments, so these discussions may have led to a mutual appreciation for the needs of both practitioners and researchers and contributed to the similarities of the rankings.
Finally, despite the efforts of the authors and multiple internal reviewers, the comparative table and narrative summaries of the five instruments did not include all possible details and clarifications about each instrument under consideration. For example, Table 1 indicates that there were no reliability or validity studies available for the DSM-IV Checklist, but a footnote could have indicated that reliability and validity studies on earlier versions of the DSM Checklist had been established.
The selection of the diagnostic instrument for the CTN’s assessment battery was a complex task that was resolved through an inclusive, multi-stage process involving the review and ranking of the instruments by investigators and providers followed by an open debate and vote. The similarity of rankings between investigators and CTPs, and the near unanimity of the final vote, indicates that the practice/research gap can be bridged, and that treatment providers and clinical investigators can find their way on to common ground. With the adoption of the CIDI-2 by the CTN, this instrument will be introduced to a broad spectrum of community-based clinicians and clinical investigators. Whether the CIDI-2—or other standardized SUD diagnostic instruments—will now be more widely used in community-based practices is unclear. The administration time and training burden associated with the use of such instruments in CTPs is substantial; with a greater appreciation of the administrative pressures and priorities of community-based providers, perhaps clinical researchers will begin to develop instruments and methods that are more compatible with realities of practice.
Acknowledgments
This project was funded in part by the National Institute on Drug Abuse Cooperative Research Grant Award 5-U10-DA-13043-03.
The authors wish to acknowledge Robert Brooner, Gloria Miele, George Woody, John Helzer, Robert Zucker, Dennis Donovan, Juris Mezinskis, Linda Cottler, John Cacciola, Thomas McLellan, Kathleen Carroll, Debbie Hasin, Jessica Peirce, Charlotte Royer-Malvestuto, and Botonya Harris for their assistance in preparation of the tables and instrument summaries. The authors also wish to acknowledge the CTN Steering Committee and CTN members that participated in the instrument reviews, ranking and selection.
Footnotes
The University of Pennsylvania Institutional Review Board determined that this study was exempt from review.
Data from respondents identifying themselves as “staff” were also obtained but are not presented in this article in order to highlight the comparison of investigators and practitioners—the principal participants in the practice/research gap. Data from staff did not differ significantly from investigators.
References
- Albanese MJ, Bartel RL, Bruno RF, Morgenbesser MW, Schatzberg AF. Comparison of measures used to determine substance abuse in an inpatient psychiatric population. American Journal of Psychiatry. 1994;151:1077–1078. doi: 10.1176/ajp.151.7.1077. [DOI] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Anton RF, Cross GM. Carbohydrate-deficient transferrin as a measure of immoderate drinking: remaining issues. Alcoholism: Clinical & Experimental Research. 1994;18:799–812. doi: 10.1111/j.1530-0277.1994.tb00043.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: Author; 2000. [Google Scholar]
- Anton RF. Carbohydrate-deficient transferrin for detection and monitoring of sustained heavy drinking. What have we learned? Where do we go from here? Alcohol. 2001;25:185–188. doi: 10.1016/s0741-8329(01)00165-3. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Implications of drug levels in body fluids: Basic concepts. In: Hawks RL, Chaing CN, editors. Urine testing for drugs of abuse. Rockville, MD: Department of Health and Human Services; 1986. (NIDA Research Monograph No. 73,pp. 62– 83) [Google Scholar]
- Cottler LB. The CICI and CIDI-substance abuse module (SAM):Cross-cultural instruments for assessing DSM-III, DSM-III-R and ICD-10 criteria. In: Harris LS, editor. Proceedings of the Committee on Problems of Drug Dependence, 1990. Rockville, MD: Department of Health and Human Services; 1991. (NIDA Research Monograph 105, DHHS Pub. No. ADM 91-1754, pp. 167–177) [PubMed] [Google Scholar]
- Cottler LB, Grant BF, Blaine J, Mavreas V, Pull C, Hasin D, Compton WM, Rubio-Stipec M, Mager D. Concordance of DSM-IV alcohol and drug use disorder criteria and diagnoses as measured by AUDADIS-ADR, CIDI and SCAN. Drug and Alcohol Dependence. 1997;47:195–205. doi: 10.1016/s0376-8716(97)00090-2. [DOI] [PubMed] [Google Scholar]
- Erdman HP, Klein MH, Greist JH, Skare SS, Justed JJ, Robins LN, Helzer JE, Goldring E, Hamburger M, Miller JP. A comparison of two computer-administered versions of the NIMH Diagnostic Interview Schedule. Journal of Psychiatric Research. 1992;26:85–95. doi: 10.1016/0022-3956(92)90019-k. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability in substance abusers. American Journal of Psychiatry. 1996;159:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Hasin D. Classification of alcohol use disorders. Alcohol Research and Health. 2003;27:5–17. [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Grant BF, Cottler L, Blaine J, Towle L, Ustun T, Sartorius N. Nosological comparisons of alcohol and drug diagnoses: A multisite, multi-instrument international study. Drug and Alcohol Dependence. 1997;47:217–226. doi: 10.1016/s0376-8716(97)00092-6. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Robins LN, McEvoy LT, Spitznagel EL, Stoltzman RK, Farmer A, Brockington IF. A comparison of clinical and DIS diagnoses: Physician reexamination of lay interviewed cases in the general population. Arch Gen Psychiatry. 1985;42:657–666. doi: 10.1001/archpsyc.1985.01790300019003. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Spitznagel EL, McEvoy L. The predictive validity of lay DIS diagnoses in the general population: A comparison with physician examiners. Archives of General Psychiatry. 1987;44:1069–1077. doi: 10.1001/archpsyc.1987.01800240045007. 1987. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Stoltzman RK, Farmer A, Brockington IF, Plesons D, Singerman B, Works J. Comparing the DIS with a DIS/DSM-III-based physician reevaluation. In: Eaton WW, Kessler LG, editors. Epidemiologic field methods in psychiatry: The NIMH Epidemiologic Catchment Area Program. New York: Academic Press; 1985. pp. 285–308. [Google Scholar]
- Hudziak JJ, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, Przybeck T. The use of the DSM-III-R Checklist for initial diagnostic assessment. Comprehensive Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Janca A, Robins LN, Bucholz KK, Early TS, Shayka JJ. Comparison of Composite International Diagnostic Interview and clinical DSM-III-R criteria checklist diagnoses. Acta Psychiatrica Scandinavica. 1992;85(6):440–443. doi: 10.1111/j.1600-0447.1992.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wittchen H-U, Abelson JM, McGonagle KA, Schwarz N, Kendler KS, Knäuper B, Zhao S. Methodological studies of the Composite International Diagnostic Interview (CIDI) in the US National Comorbidity Survey. International Journal of Methods in Psychiatric Research. 1998;7:33–55. [Google Scholar]
- Lamb S, Greenlick MR, McCarty D. Bridging the gap between practice and research: Forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Malgady RG, Rogler LH, Tryon WW. Issues of validity in the Diagnostic Interview Schedule. Journal of Psychiatric Research. 1992;26:59–67. doi: 10.1016/0022-3956(92)90016-h. [DOI] [PubMed] [Google Scholar]
- Manley MR. Psychiatric interview, history, and mental status examination. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 652–665. [Google Scholar]
- Miele G, Carpenter KM, Cockerham MS, Trautman KD, Blaine J, Hasin DS. Concurrent and predictive validity of the Substance Dependence Severity Scale. Drug and Alcohol Dependence. 2000a;59:77–88. doi: 10.1016/s0376-8716(99)00110-6. [DOI] [PubMed] [Google Scholar]
- Miele G, Carpenter KM, Cockerham MS, Trautman KD, Blaine J, Hasin DS. Substance Dependence Severity Scale (SDSS): reliability and validity of a clinician-administered interview for DSM-IV substance use disorders. Drug and Alcohol Dependence. 2000b;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. National Drug Abuse Treatment Clinical Trials Network Request for Proposals DA-99– 004. Bethesda, MD: 1999. Jan 11, p. 3. 1999. [Google Scholar]
- Peters L, Clark D, Carroll F. Are computerized interviews equivalent to human interviews: CIDI Auto versus CIDI. Psychological Medicine. 1998;28:893–901. doi: 10.1017/s0033291798006655. [DOI] [PubMed] [Google Scholar]
- Pull CB, Saunders JB, Mavreas V, Cottler L, Grant B, Hasin D, Blaine J, Mager D, Ustun BT. Concordance between ICD.10 alcohol and drug use disorder criteria and diagnoses as measured by the AUDADIS.ADR, CIDI and SCAN: Results of a cross-national study. Drug and Alcohol Dependence. 1997;47:207–216. doi: 10.1016/s0376-8716(97)00091-4. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KL. The NIMH diagnostic interview schedule: its history, characteristics and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablensky A, Pickens R, Regier DA, Sartorius N, Towle LH. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1989;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Roman PM, Blum TC. National Treatment Center Study, Institute for Behavioral Research. Athens, GA: University of Georgia; 1997. [Google Scholar]
- Spitzer RL, Williams JBW. Structured clinical interview for DSM-III-R, patient version. New York: Biometric Research Department, New York State Psychiatric Institute; 1985. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Ustun B, Compton WM, Mager D, Babor T, Baiyewu O, Chatterji S, Cottler L, Gogus A, Mavreas V, Peters L, Pull C, Saunders J, Smeets R, Stipec MR, Vrasti R, Hasin D, Room R, Van den Brink W, Regier D, Blaine J, Grant BF, Sartorius N. WHO Study on the reliability and validity of the alcohol and drug use disorder instruments: Overview of methods and results. Drug and Alcohol Dependence. 1997;47:161–169. doi: 10.1016/s0376-8716(97)00087-2. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howesx MJ, Kane J, Pope HG, Rounsaville B. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva, Switzerland: Author; International Classification of Diseases. (10th rev.) 1992